Abstract
A comprehensive understanding of the complex, autologous cellular interactions and regulatory mechanisms that occur during normal dendritic cell (DC)–stimulated immune responses is critical to optimizing DC-based immunotherapy. We have found that mature, immunogenic human monocyte-derived DCs (moDCs) up-regulate the immune-inhibitory enzyme, indoleamine 2,3-dioxygenase (IDO). Under stringent autologous culture conditions without exogenous cytokines, mature moDCs expand regulatory T cells (Tregs) by an IDO-dependent mechanism. The priming of resting T cells with autologous, IDO-expressing, mature moDCs results in up to 10-fold expansion of CD4+CD25brightFoxp3+CD127neg Tregs. Treg expansion requires moDC contact, CD80/CD86 ligation, and endogenous interleukin-2. Cytofluorographically sorted CD4+ CD25brightFoxp3+ Tregs inhibit as much as 80% to 90% of DC-stimulated autologous and allogeneic T-cell proliferation, in a dose-dependent manner at Treg:T-cell ratios of 1:1, 1:5, and as low as 1:25. CD4+CD25brightFoxp3+ Tregs also suppress the generation of cytotoxic T lymphocytes specific for the Wilms tumor antigen 1, resulting in more than an 80% decrease in specific target cell lysis. Suppression by Tregs is both contact-dependent and transforming growth factor-β–mediated. Although mature moDCs can generate Tregs by this IDO-dependent mechanism to limit otherwise unrestrained immune responses, inhibition of this counter-regulatory pathway should also prove useful in sustaining responses stimulated by DC-based immunotherapy.
Introduction
Suppressor mechanisms operating under normal physiologic conditions to modulate immunity contribute to immunologic unresponsiveness. The regulatory attributes of conventional human dendritic cells (DCs) in this respect merit further investigation. Critical to the initiation of immunity,1,2 DCs are also key participants in immune regulation. Immature DCs, for example, process self-antigens to induce and maintain tolerance.3-5 Less well characterized and of emerging importance are regulatory checkpoints intrinsic to mature, immunostimulatory DCs in controlling the degree and duration of immune responses.6,7 Understanding these regulatory mechanisms in an autologous setting free of exogenous cytokines or allogeneic stimulation is especially important given that nearly all translational applications of DCs use autologous, and not allogeneic, cells.
Regulatory T cells (Tregs) play a central role in tempering immunity and in maintaining immune homeostasis. Tregs contribute to the prevention of autoimmune diseases, the mediation of transplantation tolerance, and the down-regulation of the immune response to allergens, pathogens, and tumor cells.8,9 Several Treg subpopulations have been described, including naturally occurring Tregs, which arise from the thymus, and adaptive Tregs, which develop in the periphery.9 The transcription factor Foxp3 is among the most commonly used markers to identify Tregs.10 In addition, absent or low-level surface expression of the interleukin (IL)–7 receptor α (CD127) correlates inversely with Foxp3 expression and Treg suppressor function, offering a complementary epitope to identify human Tregs.11,12 Phenotypic markers for human Tregs, however, do not identify distinct populations as exist in mice,13 thus necessitating demonstration of suppressor function, which remains the “gold standard” for confirming Treg identity. The secretion of inhibitory cytokines and contact-dependent inhibition are among the different mechanisms of Treg-mediated suppression that have been identified.13
Indoleamine 2,3-dioxygenase (IDO) is a key immunomodulatory enzyme that promotes peripheral immune tolerance by inhibiting T-cell activation and proliferation through tryptophan catabolism.14 Initial evidence for IDO-mediated immunosuppression in vivo was demonstrated at the maternal-fetal interface.15 Subsequent studies have shown that IDO is an important regulator of immunity in infections, autoimmunity, transplantation, and cancer.16,17 Inhibition of IDO with the competitive inhibitor, 1-methyl-tryptophan (1MT), results in rejection of allogeneic fetuses15 and worsens autoimmunity in mouse models,18-20 thus supporting the role of IDO as a negative regulator of immunity in vivo.
IDO expressed specifically by DCs has also been implicated in immune regulation.16 Investigators have shown that distinct subsets of DCs expressing IDO, particularly those that express the IL-3 receptor (CD123), play a prominent role in IDO-mediated immune regulation.21-24 All conventional or myeloid DCs in humans, however, express low levels of CD123,25 whereas only plasmacytoid DCs (pDCs) are CD123bright.26 Previous studies reporting that any or all CD123+ DCs were the principal mediators of IDO activity may have therefore overestimated the contribution of pDCs, to the possible exclusion of conventional DCs. Differences in epitope expression between mouse and human pDCs may have further confounded interpretation of these data. The pattern of IDO expression and its attendant functional significance in well-defined human DC subtypes therefore remain important unknowns.
The expansion of Foxp3+ Tregs by mature, conventional monocyte-derived DCs (moDCs) has been described,27,28 but these studies did not define mechanisms of Treg induction. Investigators have recently addressed the role of IDO, but have emphasized the proliferation of allogeneic, naturally occurring CD4+CD25+ Tregs with the support of exogenous IL-2.29 The expansion of allogeneic Tregs by IDO+ pDCs has also been described.30,31 Allogeneic priming conditions and/or exogenous IL-2 therefore characterize(s) these and other studies of DC-based Treg expansion. In contrast, the generation of Tregs in an autologous system should prove more relevant to the physiologic termination of adaptive immune responses and to the therapeutic manipulation of cellular immunity against infections and tumors.
