Abstract
Insertional mutagenesis by retroviral vectors is a major impediment to the clinical application of hematopoietic stem cell gene transfer for the treatment of hematologic disorders. We recently developed an insulated self-inactivating gammaretroviral vector, RMSinOFB, which uses a novel enhancer-blocking element that significantly decreases genotoxicity of retroviral integration. In this study, we used the RMSinOFB vector to evaluate the efficacy of a newly bioengineered factor VIII (fVIII) variant (efVIII)—containing a combination of A1 domain point mutations (L303E/F309S) and an extended partial B domain for improved secretion plus A2 domain mutations (R484A/R489A/P492A) for reduced immunogenicity—toward successful treatment of murine hemophilia A. In cell lines, efVIII was secreted at up to 6-fold higher levels than an L303E/F309S A1 domain–only fVIII variant (sfVIIIΔB). Most important, when compared with a conventional gammaretroviral vector expressing sfVIIIΔB, lower doses of RMSin-efVIII-OFB–transduced hematopoietic stem cells were needed to generate comparable curative fVIII levels in hemophilia A BALB/c mice after reduced-intensity total body irradiation or nonmyeloablative chemotherapy conditioning regimens. These data suggest that the safety-augmented RMSin-efVIII-OFB platform represents an encouraging step in the development of a clinically appropriate gene addition therapy for hemophilia A.
Introduction
Hemophilia A is a common inherited bleeding disorder caused by deficiency of coagulation factor VIII (fVIII).1 There is currently no cure for hemophilia A and the main treatment is fVIII replacement therapy, which is expensive, inconvenient, and is only offered to 30% of patients with hemophilia A worldwide.2 Furthermore, up to 25% to 30% of patients with severe hemophilia A develop inhibitory antibodies to fVIII, prompting the need for immune tolerance induction in these patients.3,4
Hematopoietic stem cells (HSCs) are an attractive target cell population for hemophilia A gene therapy because they are readily accessible for ex vivo genetic modification and allow for the possibility of sustained expression of a fVIII transgene in circulating peripheral blood (PB) cells for the lifetime of the patient following bone marrow (BM) transplantation.5 Retroviral vectors have been widely used for both experimental and clinical HSC gene therapy studies because they integrate into chromosomal DNA and are therefore stably transferred during HSC self-renewal and differentiative cell divisions.6 Using a conventional long terminal repeat (LTR) gammaretroviral vector encoding a secretion enhanced B domain–deleted (BDD) human fVIII transgene (sfVIIIΔB), we previously reported successful HSC gene therapy–based correction of hemophilia A in murine BM transplantation models.7,8 Unfortunately, insertional mutagenesis has become a major hurdle to gene therapy, and improvements in retroviral vector design are an absolute necessity to increase the biosafety of gene therapy protocols.9,10 The main mechanism of insertional mutagenesis is due to the activation of growth control genes by the strong enhancer-promoter sequences located in the retroviral LTRs.11 Although the absence of these sequences in self-inactivating (SIN) gammaretroviral vectors has been shown to significantly reduce mutagenic potential,12,13 insertional side effects still remain a concern for SIN vectors,14 especially in gene therapy settings where high levels of transgene expression, achieved with strong internal enhancer-promoter sequences or by multiple copy gene transfer,15 are required to obtain clinical benefits.
Another challenging problem in the development of a successful gene therapy for hemophilia A concerns the biochemical characteristics of fVIII that result in difficulties in its production.16 For example, it was recently shown that misfolding of newly synthesized fVIII in the endoplasmic reticulum lumen causes oxidative stress and induces apoptosis in vitro and in vivo in mice.17 However, as our knowledge of fVIII expression and secretion increases, it should be possible to design more efficient proteins that overcome the current limitations in fVIII production.5,18,19 Notably, point mutations within the A1 domain (L303E/F309S) that disrupt the interaction between fVIII and the chaperone HSPA5 (previously known as BiP/GRP78) results in a 3-fold increase in fVIII secretion.20,21 Similarly, incorporation of a short segment of the B domain harboring 6 N-linked glycosylated residues has been shown to significantly increase secretion compared with BDD-fVIII.17,18,22 Alternatively, a BDD porcine fVIII (BDD-pfVIII) has been developed and demonstrated to be expressed at 10- to 14-fold greater levels than human fVIII in vitro.23 BDD-pfVIII has also been shown to be efficiently secreted in hemophilia A mice that received transplants of LTR gammaretro-viral vector–transduced HSCs.24,25
As indicated here, the development of neutralizing antibodies to fVIII is another major complication of current protein replacement therapies for hemophilia A.3,4 Thus, a potential benefit of targeting HSCs for hemophilia A gene therapy is the possibility of inducing immune hyporesponsiveness and, ideally, stable long-term tolerance to an expressed transgene product.26,27 Nonetheless, a caveat associated with the use of a BDD-pfVIII transgene for human gene therapy is that the potential risk associated with the mounting of an immune response to the xenogeneic porcine coagulation protein remains unknown. Most of the fVIII inhibitory antibodies recognize epitopes localized to the A2 and C2 domains of the molecule.28 In this regard, recombinant BDD-fVIII containing mutations at R484A/R489A/P492A within the A2 epitope was reported to produce lower inhibitory antibody titers in C57-fVIIIKO mice (fVIII exon 16–disrupted knockout mice on the C57BL/6 background) while retaining full functionality.29
In this study, we have built upon several previous reports and combinatorially incorporated the individually validated results as the basis for the rational design of an improved fVIII transgene (efVIII) that encodes a more efficiently secreted and less immunogenic protein.17,18,20,21,29 To better approximate the clinical situation, we adapted a nonmyeloablative chemotherapy conditioning regimen to transplant HSCs that are stably transduced at low copy numbers with a new safety-augmented SIN gammaretroviral vector (RMSinOFB) expressing the efVIII transgene.25,30 Furthermore, we determined the effectiveness of the strategy by using a recently described hemophilia A mouse model (BAL-fVIIIKO; fVIII exon 16–disrupted knockout mice bred onto the BALB/c background) that develops a more potent inhibitory immune response to exogenous fVIII than the C57-fVIIIKO strain and therefore may more accurately predict clinical outcome.31-33 We show that compared with sfVIIIΔB expressed from a conventional LTR gammaretroviral vector, the efVIII variant expressed from the RMSinOFB backbone requires transplantation of significantly fewer transduced HSCs to achieve similar curative levels of fVIII.
Methods
efVIII transgene and gammaretroviral vector construction
The sfVIIIΔB transgene containing the 2 point mutations L303E/F309S in the A1 domain has been described previously.7 The sfVIIIΔB cDNA was further modified to generate the efVIII transgene as follows: the A2 domain mutations R484A/R489A/P492A29 were introduced by site-directed mutagenesis using the mutagenic sense (5′-CACTGATGTCGCTCCTTTGTATTCAGCGAGATTAGCAAAAGGTG-3′) and antisense (5′-CACCTTTTGCTAATCTCGCTGAATACAAAGGAGCGACATCAGTG-3′) primers, as described by the manufacturer (Stratagene); to incorporate an NH2-terminal B domain segment containing 6 consensus sites for N-linked glycosylation,18 a 4.6-kb NcoI/SalI fragment of MSGV-sfVIIIΔB-IRES-GFP7 containing the sfVIIIΔB transgene and the A2 domain mutations was excised and subcloned into an NcoI/XbaI-digested pRL-TK-Luc plasmid (Promega; SalI and XbaI ends were blunted first). Next, the 1.74-kb KpnI/ApaI fragment of sfVIIIΔB was removed and replaced with a 1.17-kb KpnI/SacI fragment of full-length fVIII cDNA and a 1.2-kb SacI/ApaI fragment of sfVIIIΔB in a triple ligation reaction. The 1.17-kb KpnI/SacI fragment incorporates 238 amino acids of NH2-terminal B domain sequence, whereas the 1.2-kb SacI/ApaI fragment, which was polymerase chain reaction (PCR)–amplified using the sense (5′-TCGAGCTCCACCAGTCTTGAAACGC-3′) and antisense (5′-CCGGAATAATGAAGTCTG-3′) primers, recreates the remainder of the sequences up to the ApaI site. RMSin-efVIII-OFB (abbreviated FB.efVIII) was generated by cloning the 5.1-kb NcoI/NotI fragment containing the efVIII cDNA from pRL-efVIII into NcoI/SalI-digested RMSinOFB30 (NotI and SalI ends were blunted first), replacing the green fluorescent protein (GFP) coding sequence. To generate RMSin-sfVIIIΔB-OFB (abbreviated FB.sfVIIIΔB), the 4.6-kb NcoI/SalI fragment of MSGV-sfVIIIΔB-IRES-GFP (abbreviated MSGV-sfVIIIΔB) containing the sfVIIIΔB was excised and cloned into the NcoI/SalI-digested RMSinOFB vector.