Using strict autologous culture conditions, without exogenous cytokines or serum from allogeneic or xenogeneic sources, we identified an IDO-based mechanism by which human moDCs expand autologous Tregs. We defined these resultant Tregs both phenotypically and functionally using sorted populations to demonstrate dose-dependent potency. Suppression was evaluated against strong allogeneic antigens, as well as against the Wilms tumor antigen (WT1), a self-differentiation, tumor-associated antigen. Our findings define an IDO-dependent mechanism by which mature, rather than immature moDCs expand both natural and adaptive Tregs with potent suppressor activity. Whereas this may normally provide a means to curb an otherwise unchecked immune response, our results demonstrate that IDO is a rational target for overcoming immune inhibition to optimize DC-based immunotherapy.
Methods
Blood samples
Healthy donors provided peripheral blood after signing informed consent in accordance with the Declaration of Helsinki and under protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, Memorial Sloan-Kettering Cancer Center (MSKCC). Buffy coats purchased from the Greater New York Blood Center, American Red Cross, were also used as a source of cells from healthy donors.
Media, serum, and noncytokine reagents
Complete RPMI 1640 was supplemented with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% penicillin/streptomycin (Media Laboratory, MSKCC), 50 μM 2-mercaptoethanol (GibcoBRL Life Technologies), 1% l-glutamine (GibcoBRL), and heat-inactivated, autologous single-donor or pooled human serum (1% or 10% vol/vol, as specified for a particular experiment). All media and reagents were endotoxin free.
Cytokines
Sterile, recombinant, endotoxin-, pyrogen-, mycoplasma-, and carrier-free human cytokines were used to generate immature and mature blood moDCs, exactly as published.32
Cell purification and generation of moDCs
MoDC precursors were tissue culture plastic-adherent peripheral blood mononuclear cells (PBMCs), obtained by standard centrifugation over Ficoll-Paque PLUS (Amersham Biosciences) from either whole blood diluted 1/1 with buffered saline or leukocyte concentrates (MSKCC Blood Bank). PBMCs were cultured in complete RPMI 1640–1% autologous serum with granulocyte-macrophage colony-stimulating factor (1000 IU/mL) and IL-4 (500 IU/mL). Fresh medium and cytokines were replenished every 2 days. Terminal maturation of immature moDCs was accomplished from days 6 to 8 by exposure to the inflammatory cytokines IL-1 (2 ng/mL), IL-6 (1000 IU/mL), tumor necrosis factor-α (10 ng/mL), and prostaglandin E2 (5 mM/mL). MoDCs were used fresh or cryopreserved and thawed for later use without compromising phenotype or activity appropriate to their maturation state.33
T lymphocytes
T cells were obtained from tissue culture plastic-nonadherent PBMCs, further purified by nonadherence and elution from nylon wool columns (Polysciences). Purity was more than 90% to 95% based on CD3 expression.
Phenotypic analyses and cell sorting by flow cytometry
Fluorescein, phycoerythrin (PE)-, PE-cyanine-7–, and allophycocyanin (APC)–conjugated mouse anti–human monoclonal antibodies included anti-CD3, anti-CD8, anti-CD11c, anti-CD14, anti-CD25, anti-CD80, anti-CD83, anti-CD86, anti–human histocompatibility leukocyte antigen (HLA)–DR, and anti-Ki67 (BD Biosciences); PE-Texas Red (ECD; Beckman Coulter)–conjugated anti-CD4; fluorescein-conjugated anti-CD127; APC anti–human Foxp3; and APC Alexa Fluor750 anti-CD8 (eBioscience). Isotype controls included appropriate fluorochrome-conjugated mouse immunoglobulin (Ig)G1, mouse IgG2a (DakoCytomation; eBioscience), and rat IgG2a (eBioscience). Foxp3 staining was performed using a kit (eBioscience), according to the manufacturer's instructions.
Flow cytometry analyses used a FACScan (BD Immunocytometry Systems) or Cyan-adenosine 5′-diphosphate flow cytometer (DakoCytomation). Gates were set for collection and analysis of at least 20 000 live events. T cells were sorted on a FACSVantage (BD Biosciences) or FACSAria (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Determination of IDO protein expression
For flow cytometric anaylsis, cells were harvested and stained with anti-CD14, anti-CD80, anti-CD83, anti-CD86, and anti–HLA-DR antibodies. After fixation and permeabilization (Cytofix/Cytoperm; BD Biosciences), cells were stained with a rabbit anti-IDO polyclonal antibody,21 followed by PE-labeled anti–rabbit secondary antibody (Jackson ImmunoResearch Laboratories), cross-adsorbed against mouse, human, and bovine IgG. This anti-IDO antibody is specific for IDO1 and does not cross-react with recombinant human IDO2 protein (data not shown). Negative control for IDO staining was the anti-IDO antibody preadsorbed with 50-fold molar excess of the immunizing peptide. Mature moDCs were distinguished from immature cells by CD83 expression.
For Western blots, cell lysates (15 μg) were separated on a 12% bis-Tris gel (Invitrogen), and protein was transferred to a polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline/0.1% Tween 20, and then probed with a mouse anti–human IDO primary monoclonal antibody (Millipore). The loading control was a mouse anti–human Rho-GDP-dissociation inhibitor monoclonal antibody (BD Biosciences). Incubation of primary antibodies was followed by horseradish peroxidase-conjugated goat anti–mouse IgG (PerkinElmer Life Sciences). Immunoreactive protein bands were detected with an enhanced chemiluminescence detection kit (Amersham).
Determination of IDO enzyme activity
Kynurenine levels were measured to determine IDO enzyme activity. Immature and mature moDCs were harvested, washed, resuspended in Hanks buffered saline solution supplemented with 100 μM tryptophan (Calbiochem), and then incubated for up to 4 hours. Supernatants were harvested at the indicated time points. Kynurenine was detected by high performance liquid chromatography (HPLC)34 or a modified spectrophotometric assay.35,36
For HPLC analysis, 40 μL of clarified sample were injected into an Amersham reverse-phase C2/C18 column and eluted with KH2PO4 buffer (0.01 M KH2PO4 and 0.15 mM EDTA [ethylenediaminetetraacetic acid], pH 5.0) containing 10% methanol (flow rate 1.0 mL/min). The spectrophotometer was set at 254 nm to detect both kynurenine and tryptophan. Retention time was determined using standard solutions of kynurenine (Sigma-Aldrich) and tryptophan.
For spectrophotometric analysis, supernatants were mixed with 30% trichloroacetic acid (2:1), vortexed, and centrifuged at 8000g for 5 minutes. Subsequently, 75 μL of this mixture were added to an equal volume of Ehrlich reagent (100 mg of p-dimethylbenzaldehyde, 5 mL glacial acetic acid; Sigma-Aldrich) in a 96-well microtiter plate. Triplicate samples were run against a standard curve of defined kynurenine concentrations (0-100 μg/mL; Sigma-Aldrich). Optical density was measured using a Medgenix 400 AT microplate reader (SLT Lab Instruments) at 490 nm.
Autologous mixed leukocyte reactions
Mature moDCs were cultured with autologous T cells in 24- or 48-well plates (Corning Life Sciences) at a 1:10 ratio in RPMI-10% autologous serum. Heterologous, allogeneic, or xenogeneic serum or plasma was never used in these priming cultures. After 6 days, T cells were harvested and recultured with freshly thawed autologous, mature moDCs for another 6 days at the same ratio in the same medium. No exogenous cytokines were added to cultures. Mixed leukocyte reactions (MLRs) were performed with or without 400 μM 1-methyl-dl-tryptophan (dl-1MT; Sigma-Aldrich) or 1 mM 1-methyl-l-tryptophan (l-1MT; Sigma-Aldrich). In some experiments, transwell inserts (Millipore) were used to separate moDCs from T cells. Culture scheme depicted in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Blocking experiments
Mature moDCs were treated with anti-CD80 and anti-CD86 antibodies (10 μg/mL each; eBioscience) or mouse IgG1 and IgG2b isotype controls (10 μg/mL each; R&D Systems) for 45 minutes before culture with T cells. A neutralizing anti–IL-2 antibody (10 μg/mL; R&D Systems) or mouse IgG1 isotype control (10 μg/mL; R&D Systems) was added at day 1 of autologous priming. In some MLR suppression assays (see “MLR suppression assays”), neutralizing antibodies or corresponding isotype controls were added at 10 μg/mL. Antibodies included anti–transforming growth factor (TGF)-β (R&D Systems), anti–IL-10 (eBioscience), mouse IgG1 isotype control (R&D Systems), and rat IgG1 isotype control (eBioscience).
MLR suppression assays
Bulk T cells harvested after 2 rounds of autologous priming were added to separate allogeneic MLRs composed of fresh autologous moDCs with responder allogeneic T cells (supplemental Figure 1). MoDCs and new responder T cells were designated either allogeneic or autologous relative to the candidate primed suppressor T cells. Stimulator moDCs and responder T cells were cultured in 96-well round-bottom plates (Corning) at a ratio of 1:30 in RPMI-10% pooled human serum. Primed bulk T cells were added at a ratio of 1:1, 1:2, and 1:10 (suppressor:responder). Responder T-cell proliferation was measured by incorporation of methyl-[3H]thymidine ([3H]TdR, 1 μCi/well; New England Nuclear, Division of PerkinElmer Life Sciences) during the last 8 hours of a 4- to 5-day culture, as measured in a beta scintillation counter (Betaplate; Wallac, Division of PerkinElmer Life Sciences). Percentage of inhibition was calculated by comparing with controls containing no suppressor cells.
Bulk T cells primed by autologous moDCs were cytofluorographically sorted into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subpopulations. These cells were added to allogeneic MLRs composed of either fresh autologous moDCs with new allogeneic T cells or fresh autologous T cells with new allogeneic moDCs (supplemental Figure 1). Stimulator moDCs and responder T cells were cultured in 96-well round-bottom plates at a 1:30 ratio. Candidate Tregs were added at a ratio of 1:25, 1:5, or 1:1 (suppressor:responder). Controls included MLRs without suppressor cells and moDCs cultured with suppressor cells without responder T cells. In some experiments, transwell inserts (Corning) were used to separate CD4+CD25bright cells from responder T cells. Responder T-cell proliferation was measured by [3H]TdR incorporation during the last 8 hours of a 4- to 5-day culture.
Suppression of WT1-specific cytotoxic T lymphocytes by Tregs expanded by autologous moDCs
WT1 mRNA-electroporated, mature moDCs (E.R., M.R., Rosa Barreira da Silva, Francesca Avogadri, Dana E. Pepe, Sharanya Chandrasekhar, Christian Munz, Glenn Heller, J.Y., and J.W.Y., manuscript submitted, May 2009) were cocultured with 105 autologous T cells in 96-well round-bottom plates at a ratio of 10:1 (T:DC) in complete RPMI 1640–10% autologous serum supplemented with recombinant human IL-15 (10 ng/mL; R&D Systems). After 7 days, T cells were tested for WT1-specific cytolytic activity generated in primary culture using a colorimetric cytotoxic T lymphocyte (CTL) assay.37 Target cells were 697 (WT1+ HLA-A*0201+ cell line) or LCL 721.221 (WT1neg HLA-A*0201neg NK cell-sensitive lymphoblastoid cell line). Target cells were stained with PKH26 membrane dye (Sigma-Aldrich) and added to wells containing candidate CTLs. After 4 to 5 hours, cells were harvested, exposed to a membrane-impermeable DNA stain (TO-PRO-3 iodide, 1 μM final concentration; Invitrogen), and analyzed by flow cytometry. Target cells were gated for PKH26 red fluorescence, and lysed targets were costained with TO-PRO-3. Unlysed targets excluded this membrane-impermeable stain. Background and maximum TO-PRO-3 staining were obtained by incubation with medium and detergent-exposed targets, respectively.