Cell lines
NIH3T3 fibroblasts (ATCC No. CRL-1658), Phoenix-Eco packaging cells (ATCC No. SD 3444), and 293T/17 cells (ATCC No. CRL-11 268) were grown in Dulbecco modified Eagle medium (Mediatech Inc) and supplemented with 4.5 g/L glucose, 4 mM l-glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS; Cambrex BioScience Walkersville Inc). K562 human erythroleukemic cells (ATCC No. CCL-243) were cultured in Iscove modified Dulbecco medium (IMDM; Mediatech Inc) plus 10% FBS, 4 mM l-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin. All cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Production of retroviral vector particles
Ecotropic vector particles were prepared by transient transfection of the Phoenix-Eco packaging cell line as previously described.7 When required, vector particles were concentrated by overnight centrifugation at 9000g. The MSGV-sfVIIIΔB vector titers were determined on NIH3T3 cells by flow cytometric analysis of GFP fluorescence using a FACSCalibur analyzer (BD Biosciences) as described previously.34-36 FB.efVIII vector titers were determined on NIH3T3 cells using Southern blot analysis as described previously.30
Transient and stable expression of efVIII and sfVIIIΔB
For transient fVIII expression, 4 × 106 293T/17 cells were transiently transfected with 10 μg of each plasmid DNA by calcium phosphate coprecipitation as described previously.37 At 24 hours after transfection, the cells were rinsed twice with phosphate-buffered saline (PBS) and cultured in fresh medium for another 24 hours before the conditioned medium was harvested and used to measure the fVIII concentrations. For stable fVIII expression, K562 cells were transduced at low multiplicities of infection (MOIs) using amphotropic vector particles prepared by transient transfection of the 293T/17 cells.30
fVIII-deficient mice
fVIII-deficient male or female BAL-fVIIIKO mice aged 8 to 12 weeks (breeding pairs generously provided by David Lillicrap, Queen's University, Kingston, ON)31 were used as BM transplantation donors and recipients. All animal procedures were carried out in accordance with our Institutional Animal Care and Use Committee guidelines.
BM cell transduction and transplantation
BM cells were harvested from BAL-fVIIIKO mice at 8 to 12 weeks of age 4 days after intraperitoneal injection of 5-fluorouracil (150 mg/kg body weight; InvivoGen).38 BM processing, transduction, and GFP expression analysis were carried out as described in detail previously.7,30,38,39 The MSGV-sfVIIIΔB and the FB.efVIII vector particles were used to transduce BM cells daily at MOIs of 5 and 0.5, respectively, for 3 consecutive days.
Recipient mice received either a sublethal dose (550 cGy) of total body irradiation (TBI) on the day of the transplantation, or a nonmyeloablative chemotherapy conditioning regimen with busulfan (BU; MP Biomedicals) plus transient immunosuppression by anti (murine)–thymocyte serum (ATS) treatment (Inter-Cell Technologies), as described previously.25 BU was given intraperitoneally at 17.5 mg/kg per day on days −3 and −2 (35 mg/kg total), and ATS was given intraperitoneally on days −1 and 0 (4 hours before transplantation) at 30 mg/kg. Conditioned mice received 2 × 106 unsorted cells via tail vein injection on day 0. Transduction and engraftment efficiencies were analyzed by flow cytometry (for GFP expression) and Southern blot analysis (using a fVIII-specific probe). Representative mice from each experimental group were killed at 24 weeks after transplantation and their BM cells (∼ 2 × 107 cells) were injected into 2 lethally irradiated (800 cGy) secondary transplant recipients each. Both primary and secondary recipients were evaluated for phenotypic correction by tail clipping at 24 weeks after primary transplantation and 16 weeks after secondary transplantation, respectively.7
Detection of fVIII and anti-fVIII antibodies
fVIII antigen levels in murine plasma were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cedarlane Laboratories Ltd) as described previously.8 fVIII activity was determined by a chromogenic functional assay (COATEST VIII:C/4; DiaPharma Group Inc) as described previously.7 Details of the assay and the measurement of anti-fVIII antibodies are described in “Detection of fVIII and anti-fVIII antibodies” in supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Estimation of transduction efficiency by Southern blot analysis
Southern blot analysis was carried out as previously described.30,34-36 For each sample, 10 μg genomic DNA was digested with ApaI and BstBI and subjected to Southern blot analysis. The probe, prepared by random oligonucleotide priming, contained fVIII cDNA sequences corresponding to nucleotides 4207 to 4987 and 3553 to 4333 of efVIII and sfVIIIΔB, respectively. The copy number control standards were prepared by adding 0.0017 pg, 0.017 pg, and 0.17 pg vector plasmid DNAs, equivalent to 0.1, 1, and 10 vector copies per cell, to 10 μg control K562 genomic DNA and digesting with ApaI and BstBI. Approximate vector copy number per cell ratios were determined by acquiring digital images using a Storm 860 PhosphorImager, quantifying band intensities using ImageQuant software (Amersham Biosciences Corp), and comparing to copy number standards following normalization to glyceraldehyde-3-phosphate dehydrogenase controls.
Determination of vector copy number by real-time quantitative PCR
Genomic DNA was extracted using the GenElute Kit (Sigma-Aldrich). Real-time quantitative PCR was performed on a 7300 Real-Time PCR system (Applied Biosystems) using the Power SYBR Green PCR Master Mix kit (Applied Biosystems). Details are described in “Determination of vector copy number by real-time quantitative PCR” in supplemental methods.
Statistical analysis
Data from experiments are expressed as mean values plus or minus SD. The Student paired t test was used to compare differences between the indicated groups. A P value less than .05 was considered significant.