Cytofluorographically sorted CD4+CD25bright or CD4+CD25neg T cells, primed by autologous IDO+ moDCs, were added to the above cultures for generating WT1-specific CTL at a ratio of 1:1 (suppressor:responder T cell). Controls included cultures without candidate Tregs. After 7 days, T cells were tested for WT1-specific cytolytic activity generated in primary culture using a colorimetric assay by direct incubation with PKH26-labeled target cells, as described above.
Detection of interferon-γ secretion
MLR suppression assays were performed, as described above. Culture supernatant from each condition was collected at day 4. Supernatant samples were diluted 1/50, and interferon (IFN)–γ levels were measured using an MSD Cytokine Assay Kit and Multi-Spot plates (Meso Scale Discovery). Preparation of samples, controls, and calibration curves was done according to the manufacturer's instructions. IFN-γ concentration was measured using a Sector Imager 2400A (Meso Scale Discovery).
Statistics
Student t test was used for each pairwise comparison. One-way analysis of variance was used for multiple group comparisons. A P value less than .05 was considered statistically significant.
Results
Mature moDCs exert significantly greater IDO activity than immature moDCs and prime autologous T cells to suppress allogeneic T-cell proliferation
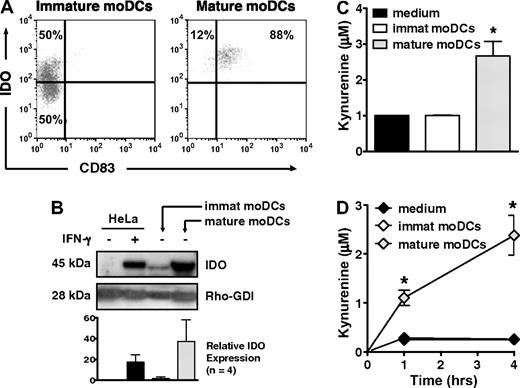
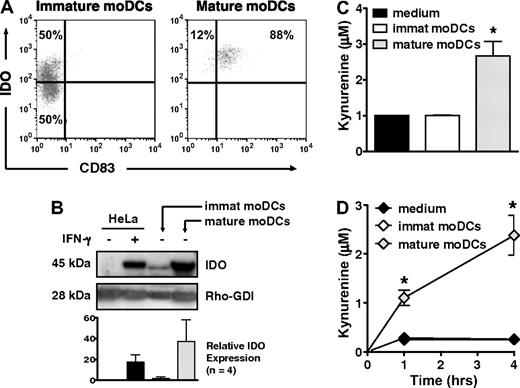
Only 50% of immature, CD83neg moDCs, but nearly all mature, CD83+ moDCs expressed IDO protein by flow cytometry (Figure 1A), a finding confirmed by Western blot (Figure 1B). Mature moDCs also generated substantial amounts of kynurenine, the first stable downstream product of tryptophan catabolism by IDO, whether detected by HPLC (Figure 1C) or a modified spectrophotometric assay (Figure 1D). In contrast, despite the presence of IDO protein in approximately half of the immature moDCs, IDO activity was no greater than in control samples containing only tryptophan-enriched media (Figure 1C-D). Hence, whereas both immature and mature moDCs expressed IDO protein, the enzyme was only active in mature moDCs.
IDO protein expression and activity increase with maturation of human moDCs. (A) IDO protein expression was assessed by flow cytometry in immature, CD83neg moDCs and in mature, CD83+ moDCs. Data are representative of 1 of 10 experiments. (B) IDO protein expression was assessed by Western blot in cell extracts from immature and mature moDCs. Untreated and IFN-γ–treated HeLa cells served as negative and positive controls, respectively. A representative blot from 1 experiment is shown along with pooled densitometry data from 4 separate experiments (mean ± SD; P = NS) showing relative IDO expression between groups. Densitometry values for each group were normalized to Rho-GDI (internal control). Relative IDO expression indicates fold increase above baseline IDO expression in untreated HeLa cells. (C) Supernatants from equal numbers of immature and mature moDCs were resuspended in tryptophan-enriched medium and tested after 4 hours by HPLC for production of the tryptophan catabolite, kynurenine, as an index of IDO activity (average of triplicate means ± SEM, n = 4 independent experiments). *P < .01 vs immature moDCs. (D) Kynurenine concentrations were determined by a spectrophotometric assay at 1 and 4 hours, using culture parameters identical to those in panel C (average of triplicate means ± SEM, n = 3 independent experiments). *P < .001 vs immature moDCs.
IDO protein expression and activity increase with maturation of human moDCs. (A) IDO protein expression was assessed by flow cytometry in immature, CD83neg moDCs and in mature, CD83+ moDCs. Data are representative of 1 of 10 experiments. (B) IDO protein expression was assessed by Western blot in cell extracts from immature and mature moDCs. Untreated and IFN-γ–treated HeLa cells served as negative and positive controls, respectively. A representative blot from 1 experiment is shown along with pooled densitometry data from 4 separate experiments (mean ± SD; P = NS) showing relative IDO expression between groups. Densitometry values for each group were normalized to Rho-GDI (internal control). Relative IDO expression indicates fold increase above baseline IDO expression in untreated HeLa cells. (C) Supernatants from equal numbers of immature and mature moDCs were resuspended in tryptophan-enriched medium and tested after 4 hours by HPLC for production of the tryptophan catabolite, kynurenine, as an index of IDO activity (average of triplicate means ± SEM, n = 4 independent experiments). *P < .01 vs immature moDCs. (D) Kynurenine concentrations were determined by a spectrophotometric assay at 1 and 4 hours, using culture parameters identical to those in panel C (average of triplicate means ± SEM, n = 3 independent experiments). *P < .001 vs immature moDCs.