Results
Bioengineering of the efVIII variant and generation of an efVIII-expressing safety-augmented SIN gammaretroviral vector
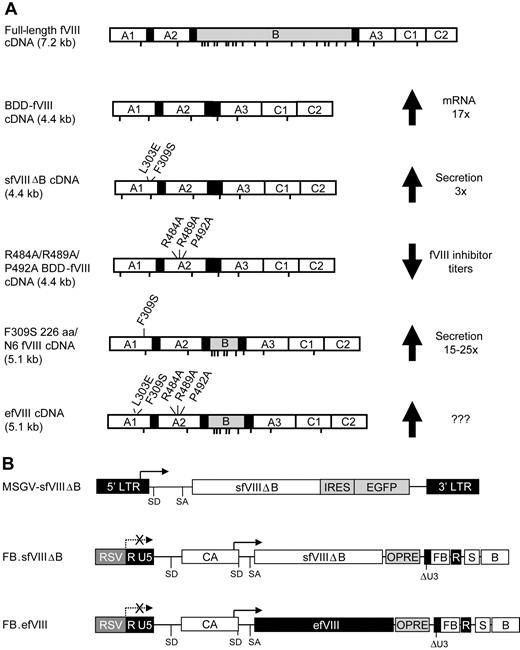
The fVIII transgene used in our previous HSC gene therapy studies is a translation- and secretion-enhanced BDD-fVIII variant (sfVIIIΔB) that contains 2 point mutations (L303E/F309S) in the HSPA5-binding site within the A1 domain (Figure 1A).7,21 For the current study, the sfVIIIΔB transgene was further modified to include the R484A/R489A/P492A mutations that have been shown to reduce the inhibitory antibody response to an immunodominant B-cell epitope within the A2 domain.29 Next, we incorporated a B domain sequence extension containing 6 consensus sites for N-linked glycosylation, comparable to the 226aa/N6 LMAN1-faciliated high-efficiency secretion variant described previously,18 giving rise to the efVIII variant (Figure 1A).
Schematic representation of fVIII and gammaretroviral vector constructs. (A) Full-length fVIII and bioengineered fVIII molecules with improved secretion and reduced immunogenicity. The sfVIIIΔB variant is a BDD-fVIII transgene that has the HSPA5 binding site double mutation F309S/L303E for enhanced secretion of recombinant fVIII.21 The R484A/R489A/P492A BDD-fVIII variant displays reduced immunogenicity in C57-fVIIIKO mice.29 The F309S/N6 BDD-fVIII variant was secreted approximately 14-fold more efficiently than BDD-fVIII (15- to 25-fold more efficiently than full-length fVIII) from COS-1 cells.18 The efVIII cDNA contains the F309S/L309E, the R484A/R489A/P492A, and the N6 modifications. ??? indicates extent of improvement to be determined. Potential N-linked glycosylation sites are indicated. (B) Schematic representation of the MSGV-sfVIIIΔB-IRES-GFP (abbreviated MSGV-sfVIIIΔB), RMSin-sfVIIIΔB-OFB (referred to as FB.sfVIIIΔB in the text), and RMSin-efVIII-OFB (referred to as FB.efVIII in the text) gammaretroviral vectors. Plasmid forms of the vectors are illustrated. Arrows depict transcription initiation sites. LTR indicates long terminal repeat; RSV, Rous sarcoma virus enhancer-promoter sequences; RMSin, RSV enhancer-promoter–driven self-inactivating MSGV-based retroviral vector backbone; SD, splice donor; SA, splice acceptor; CA, internal promoter consisting of the human cytomegalovirus immediate early region enhancer linked to the chicken β-actin promoter; sfVIIIΔB, secretion-enhanced BDD-fVIII gene; efVIII, glycosylation-facilitated secretion-enhanced fVIII gene with reduced immunogenicity; IRES, internal ribosome entry site; EGFP, enhanced GFP gene; OPRE, safety-optimized woodchuck hepatitis virus posttranscriptional regulatory element; ΔU3, deletion in the U3 region of the 3′ LTR; FB, FII enhancer-blocking component of the chicken β-globin 5′HS4 insulator and the BEAD-A homologous region from the human T-cell receptor α/δ BEAD-I insulator; S, simian virus 40 late poly(A) signal; and B, bovine growth hormone poly(A) signal. Transfer of the deletion in the U3 region of the 3′ LTR to the 5′ LTR after reverse transcription self-inactivates the vector as denoted by the dashed arrow with an X through it.
Schematic representation of fVIII and gammaretroviral vector constructs. (A) Full-length fVIII and bioengineered fVIII molecules with improved secretion and reduced immunogenicity. The sfVIIIΔB variant is a BDD-fVIII transgene that has the HSPA5 binding site double mutation F309S/L303E for enhanced secretion of recombinant fVIII.21 The R484A/R489A/P492A BDD-fVIII variant displays reduced immunogenicity in C57-fVIIIKO mice.29 The F309S/N6 BDD-fVIII variant was secreted approximately 14-fold more efficiently than BDD-fVIII (15- to 25-fold more efficiently than full-length fVIII) from COS-1 cells.18 The efVIII cDNA contains the F309S/L309E, the R484A/R489A/P492A, and the N6 modifications. ??? indicates extent of improvement to be determined. Potential N-linked glycosylation sites are indicated. (B) Schematic representation of the MSGV-sfVIIIΔB-IRES-GFP (abbreviated MSGV-sfVIIIΔB), RMSin-sfVIIIΔB-OFB (referred to as FB.sfVIIIΔB in the text), and RMSin-efVIII-OFB (referred to as FB.efVIII in the text) gammaretroviral vectors. Plasmid forms of the vectors are illustrated. Arrows depict transcription initiation sites. LTR indicates long terminal repeat; RSV, Rous sarcoma virus enhancer-promoter sequences; RMSin, RSV enhancer-promoter–driven self-inactivating MSGV-based retroviral vector backbone; SD, splice donor; SA, splice acceptor; CA, internal promoter consisting of the human cytomegalovirus immediate early region enhancer linked to the chicken β-actin promoter; sfVIIIΔB, secretion-enhanced BDD-fVIII gene; efVIII, glycosylation-facilitated secretion-enhanced fVIII gene with reduced immunogenicity; IRES, internal ribosome entry site; EGFP, enhanced GFP gene; OPRE, safety-optimized woodchuck hepatitis virus posttranscriptional regulatory element; ΔU3, deletion in the U3 region of the 3′ LTR; FB, FII enhancer-blocking component of the chicken β-globin 5′HS4 insulator and the BEAD-A homologous region from the human T-cell receptor α/δ BEAD-I insulator; S, simian virus 40 late poly(A) signal; and B, bovine growth hormone poly(A) signal. Transfer of the deletion in the U3 region of the 3′ LTR to the 5′ LTR after reverse transcription self-inactivates the vector as denoted by the dashed arrow with an X through it.
An efVIII-expressing gammaretroviral vector RMSin-efVIII-OFB (referred to herein as FB.efVIII for simplicity) was constructed by substituting the efVIII cDNA for the GFP transgene in our recently described RMSinOFB vector (Figure 1B). RMSinOFB is a safety-augmented SIN vector harboring a novel 77-bp enhancer-blocking element in its deleted 3′ U3 region (designated FB for FII/BEAD-A) composed of the minimal enhancer-blocking components of the chicken β-globin 5′HS4 insulator (FII) and a homologous region from the human T-cell receptor α/δ BEAD-1 insulator (BEAD-A).30 As a consequence of the mechanism of retroviral replication, the FB element is transferred to the 5′ LTR during reverse transcription, flanking the vector upon integration. Using a sensitive cell-culture assay of retroviral insertional mutagenesis,12,13 we previously showed that FB-insulated RMSinOFB was essentially devoid of transforming activity when tested on BM-derived hematopoietic progenitor cells from both wild-type and BAL-fVIIIKO hemophilia A mice.30,36 We were interested therefore in exploring the curative potential of an HSC-based gene therapy approach using the RMSinOFB vector system to express efVIII in the BAL-fVIIIKO hemophilia A model. For comparison purposes, we also inserted a copy of sfVIIIΔB into RMSinOFB, giving rise to RMSin-sfVIIIΔB-OFB (hereafter referred to as FB.sfVIIIΔB). Ecotropic pseudotypes of FB.efVIII and FB.sfVIIIΔB exhibited titers of 7.5 plus or minus 0.5 × 105 TU/mL and 8.5 plus or minus 0.5 × 105 TU/mL, respectively, which were only slightly lower than that of the similarly packaged LTR vector MSGV-sfVIIIΔB (1.1 ± 0.1 × 106 TU/mL).