We next evaluated the functional consequences of IDO activity exerted by mature moDCs. Bulk T cells primed by 2 rounds of stimulation with autologous, mature moDCs, with or without the IDO inhibitor, dl-1MT, were assessed for suppression of response to a strong stimulus like alloantigen. When added to secondary allogeneic mixed leukocyte reactions (MLRs), bulk T cells primed by autologous, mature moDCs suppressed, in a dose-dependent manner, the proliferation of allogeneic T cells stimulated by mature moDCs, which were autologous to the candidate suppressor T cells (Figure 2A left; supplemental Figure 1). The presence of dl-1MT during autologous T-cell priming reduced suppressor activity, indicating a role for IDO (Figure 2A right). The levo (l) stereoisomer of 1MT, rather than the dextro (d) form, was subsequently found to be the more efficient inhibitor of IDO activity in human moDCs.38 This, together with the use of bulk T cells rather than sorted Tregs, explained the incomplete reversal of suppression observed with dl-1MT. Ensuing experiments therefore used l-1MT to inhibit IDO and demonstrate its role in moDC-based Treg expansion. The addition of 1MT (dl mixture or l stereoisomer) to priming cultures did not have a direct effect on T-cell viability (Figure 2B). The absolute number of cells declined over time due to nonspecific cell losses related to multiple harvests and culture manipulations, but was similar between untreated and 1MT-treated conditions. In summary, T cells primed by autologous, mature moDCs suppressed allogeneic T-cell proliferation in secondary MLRs by an IDO-dependent mechanism.
IDO-expressing, mature moDCs prime autologous T cells to suppress allogeneic T-cell responses. (A) T cells were cultured twice for 6 days, each with fresh autologous, mature moDCs, with or without the IDO inhibitor, dl-1MT. These primed T cells (Auto) from either the untreated or dl-1MT–treated group were harvested, washed, and added to separate allogeneic MLRs composed of fresh autologous moDCs with new allogeneic responder T cells (Allo), but no dl-1MT. MoDC to new allogeneic responder T-cell ratio was 1:30; and candidate suppressor T-cell (Auto) to responder allogeneic T-cell (Allo) ratios were 1:10 (□), 1:2 (▩), and 1:1 (▤). Controls were MLRs without suppressor cells (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of triplicate means ± SEM, n = 6 independent experiments; P = NS). (B) T-cell viability was determined by trypan blue exclusion and was compared in autologous priming cultures with or without dl-1MT or l-1MT. T-cell counts were determined at day 0 and after 6 days of culture (average of triplicate means ± SEM, n = 6 independent experiments; P = NS).
IDO-expressing, mature moDCs prime autologous T cells to suppress allogeneic T-cell responses. (A) T cells were cultured twice for 6 days, each with fresh autologous, mature moDCs, with or without the IDO inhibitor, dl-1MT. These primed T cells (Auto) from either the untreated or dl-1MT–treated group were harvested, washed, and added to separate allogeneic MLRs composed of fresh autologous moDCs with new allogeneic responder T cells (Allo), but no dl-1MT. MoDC to new allogeneic responder T-cell ratio was 1:30; and candidate suppressor T-cell (Auto) to responder allogeneic T-cell (Allo) ratios were 1:10 (□), 1:2 (▩), and 1:1 (▤). Controls were MLRs without suppressor cells (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of triplicate means ± SEM, n = 6 independent experiments; P = NS). (B) T-cell viability was determined by trypan blue exclusion and was compared in autologous priming cultures with or without dl-1MT or l-1MT. T-cell counts were determined at day 0 and after 6 days of culture (average of triplicate means ± SEM, n = 6 independent experiments; P = NS).
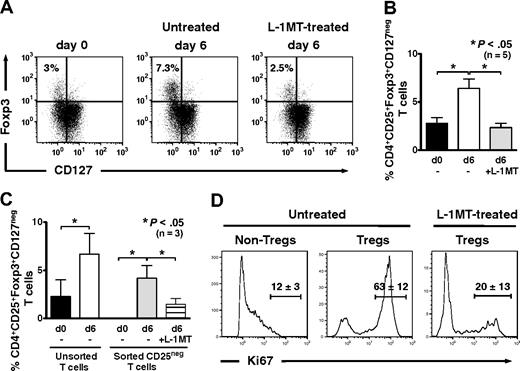
IDO-expressing, mature moDCs expand Tregs in autologous culture
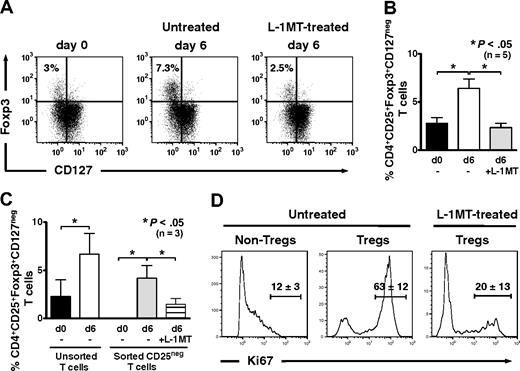
Compared with CD4+ T cells at baseline, the percentage of CD4+CD25brightFoxp3+CD127neg Tregs increased significantly after 6 days of priming in autologous MLRs in the absence of exogenous IL-2 (Figure 3A). The addition of l-1MT to priming cultures, in contrast, prevented Treg expansion from baseline levels (Figure 3A-B). Thus, autologous mature moDCs use an IDO-dependent mechanism to expand CD4+CD25brightFoxp3+CD127neg Tregs.