Transient and stable expression of efVIII in vitro
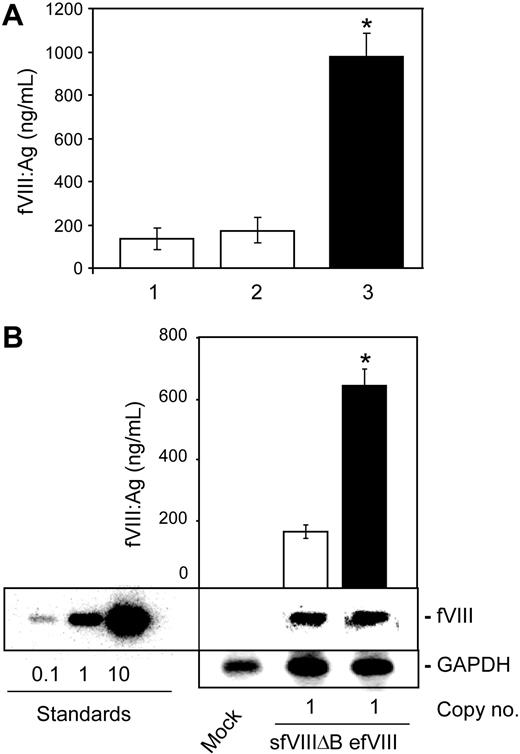
FB.efVIII-directed efVIII expression was first measured and compared with that of the sfVIIIΔB variant expressed from either FB.sfVIIIΔB (ie, from the same vector backbone as efVIII) or from the LTR vector MSGV-sfVIIIΔB in the human embryonic kidney cell line 293T/17. The cells were transiently transfected with these vectors and cultured for 24 hours, after which time fVIII levels in the conditioned medium were measured by ELISA (Figure 2A). While the medium collected from the cells transfected with either one of the sfVIIIΔB-expressing vectors contained similar levels of fVIII protein (135 ± 50 and 175 ± 60 ng/mL/106 cells/24 hours for MSGV-sfVIIIΔB and FB.sfVIIIΔB, respectively), medium obtained from FB.efVIII-transfected cells had nearly 6-fold higher fVIII levels (980 ± 50 ng/mL/106 cells/24 hours; P < .001).
efVIII variant is expressed more efficiently than sfVIIIΔB in 293T and K562 cells. (A) efVIII and sfVIIIΔB expression levels in transiently transfected 293T cells. 293T cells were transiently transfected using 10 μg MSGV-sfVIIIΔB (column 1), FB.sfVIIIΔB (column 2), or FB.efVIII (column 3) plasmid DNAs. At 36 hours later, the conditioned medium was assayed for fVIII antigen levels. *Significantly higher levels (P < .001). (B) efVIII and sfVIIIΔB expression levels in stably transduced K562 cells. K562 cells were transduced with the FB.efVIII or MSGV-sfVIIIΔB vectors at an MOI of 1. DNA was isolated from the transduced cells 2 weeks later, digested with ApaI and BstBI, and subjected to Southern blot analysis. Pools of stably transduced cells with an average of one vector copy per cell were selected and their conditioned medium assayed for fVIII antigen levels. The standards were generated by digesting MSGV-sfVIIIΔB plasmid DNA in amounts equivalent to 0.1, 1, and 10 vector copies per cell. Vector copy numbers were calculated by PhosphoImager quantification and comparison with copy number standards and normalization to GAPDH controls. The approximate vector copy numbers per cell are indicated below the lanes. fVIII antigen levels were determined by a commercial ELISA (fVIII:C Ag). Data presented are the mean values of several independent experiments (n = 3) ± SDs. *Significantly higher levels (P < .001).
efVIII variant is expressed more efficiently than sfVIIIΔB in 293T and K562 cells. (A) efVIII and sfVIIIΔB expression levels in transiently transfected 293T cells. 293T cells were transiently transfected using 10 μg MSGV-sfVIIIΔB (column 1), FB.sfVIIIΔB (column 2), or FB.efVIII (column 3) plasmid DNAs. At 36 hours later, the conditioned medium was assayed for fVIII antigen levels. *Significantly higher levels (P < .001). (B) efVIII and sfVIIIΔB expression levels in stably transduced K562 cells. K562 cells were transduced with the FB.efVIII or MSGV-sfVIIIΔB vectors at an MOI of 1. DNA was isolated from the transduced cells 2 weeks later, digested with ApaI and BstBI, and subjected to Southern blot analysis. Pools of stably transduced cells with an average of one vector copy per cell were selected and their conditioned medium assayed for fVIII antigen levels. The standards were generated by digesting MSGV-sfVIIIΔB plasmid DNA in amounts equivalent to 0.1, 1, and 10 vector copies per cell. Vector copy numbers were calculated by PhosphoImager quantification and comparison with copy number standards and normalization to GAPDH controls. The approximate vector copy numbers per cell are indicated below the lanes. fVIII antigen levels were determined by a commercial ELISA (fVIII:C Ag). Data presented are the mean values of several independent experiments (n = 3) ± SDs. *Significantly higher levels (P < .001).
FB.efVIII-directed efVIII expression was next measured in the human myeloerythroid cell line K562.40 The cells were stably transduced with amphotropic FB.efVIII and MSGV-sfVIIIΔB vector particles at low MOI (Figure 2B). At 2 weeks later, genomic DNA was isolated from transduced cells and subjected to Southern blot analysis. The approximate vector DNA copy number per cell was estimated by quantifying band intensities and comparing with copy number standards. Transduced cultures with an average of one vector copy per cell were selected for further analysis. Medium conditioned for 24 hours was collected from FB.efVIII- and MSGV-sfVIIIΔB–transduced K562 cells and subjected to fVIII ELISA (Figure 2B). Similar to the transient expression analyses, fVIII levels measured for the FB.efVIII and MSGV-sfVIIIΔB vector–transduced cells were significantly different, with efVIII levels more than 3-fold higher than those of sfVIIIΔB (184 ± 23 and 616 ± 31 ng/mL/106 cells/24 hours, respectively; P < .001).
Engraftment of FB.efVIII-transduced BM cells in BAL-fVIIIKO mice following reduced-intensity TBI or nonmyeloablative BU-ATS conditioning regimens corrects their hemophilia
Besides modifications to retroviral vectors, additional strategies such as limiting the number of vector copies per transduced cell and limiting the dose of transduced cells transplanted have been proposed to further improve the biosafety of gene therapy protocols.11 Unfortunately, such strategies are often difficult to implement, especially for an inefficiently produced protein such as human fVIII. In light of the enhanced in vitro secretion levels of efVIII, we anticipated the possibility of obtaining therapeutic efVIII levels in vivo following transplantation of a limited number of transduced HSCs. To achieve this, we adjusted the MOIs such that only an estimated 10% of the BM cells would be transduced with the FB.efVIII vector. The low transduction efficiency also made it unlikely that any transduced cell would carry more than one vector copy.