IDO-expressing, mature moDCs expand Foxp3+CD127neg Tregs and induce Tregs from CD4+CD25neg precursors in autologous culture. (A) Baseline expression of Foxp3 and CD127 by unprimed bulk CD4+ T cells was assessed by flow cytometry. T cells were cultured with or without the IDO inhibitor, l-1MT, and stimulated by autologous, mature moDCs for 6 days. The percentage of CD4+CD25+Foxp3+CD127neg Tregs among all CD4+ T cells was assessed by flow cytometry at day 6. (B) Pooled data from 5 independent experiments show the percentage of CD4+CD25+Foxp3+CD127neg Tregs at baseline before priming (d0) and after 6 days' stimulation by autologous, mature moDCs either without (□) or with (▩) [l-1MT (mean ± SEM, number of independent experiments, and P value indicated on graph). (C) Unprimed bulk T cells were sorted cytofluorographically to collect CD4+CD25neg T cells. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (day 0) and after 6 days' stimulation by autologous moDCs either with (▤) or without (▩) l-1MT. Controls were unmanipulated bulk T cells at day 0 (■) and after 6 days' stimulation by autologous moDCs (□; mean ± SEM; number of independent experiments and P value indicated on graph). (D) Representative histograms show percentages of Ki67-positive T cells in CD4+CD25low/intFoxpneg (Non-Tregs) and CD4+CD25brightFoxp+ (Tregs) cells from cultures without l-1MT, and in Tregs from l-1MT–treated cultures (mean ± SD; n = 2 independent experiments).
IDO-expressing, mature moDCs expand Foxp3+CD127neg Tregs and induce Tregs from CD4+CD25neg precursors in autologous culture. (A) Baseline expression of Foxp3 and CD127 by unprimed bulk CD4+ T cells was assessed by flow cytometry. T cells were cultured with or without the IDO inhibitor, l-1MT, and stimulated by autologous, mature moDCs for 6 days. The percentage of CD4+CD25+Foxp3+CD127neg Tregs among all CD4+ T cells was assessed by flow cytometry at day 6. (B) Pooled data from 5 independent experiments show the percentage of CD4+CD25+Foxp3+CD127neg Tregs at baseline before priming (d0) and after 6 days' stimulation by autologous, mature moDCs either without (□) or with (▩) [l-1MT (mean ± SEM, number of independent experiments, and P value indicated on graph). (C) Unprimed bulk T cells were sorted cytofluorographically to collect CD4+CD25neg T cells. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (day 0) and after 6 days' stimulation by autologous moDCs either with (▤) or without (▩) l-1MT. Controls were unmanipulated bulk T cells at day 0 (■) and after 6 days' stimulation by autologous moDCs (□; mean ± SEM; number of independent experiments and P value indicated on graph). (D) Representative histograms show percentages of Ki67-positive T cells in CD4+CD25low/intFoxpneg (Non-Tregs) and CD4+CD25brightFoxp+ (Tregs) cells from cultures without l-1MT, and in Tregs from l-1MT–treated cultures (mean ± SD; n = 2 independent experiments).
To determine the Treg precursors responding to stimulation by moDCs, we cytofluorographically sorted bulk T cells using stringent gating to isolate CD4+CD25negFoxp3neg T cells (Figure 3C). Culture for 6 days with mature moDCs in autologous MLRs resulted in the generation of CD4+CD25brightFoxp3+CD127neg Tregs (mean, 4.2%; range, 1.6%-5.5%; n = 3, P < .05; Figure 3C), along with a corresponding increase in expression of the proliferation marker, Ki67 (Figure 3D). The addition of l-1MT to priming cultures decreased Treg induction (mean, 1.5%; range, 0.51%-2.6%; n = 3, P < .05; Figure 3C) and strongly inhibited their expression of Ki67 (Figure 3D). This provided further evidence of an IDO-dependent mechanism by which moDCs could expand Foxp3+ Tregs, in this case from Foxp3neg precursors.
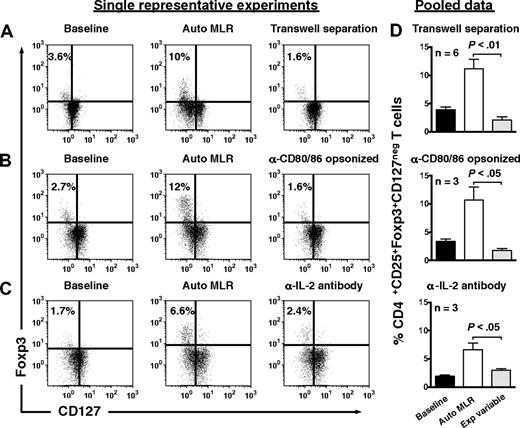
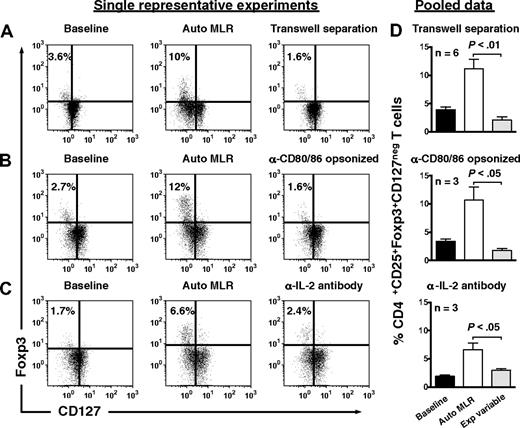
Treg expansion required contact with autologous moDCs, as separation in transwells prevented expansion (Figure 4A). Opsonization of autologous moDCs by anti-CD80 and anti-CD86 antibodies also abrogated Treg expansion (Figure 4B), as did neutralization of endogenous IL-2 (Figure 4C).