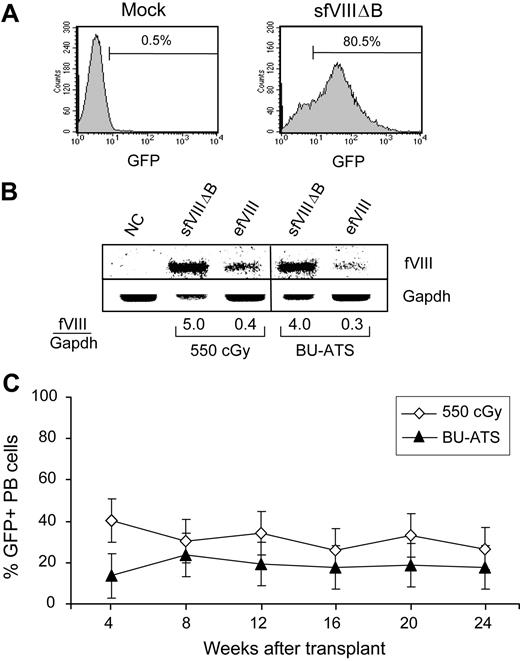
BM cells from BAL-fVIIIKO mice, enriched for hematopoietic stem/progenitor cells by 5-fluorouracil pretreatment,38 were transduced ex vivo with the FB.efVIII or MSGV-sfVIIIΔB vectors daily for 3 consecutive days at MOIs of 0.5 and 5, respectively. Before transplantation, the recipient mice were conditioned by sublethal (550 cGy) TBI on the day of transplantation or by treatment with BU at 17.5 mg/kg on days −3 and −2 in combination with the immunosuppressive agent ATS on days −1 and 0, as described previously.25 The transduction efficiency of the MSGV-sfVIIIΔB–transduced BM cells was approximately 80% as determined by analyzing aliquots of cells for GFP expression using flow cytometry (Figure 3A). Because the FB.efVIII vector lacks a GFP reporter, transduction efficiency was estimated by Southern blot analysis of vector DNA in genomic DNA isolated from an aliquot of transduced BM cells and comparison of the relative band intensity to that of MSGV-sfVIIIΔB–transduced BM cells (Figure 3B). The results indicated that the 10-fold lower MOI used to transduce BM cells with the FB.efVIII vector indeed translated into approximately 8- to 10-fold lower transduction levels. A total of 2 × 106 BM cells were transplanted into each of the conditioned recipient mice.
Comparison of BM transduction efficiencies and donor cell engraftment in hemophilia A mice following reduced-intensity TBI or nonmyeloablative BU-ATS conditioning regimens. (A) Flow cytometric histogram of GFP fluorescence 1 week after the third transduction with the MSGV-sfVIIIΔB vector at an MOI of 5 (sfVIIIΔB). The percentage of GFP+ transduced BM cells is indicated. (B) Southern blot analyses of ApaI/BstBI-digested genomic DNAs hybridized with a fVIII probe (top panel) and a Gapdh probe (bottom panel) showing the relative vector copy number per cell for the transduced BM cell populations that were transplanted into 550 cGy TBI– or BU-ATS–conditioned BAL-fVIIIKO mice. The results presented are representative of 2 independent experiments. (C) The percentage of GFP+ PB cells in 550 cGy TBI– or BU-ATS–conditioned hemophilia A mice that received MSGV-sfVIIIΔB–transduced BM cells as determined by quantitative flow cytometric analysis at various time points after transplantation. Error bars represent the SEM.
Comparison of BM transduction efficiencies and donor cell engraftment in hemophilia A mice following reduced-intensity TBI or nonmyeloablative BU-ATS conditioning regimens. (A) Flow cytometric histogram of GFP fluorescence 1 week after the third transduction with the MSGV-sfVIIIΔB vector at an MOI of 5 (sfVIIIΔB). The percentage of GFP+ transduced BM cells is indicated. (B) Southern blot analyses of ApaI/BstBI-digested genomic DNAs hybridized with a fVIII probe (top panel) and a Gapdh probe (bottom panel) showing the relative vector copy number per cell for the transduced BM cell populations that were transplanted into 550 cGy TBI– or BU-ATS–conditioned BAL-fVIIIKO mice. The results presented are representative of 2 independent experiments. (C) The percentage of GFP+ PB cells in 550 cGy TBI– or BU-ATS–conditioned hemophilia A mice that received MSGV-sfVIIIΔB–transduced BM cells as determined by quantitative flow cytometric analysis at various time points after transplantation. Error bars represent the SEM.
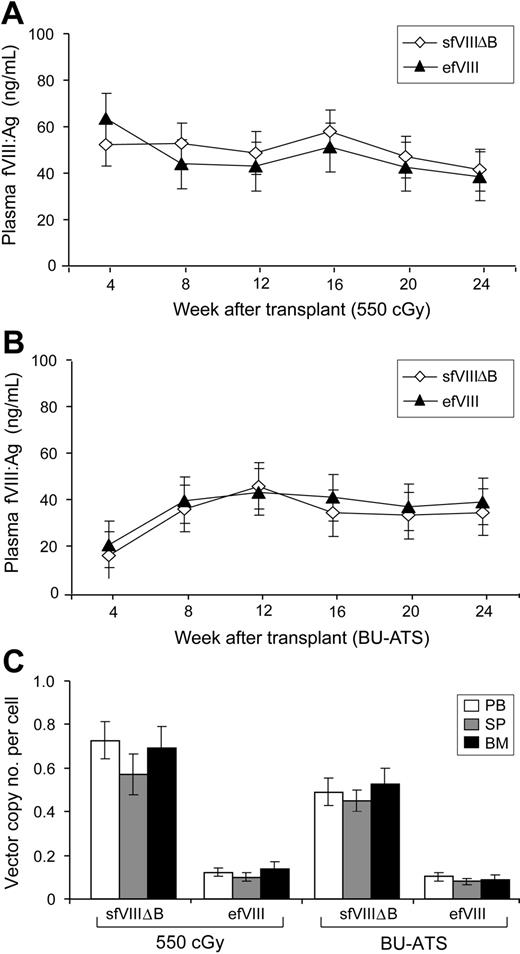
All mice conditioned with 550 cGy TBI that received MSGV-sfVIIIΔB–transduced cells (n = 4) showed high levels of engraftment in PB cells (Figure 3C): engraftment levels were 32% plus or minus 15% (range, 27%-40%) for up to 24 weeks after transplantation (the last time point analyzed) as determined by flow cytometric analysis of GFP expression. All of these mice expressed stable therapeutic levels of sfVIIIΔB, with an average concentration of 50 plus or minus 13 ng/mL (equivalent to 250 mIU fVIII activity; 25% of normal) during the 24-week observation period (Figure 4A). All mice conditioned with 550 cGy TBI that received transplants of FB.efVIII-transduced cells (n = 6) also showed stable levels of efVIII expression that were comparable with those obtained in mice that received transplants of MSGV-sfVIIIΔB–transduced BM cells. efVIII concentrations averaged around 47 plus or minus 15 ng/mL (equivalent to 235 mIU of fVIII activity; 24% of normal) for up to 24 weeks after transplantation.
fVIII expression and vector copy number analysis in hemophilia A mice following reduced-intensity TBI or BU-ATS conditioning regimens. Human efVIII and sfVIIIΔB antigen (fVIII:Ag) levels in the plasma of 550 cGy TBI–conditioned (A) or BU-ATS–conditioned (B) hemophilia A mice that received BM cells transduced with FB.efVIII- or MSGV-sfVIIIΔB vectors (at 0.5 MOI × 3 cycles and 5 MOI × 3 cycles, respectively), as measured by ELISA and presented as mean values ± SDs at various time points after transplantation. (C) Vector copy number in the genomic DNA of PB, spleen (SP), and BM cells was quantified by real-time PCR using human fVIII-specific primers at 6 months after transplantation. The vector copy numbers per cell for mice that received MSGV-sfVIIIΔB–transduced BM cells were significantly higher than those that received the FB.efVIII-transduced BM in both the 550 cGy TBI–conditioned (P = .004) and BU-ATS–conditioned (P = .001) groups. Data presented are the mean values of 2 independent experiments ± SDs.
fVIII expression and vector copy number analysis in hemophilia A mice following reduced-intensity TBI or BU-ATS conditioning regimens. Human efVIII and sfVIIIΔB antigen (fVIII:Ag) levels in the plasma of 550 cGy TBI–conditioned (A) or BU-ATS–conditioned (B) hemophilia A mice that received BM cells transduced with FB.efVIII- or MSGV-sfVIIIΔB vectors (at 0.5 MOI × 3 cycles and 5 MOI × 3 cycles, respectively), as measured by ELISA and presented as mean values ± SDs at various time points after transplantation. (C) Vector copy number in the genomic DNA of PB, spleen (SP), and BM cells was quantified by real-time PCR using human fVIII-specific primers at 6 months after transplantation. The vector copy numbers per cell for mice that received MSGV-sfVIIIΔB–transduced BM cells were significantly higher than those that received the FB.efVIII-transduced BM in both the 550 cGy TBI–conditioned (P = .004) and BU-ATS–conditioned (P = .001) groups. Data presented are the mean values of 2 independent experiments ± SDs.
Mice conditioned with BU-ATS that received MSGV-sfVIIIΔB–transduced cells (n = 8) were also all stably engrafted although, as anticipated,8 at lower levels than observed in the 550 cGy TBI–conditioned animals (19% ± 8%; range, 14%-24%). Stable therapeutic levels of sfVIIIΔB, with an average concentration of 33 plus or minus 22 ng/mL (equivalent to 165 mIU of fVIII activity; 16.5% of normal), were observed in these mice during a 24-week observation period (Figure 4B). Moreover, all mice conditioned with BU-ATS that received transplants of FB.efVIII-transduced cells (n = 8) also had high levels of efVIII, which were similar to those obtained in mice that received transplants of MSGV-sfVIIIΔB–transduced BM cells: efVIII concentrations averaged around 36 plus or minus 15 ng/mL (equivalent to 180 mIU fVIII activity; 18% of normal) for up to 24 weeks after transplantation.
At the 24-week time point, the tails of the primary BM transplant recipients were clipped for coagulation analysis using a stringent survival assay. Notably, irrespective of the vector or conditioning regimen, all BAL-fVIIIKO mice treated by fVIII gene therapy exhibited clot formation and survived tail clipping, indicating correction of their hemophilic phenotype (Table 1). In sharp contrast, none of the control untreated BAL-fVIIIKO hemophilia A mice survived tail clipping, dying from exsanguination between 2 and 12 hours.
Phenotypic correction of BAL-fVIIIKO hemophilia A mice
| Mouse . | Conditioning . | Vector . | Weeks after transplantation . | % GFP+ . | fVIII, ng/mL . | fVIII, mIU/mL . | Survival . |
|---|---|---|---|---|---|---|---|
| Primaries | |||||||
| 1 | 550 cGy | MSGV-sfVIIIΔB | 24 | 14.3 | 27.2 | 181 | + |
| 2 | 550 cGy | MSGV-sfVIIIΔB | 24 | 19.3 | 35.9 | 153 | + |
| 3 | 550 cGy | MSGV-sfVIIIΔB | 24 | 35.4 | 57.6 | 339 | + |
| 4 | 550 cGy | MSGV-sfVIIIΔB | 24 | 29.8 | 44.3 | 256 | + |
| 5 | 550 cGy | FB.efVIII | 24 | NA | 59.3 | 292 | + |
| 6 | 550 cGy | FB.efVIII | 24 | NA | 31 | 201 | + |
| 7 | 550 cGy | FB.efVIII | 24 | NA | 25.5 | 177 | + |
| 8 | 550 cGy | FB.efVIII | 24 | NA | 17.3 | 218 | + |
| 9 | 550 cGy | FB.efVIII | 24 | NA | 49.8 | 407 | + |
| 10 | 550 cGy | FB.efVIII | 24 | NA | 47.8 | 231 | + |
| 11 | BU-ATS | MSGV-sfVIIIΔB | 24 | 11.3 | 41.1 | 86 | + |
| 12 | BU-ATS | MSGV-sfVIIIΔB | 24 | 14.4 | 38.9 | 337 | + |
| 13 | BU-ATS | MSGV-sfVIIIΔB | 24 | 21.4 | 42.7 | 241 | + |
| 14 | BU-ATS | MSGV-sfVIIIΔB | 24 | 33.9 | 25.6 | 181 | + |
| 15 | BU-ATS | MSGV-sfVIIIΔB | 24 | 15.7 | 27.7 | 123 | + |
| 16 | BU-ATS | MSGV-sfVIIIΔB | 24 | 9.6 | 31.4 | 189 | + |
| 17 | BU-ATS | MSGV-sfVIIIΔB | 12 | 12.5 | 49.5 | 335 | ND |
| 18 | BU-ATS | MSGV-sfVIIIΔB | 8 | 21.5 | 36.6 | 184 | ND |
| 19 | BU-ATS | FB.efVIII | 24 | NA | 52.2 | 143 | + |
| 20 | BU-ATS | FB.efVIII | 24 | NA | 33.4 | 111 | + |
| 21 | BU-ATS | FB.efVIII | 24 | NA | 31.9 | 172 | + |
| 22 | BU-ATS | FB.efVIII | 24 | NA | 28.9 | 151 | + |
| 23 | BU-ATS | FB.efVIII | 24 | NA | 44.1 | 277 | + |
| 24 | BU-ATS | FB.efVIII | 24 | NA | 53.7 | 133 | + |
| 25 | BU-ATS | FB.efVIII | 24 | NA | 39.8 | 255 | + |
| 26 | BU-ATS | FB.efVIII | 24 | NA | 29.1 | 103 | + |
| Secondaries | |||||||
| 1 | 800 cGy | MSGV-sfVIIIΔB | 16 | 9.1 | 25.3 | 121 | + |
| 2 | 800 cGy | MSGV-sfVIIIΔB | 16 | 5.4 | 18.6 | 73 | + |
| 3 | 800 cGy | FB.efVIII | 16 | NA | 14.8 | 128 | + |
| 4 | 800 cGy | FB.efVIII | 16 | NA | 19.4 | 177 | + |
| 5 | 800 cGy | MSGV-sfVIIIΔB | 16 | 8.2 | 21.7 | 155 | + |
| 6 | 800 cGy | MSGV-sfVIIIΔB | 16 | 7 | 11.9 | 81 | + |
| 7 | 800 cGy | FB.efVIII | 16 | NA | 13.5 | 59 | + |
| 8 | 800 cGy | FB.efVIII | 16 | NA | 16.3 | 126 | + |
| Controls | |||||||
| BAL-fVIIIKO | 0/5 | ||||||
| Wild type | 5/5 |
| Mouse . | Conditioning . | Vector . | Weeks after transplantation . | % GFP+ . | fVIII, ng/mL . | fVIII, mIU/mL . | Survival . |
|---|---|---|---|---|---|---|---|
| Primaries | |||||||
| 1 | 550 cGy | MSGV-sfVIIIΔB | 24 | 14.3 | 27.2 | 181 | + |
| 2 | 550 cGy | MSGV-sfVIIIΔB | 24 | 19.3 | 35.9 | 153 | + |
| 3 | 550 cGy | MSGV-sfVIIIΔB | 24 | 35.4 | 57.6 | 339 | + |
| 4 | 550 cGy | MSGV-sfVIIIΔB | 24 | 29.8 | 44.3 | 256 | + |
| 5 | 550 cGy | FB.efVIII | 24 | NA | 59.3 | 292 | + |
| 6 | 550 cGy | FB.efVIII | 24 | NA | 31 | 201 | + |
| 7 | 550 cGy | FB.efVIII | 24 | NA | 25.5 | 177 | + |
| 8 | 550 cGy | FB.efVIII | 24 | NA | 17.3 | 218 | + |
| 9 | 550 cGy | FB.efVIII | 24 | NA | 49.8 | 407 | + |
| 10 | 550 cGy | FB.efVIII | 24 | NA | 47.8 | 231 | + |
| 11 | BU-ATS | MSGV-sfVIIIΔB | 24 | 11.3 | 41.1 | 86 | + |
| 12 | BU-ATS | MSGV-sfVIIIΔB | 24 | 14.4 | 38.9 | 337 | + |
| 13 | BU-ATS | MSGV-sfVIIIΔB | 24 | 21.4 | 42.7 | 241 | + |
| 14 | BU-ATS | MSGV-sfVIIIΔB | 24 | 33.9 | 25.6 | 181 | + |
| 15 | BU-ATS | MSGV-sfVIIIΔB | 24 | 15.7 | 27.7 | 123 | + |
| 16 | BU-ATS | MSGV-sfVIIIΔB | 24 | 9.6 | 31.4 | 189 | + |
| 17 | BU-ATS | MSGV-sfVIIIΔB | 12 | 12.5 | 49.5 | 335 | ND |
| 18 | BU-ATS | MSGV-sfVIIIΔB | 8 | 21.5 | 36.6 | 184 | ND |
| 19 | BU-ATS | FB.efVIII | 24 | NA | 52.2 | 143 | + |
| 20 | BU-ATS | FB.efVIII | 24 | NA | 33.4 | 111 | + |
| 21 | BU-ATS | FB.efVIII | 24 | NA | 31.9 | 172 | + |
| 22 | BU-ATS | FB.efVIII | 24 | NA | 28.9 | 151 | + |
| 23 | BU-ATS | FB.efVIII | 24 | NA | 44.1 | 277 | + |
| 24 | BU-ATS | FB.efVIII | 24 | NA | 53.7 | 133 | + |
| 25 | BU-ATS | FB.efVIII | 24 | NA | 39.8 | 255 | + |
| 26 | BU-ATS | FB.efVIII | 24 | NA | 29.1 | 103 | + |
| Secondaries | |||||||
| 1 | 800 cGy | MSGV-sfVIIIΔB | 16 | 9.1 | 25.3 | 121 | + |
| 2 | 800 cGy | MSGV-sfVIIIΔB | 16 | 5.4 | 18.6 | 73 | + |
| 3 | 800 cGy | FB.efVIII | 16 | NA | 14.8 | 128 | + |
| 4 | 800 cGy | FB.efVIII | 16 | NA | 19.4 | 177 | + |