Expansion of Tregs by autologous, IDO-expressing, mature moDCs involves cell-to-cell contact, CD80/86 ligation, and IL-2. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (left panels), after 6 days' stimulation by autologous DCs (Auto MLR, middle panels), and after 6 days' stimulation by autologous moDCs (A) with transwell separation of moDCs from responder T cells (transwell separation), (B) after opsonization of moDCs with anti-CD80 and anti-CD86 a priori (α-CD80/86 opsonized), and (C) in the presence of neutralizing anti–IL-2 (α-IL-2 antibody; right panel for each condition). Isotype-matched, nonreactive antibodies were added to the control auto-MLRs in panels B and C. (D) Pooled data are shown for each experimental variable in rows A through C (mean ± SEM; number of independent experiments and P value indicated on each graph).
Expansion of Tregs by autologous, IDO-expressing, mature moDCs involves cell-to-cell contact, CD80/86 ligation, and IL-2. CD4+CD25+ T cells were assessed for their content of Foxp3+CD127neg T cells at baseline (left panels), after 6 days' stimulation by autologous DCs (Auto MLR, middle panels), and after 6 days' stimulation by autologous moDCs (A) with transwell separation of moDCs from responder T cells (transwell separation), (B) after opsonization of moDCs with anti-CD80 and anti-CD86 a priori (α-CD80/86 opsonized), and (C) in the presence of neutralizing anti–IL-2 (α-IL-2 antibody; right panel for each condition). Isotype-matched, nonreactive antibodies were added to the control auto-MLRs in panels B and C. (D) Pooled data are shown for each experimental variable in rows A through C (mean ± SEM; number of independent experiments and P value indicated on each graph).
CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs are potent inhibitors of both autologous and allogeneic T-cell proliferation
Because phenotypic markers alone are an imperfect gauge of Treg function, we next measured the capacity of the expanded Treg population to suppress T-cell proliferation in secondary MLRs, using a strong stimulus like alloantigen to challenge the model in vitro (supplemental Figure 1). T cells harvested after 2 rounds of autologous priming were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups (Figure 5A). Postsort flow cytometric analysis confirmed that the majority of CD4+CD25bright T cells expressed Foxp3, which was reduced by approximately half in CD4+CD25int T cells and was undetectable in CD4+CD25neg T cells (Figure 5B). When added to allogeneic MLRs composed of autologous moDCs and allogeneic T cells, relative to the candidate Tregs, CD4+CD25bright T cells inhibited T-cell proliferation in a dose-dependent manner with more than 90% inhibition at a suppressor to responder T-cell ratio of 1:1 and up to 54% at a ratio of 1:25 (mean, 43%; range, 33%–54%; n = 4, P < .05; Figure 5C top graph). Relatively high candidate suppressor to responder T-cell ratios were therefore used in these and subsequent assays to demonstrate unequivocal inhibitory activity where present. CD4+CD25int T cells inhibited proliferation to a lesser degree because of the presence of fewer Foxp3+ Tregs (Figure 5C middle graph), whereas CD4+CD25neg T cells were not at all inhibitory (Figure 5C bottom graph). A similar pattern of inhibition was observed when the cells in the allogeneic MLR were of reverse origin, with moDCs allogeneic and responder T cells autologous to the candidate Tregs (Figure 5D). Whereas CD4+CD25bright T cells, and to a lesser extent CD4+CD25int T cells, suppressed the proliferative response of alloreactive T cells in the MLRs, they were themselves anergic to stimulation by the allogeneic moDCs in proportion to CD25 and Foxp3 expression (Figure 5D Ctrl#2). CD4+CD25bright T cells also significantly reduced IFN-γ levels in culture supernatants, further supporting their suppressive effects on immunity (supplemental Figure 2). The expanded CD4+CD25brightFoxp3+ Tregs therefore significantly suppressed the proliferation of both allogeneic and autologous T cells responding de novo to a strong alloantigenic stimulus presented by mature moDCs.
Suppression of both autologous and allogeneic T-cell responses segregates with the CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs. (A) T cells harvested after two 6-day rounds of priming with autologous, mature moDCs were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups, and (B) assessed for Foxp3 expression postsort. Data are from 1 representative experiment of 4. (C-D) Cytofluorographically sorted CD4+CD25bright, CD4+CD25int, and CD4+CD25neg T cells were added to allogeneic MLRs composed of either fresh autologous moDCs and new allogeneic T cells (C) or fresh autologous T cells with new allogeneic moDCs (D). MoDC to responder T-cell ratio was 1:30 throughout, and candidate suppressor T-cell to responder T-cell ratios were 1:25 (□), 1:5 (▩), and 1:1 (▤). Controls included MLRs without suppressor cells (Ctrl#1, ■) and moDCs cultured with candidate suppressor cells without responder T cells (Ctrl#2,  ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
Suppression of both autologous and allogeneic T-cell responses segregates with the CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs. (A) T cells harvested after two 6-day rounds of priming with autologous, mature moDCs were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups, and (B) assessed for Foxp3 expression postsort. Data are from 1 representative experiment of 4. (C-D) Cytofluorographically sorted CD4+CD25bright, CD4+CD25int, and CD4+CD25neg T cells were added to allogeneic MLRs composed of either fresh autologous moDCs and new allogeneic T cells (C) or fresh autologous T cells with new allogeneic moDCs (D). MoDC to responder T-cell ratio was 1:30 throughout, and candidate suppressor T-cell to responder T-cell ratios were 1:25 (□), 1:5 (▩), and 1:1 (▤). Controls included MLRs without suppressor cells (Ctrl#1, ■) and moDCs cultured with candidate suppressor cells without responder T cells (Ctrl#2,  ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
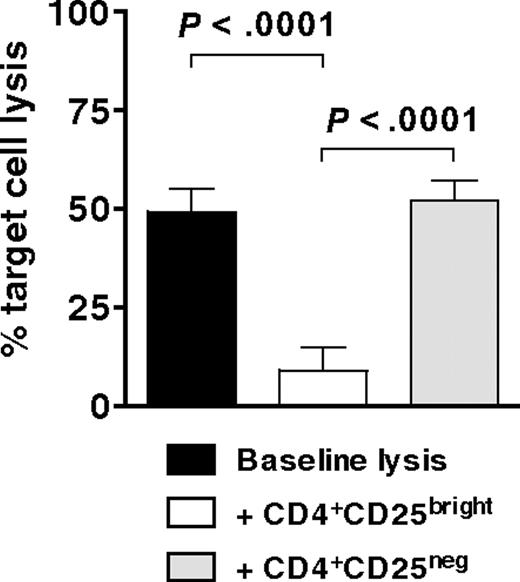
CD4+CD25brightFoxp3+ Tregs suppress the generation of antigen-specific CTLs
Having demonstrated that CD4+CD25bright T cells are potent inhibitors of both allogeneic and autologous T-cell proliferation in secondary MLRs, we then tested cytofluorographically sorted CD4+CD25bright T cells for their ability to suppress the development of WT1-specific CTLs. MoDCs, electroporated with mRNA for WT1 and supplemented with recombinant human IL-15, stimulated autologous T cells (E.R., M.R., Rosa Barreira da Silva, Francesca Avogadri, Dana E. Pepe, Sharanya Chandrasekhar, Christian Munz, Glenn Heller, J.Y., and J.W.Y., manuscript submitted May 2009) to which allogeneic CD4+CD25bright or CD4+CD25neg T cells (as per Figure 5B top and bottom panels) were added at a suppressor to responder T-cell ratio of 1:1. Specific target cell lysis of a WT1-expressing cell line was measured after 6 to 7 days by a flow cytometric PKH-26 cell lysis assay. Target cell lysis by CTLs generated in the presence of CD4+CD25bright T cells decreased by more than 80% (Figure 6). In contrast, the lytic activity of CTLs generated in the presence of CD4+CD25neg T cells did not change from baseline (Figure 6). Hence, CD4+CD25brightFoxp3+ Tregs expanded by IDO-expressing mature moDCs significantly suppressed the development of tumor antigen-specific CTLs.
Inhibition of antigen-specific CTL generation by CD4+CD25brightFoxp3+ Tregs. Bulk T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright and CD4+CD25neg candidate Tregs. These were then added 1:1 with responder T cells to mature moDCs that were electroporated before terminal maturation with WT1 mRNA. After 7 days' culture, WT1-specific target cell lysis was measured by a flow cytometric PKH-26 cell lysis assay and compared between cultures containing no candidate Tregs (baseline lysis, ■), CD4+CD25bright T cells (□), and CD4+CD25neg T cells (▩; average of triplicate means ± SEM, n = 3 independent experiments; P values indicated on graph).
Inhibition of antigen-specific CTL generation by CD4+CD25brightFoxp3+ Tregs. Bulk T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright and CD4+CD25neg candidate Tregs. These were then added 1:1 with responder T cells to mature moDCs that were electroporated before terminal maturation with WT1 mRNA. After 7 days' culture, WT1-specific target cell lysis was measured by a flow cytometric PKH-26 cell lysis assay and compared between cultures containing no candidate Tregs (baseline lysis, ■), CD4+CD25bright T cells (□), and CD4+CD25neg T cells (▩; average of triplicate means ± SEM, n = 3 independent experiments; P values indicated on graph).
CD4+CD25brightFoxp3+ Tregs inhibit responder T-cell proliferation by both contact-dependent and contact-independent mechanisms
Cytofluorographically sorted CD4+CD25bright T cells (Figure 5B top panel) were added to allogeneic MLRs composed of either autologous DCs with allogeneic T cells (Figure 7A left panel) or allogeneic DCs with autologous T cells (Figure 7A right panel). In the absence of transwell inserts, CD4+CD25bright T cells markedly inhibited T-cell proliferation (Figure 7A). Transwell inserts that prevented Treg contact with cells in the MLRs almost completely restored responder T-cell proliferation (Figure 7A). The addition of an anti–TGF-β–neutralizing antibody to MLR suppression assays also diminished, but did not completely prevent Treg-mediated suppression (Figure 7B). In contrast, an anti–IL-10–neutralizing antibody had no effect (Figure 7B). The inhibitory activity of CD4+CD25brightFoxp3+ Tregs was therefore mainly contact dependent, with measurable contribution from a contact-independent mechanism of suppression that involved TGF-β.
Suppression of T-cell proliferation by CD4+CD25brightFoxp3+ Tregs involves both contact-dependent and contact-independent mechanisms. (A) Autologous T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright candidate Tregs. These were then added 1:1 with either (A left panel) new allogeneic responder T cells stimulated by freshly thawed, mature moDCs, autologous to the candidate Tregs, or (A right panel) new responder T cells, autologous to the candidate Tregs, stimulated by new