| 5 | 800 cGy | MSGV-sfVIIIΔB | 16 | 8.2 | 21.7 | 155 | + |
| 6 | 800 cGy | MSGV-sfVIIIΔB | 16 | 7 | 11.9 | 81 | + |
| 7 | 800 cGy | FB.efVIII | 16 | NA | 13.5 | 59 | + |
| 8 | 800 cGy | FB.efVIII | 16 | NA | 16.3 | 126 | + |
| Controls | |||||||
| BAL-fVIIIKO | 0/5 | ||||||
| Wild type | 5/5 |
NA indicates not applicable; and ND, not done.
Plasma samples collected at the time of tail clipping were analyzed for fVIII activity by the chromogenic functional (COATEST) assay. We detected 254 plus or minus 84 mIU/mL and 232 plus or minus 83 mIU/mL fVIII activity in the 550 cGy TBI–conditioned mice that received transplants of FB.efVIII- and MSGV-sfVIIIΔB–transduced BM cells, respectively (Table 1). fVIII activity was also detected in all of the BU-ATS–conditioned mice, although at slightly lower levels: 168 plus or minus 64 mIU/mL and 210 plus or minus 91 mIU/mL fVIII activity in mice that received transplants of FB.efVIII- and MSGV-sfVIIIΔB–transduced BM cells, respectively (Table 1). Although there was some variability on an individual basis, there was a strong concordance overall between the values obtained for fVIII activity in the COATEST assay and those predicted by the fVIII ELISA (Table 1).
We subsequently killed the mice and determined vector copy numbers in their PB, BM, and spleen cells by real-time PCR (Figure 4C). At 24 weeks after transplantation, PB cells of the 550 cGy TBI– and BU-ATS–conditioned mice that received transplants of FB.efVIII-transduced BM cells contained average vector copy numbers of 0.12 plus or minus 0.02 and 0.10 plus or minus 0.02 per diploid genome equivalent, respectively. More than 5-fold higher vector copy numbers were detected in the PB cells of the 550 cGy TBI– and BU-ATS–conditioned mice that received transplants of MSGV-sfVIIIΔB–transduced BM cells, with averages of 0.73 plus or minus 0.09 and 0.49 plus or minus 0.06 copies per diploid genome equivalent, respectively (P < .001; Figure 4C). Normalizing the MSGV-sfVIIIΔB values to the GFP+ fraction (27% ± 10% and 18% ± 6% GFP+ PB cells for the 550 cGy TBI– and BU-ATS–conditioned recipients, respectively, at time of death) yielded an average of approximately 3 vector copies per MSGV-sfVIIIΔB–transduced donor engrafting cell. In all cases, essentially similar vector copy numbers to those determined for PB cells were obtained for BM and spleen cells of the mice (Figure 4C). Although it was not possible to likewise ascertain the percentage of FB.efVIII-transduced donor cell engraftment, these data clearly demonstrated successful correction of the hemophilic phenotype of BAL-fVIIIKO mice with a low vector copy number per cell.
Transplantation of transduced primary BM into secondary BAL-fVIIIKO recipients
To determine whether the sustained presence of efVIII and sfVIIIΔB in the plasma of the corrected hemophilia A mice was due to transduction of HSCs, lethally irradiated (800 cGy) BAL-fVIIIKO animals received transplants of BM cells obtained from representative primary recipients killed 24 weeks after transplantation. Monitoring of GFP expression showed that all MSGV-sfVIIIΔB secondary recipients contained transcriptionally active vectors in their PB cells during a 16-week observation period (7.9% ± 3.4% GFP+ for recipients of BM from the 550 cGy TBI–conditioned group and 7.3% ± 1.2% GFP+ for recipients of BM from the BU-ATS–conditioned group; n = 2 from each group; supplemental Figure 1). Furthermore, all of the secondary mice that received transplants of BM from FB.efVIII primary mice (n = 2 from each 550 cGy TBI– and BU-ATS–conditioned groups) as well as those that received transplants of BM from MSGV-sfVIIIΔB primary mice showed persistent fVIII expression (Figure 5). efVIII and fVIIIΔB concentrations averaged around 17 plus or minus 3 ng/mL (equivalent to 85 mIU fVIII activity; 8.5% of normal) and 19 plus or minus 4 ng/mL (equivalent to 95 mIU fVIII activity; 9.5% of normal), respectively, which remained stable throughout the 16 weeks following transplantation. When the tail was clipped, all secondary recipients exhibited clot formation and survived the procedure, indicating correction of their hemophilic phenotype (Table 1).
Sustained fVIII expression levels in secondary hemophilia A transplant recipients demonstrating successful genetic modification at the level of primary HSCs. Human efVIII and sfVIIIΔB antigen (fVIII:Ag) levels in the plasma of secondary transplant recipients that received BM cells from FB.efVIII- or MSGV-sfVIIIΔB–corrected primary BAL-fVIIIKO mice measured by ELISA. Mice were conditioned by lethal (800 cGy) TBI before transplantation with BM cells from primary corrected hemophilia A mice as indicated: I indicates BM cells from primary transplant recipients that had received a 550 cGy TBI conditioning regimen; B, BM cells from primary transplant recipients that had received BU-ATS conditioning.
Sustained fVIII expression levels in secondary hemophilia A transplant recipients demonstrating successful genetic modification at the level of primary HSCs. Human efVIII and sfVIIIΔB antigen (fVIII:Ag) levels in the plasma of secondary transplant recipients that received BM cells from FB.efVIII- or MSGV-sfVIIIΔB–corrected primary BAL-fVIIIKO mice measured by ELISA. Mice were conditioned by lethal (800 cGy) TBI before transplantation with BM cells from primary corrected hemophilia A mice as indicated: I indicates BM cells from primary transplant recipients that had received a 550 cGy TBI conditioning regimen; B, BM cells from primary transplant recipients that had received BU-ATS conditioning.
Analysis of plasma samples collected from secondary recipients at the time of tail clipping by COATEST assay revealed relatively similar levels of fVIII activity in the FB.efVIII and the MSGV-sfVIIIΔB animals, with an average activity of 115 plus or minus 41 mIU/mL (Table 1). As for the primary animals, when vector copy numbers were determined by real-time PCR in the PB cells obtained from these secondary recipients, significantly fewer copies were again detected in mice engrafted with FB.efVIII-transduced cells (0.06 ± 0.01 per diploid genome equivalent) compared with mice engrafted with MSGV-sfVIIIΔB–transduced cells (0.25 ± 0.05 per diploid genome equivalent; approximately 3 vector copies per cell; P < .001; results not shown).
Analysis of the anti-fVIII immune response in corrected BAL-fVIIIKO hemophilia A transplant recipients
Our previous HSC gene therapy modeling with the MSGV-sfVIIIΔB vector was carried out in C57-fVIIIKO mice (fVIII exon 16–disrupted knockout mice bred onto the C57BL/6 background).8 In the present study, we used BAL-fVIIIKO mice (fVIII exon 16–disrupted knockout mice bred onto the BALB/c background), which have been shown to develop 3- to 4-fold higher anti-fVIII inhibitor titers following adenovirus-mediated fVIII gene delivery or subcutaneous injection of recombinant human fVIII than those congenic with the C57BL/6 strain.31-33 In the current experiments, sustained expression of efVIII as well as sfVIIIΔB in the plasma of all immunocompetent hemophilia A BM transplant recipients for up to 24 weeks after transplantation implied lack of stimulation of a potent inhibitory immune response to either fVIII protein. To confirm this notion, we measured human fVIII-specific antibodies (IgG fraction) in the plasma of the mice by ELISA at the 24-week time point. All mice had less than 0.5 μg/mL anti-fVIII IgG antibodies in their plasma, similar to naive BAL-fVIIIKO hemophilia A mice (supplemental Figure 2). As a positive control group, 5 age-matched naive BAL-fVIIIKO mice were immunized with purified human fVIII and their plasma analyzed for the presence of anti-fVIII IgG by ELISA before the first injection and 1 week after the fourth fVIII injection. We observed a vigorous humoral immune response to human fVIII in these immunized BAL-fVIIIKO mice, with an average anti-fVIII IgG antibody titer of 5285 plus or minus 468 μg/mL (supplemental Figure 2).
Discussion
We previously reported correction of the bleeding disorder in C57-fVIIIKO hemophilia A mice by HSC-targeted delivery of the sfVIIIΔB (L303E/F309S) variant using a conventional LTR gammaretroviral vector, MSGV-sfVIIIΔB.7,8 The goal of the present study was to obtain comparable results by using a more clinically relevant HSC gene therapy protocol that (1) uses a less genotoxic transgene delivery system, (2) minimizes both the number of vector copies per transduced cell and the dose of transduced cells that are transplanted, (3) incorporates reduced-intensity transplant conditioning regimens, and (4) uses the BAL-fVIIIKO strain of hemophilia A mice, which mounts a more potent anti-fVIII inhibitor response to exogenous fVIII than the C57-fVIIIKO strain.31-33
To minimize the number of genetically modified cells needed to produce therapeutic levels of fVIII, we constructed an enhanced fVIII variant, efVIII. Several fVIII variants have been bioengineered previously which exhibit increased secretion, altered immunogenicity, or decreased degradation.5,18,19,21,29 efVIII is a BDD variant that combines many of these features, including the A1 domain L303E/F309S point mutations and the partial B domain (N6) for more efficient glycosylation-facilitated secretion as well as A2 domain mutations (R484A/R489A/P492A) for reduced immunogenicity.7,18,29 We found that efVIII is expressed at levels up to 6-fold greater than the sfVIIIΔB variant in vitro using transiently transfected 293T/17 cells, and stable expression of efVIII in pools of transduced K562 cells resulted in greater than 3-fold higher levels compared with similarly transduced sfVIIIΔB-expressing K562 cells.
Recently, we demonstrated that the hematopoietic transforming activity of the RMSinOFB SIN gammaretroviral vector could be virtually eliminated by incorporation of the novel 77-bp FB enhancer-blocking element in the deleted U3 region of the 3′ LTR.30 Flanking the therapeutic transgene cassette with the FB enhancer-blocking element allows usage of a strong internal enhancer-promoter combination,30 which is often needed to obtain sufficiently high gene expression levels to confer clinical benefits from a single copy of the vector. Therefore, in this study, we transduced BM cells at a low MOI (0.5) with the safety-augmented FB.efVIII vector derived from RMSinOFB. Of note, we obtained similar therapeutic levels of efVIII and sfVIIIΔB in the plasma of all 550 cGy–sublethally irradiated (24% and 25% of normal, respectively) and BU-ATS–treated (18% and 16.5% of normal, respectively) transplant recipients for the duration of the study (24 weeks) despite using 10-fold lower MOIs than the conventional MSCV-sfVIIIΔB gammaretroviral vector. Circulating fVIII activity determined by functional COATEST assay for efVIII corresponded very closely to that predicted by total protein levels measured by ELISA.
The development of neutralizing antibodies to fVIII is one of the most serious complications of fVIII protein therapy, which often leaves patients with very limited treatment alternatives.3,4 A recombinant fVIII variant with mutations in residues that are frequently the target of inhibitory antibodies would be a significant advantage if it could avoid or lower the immune response.28,29 Although the humoral reaction to intravenously injected recombinant fVIII that was stimulated in naive BAL-fVIIIKO mice was much more vigorous than what we had detected previously in C57-fVIIIKO mice,8 we did not observe significant differences in the anti-fVIII immune responses to vector-directed efVIII and sfVIIIΔB in BAL-fVIIIKO transplant recipients, even following nonmyeloablative conditioning. A notable difference between the nonmyeloablative BU conditioning regimen used in the current experiments and our previous protocol was the addition of ATS to induce transient immunosuppression and enhance donor cell engraftment.25,41 As mentioned, induction of transplantation tolerance is a specific objective of the HSC-based gene therapy approach.26,27 It will therefore be of much interest to extend these studies to BAL-fVIIIKO mice with pre-existing inhibitory antibodies against fVIII to determine whether any immunologic advantages are conferred by efVIII over the sfVIIIΔB variant within the context of this experimental paradigm.
In conclusion, we demonstrated that usage of a rationally designed fVIII molecule—which overcomes most of the inherent limitations of native fVIII protein biosynthesis—in combination with a safety-augmented SIN gammaretroviral vector system allowed implementation of a reduced-risk HSC-based gene addition strategy that effectively achieves sustained fVIII levels in the therapeutic window using a stringent mouse model of hemophilia A. These findings represent an encouraging step toward potential clinical application of a curative alternative to prophylactic treatment of this otherwise progressively debilitating, life-threatening bleeding disorder.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sara Karandish for technical assistance.
This study was supported in part by National Institutes of Health grants R01HL65519 and R01HL66305, and by an Elaine H. Snyder Cancer Research Award and a King Fahd Endowed Professorship (to R.G.H.) from The George Washington University Medical Center.
National Institutes of Health
Authorship
Contribution: A.R. was responsible for conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; and R.G.H. was responsible for conception and design, financial support, data analysis and interpretation, and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert G. Hawley, Department of Anatomy and Regenerative Biology, The George Washington University Medical Center, 2300 I Street NW, Washington, DC 20037; e-mail: rghawley@gwu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal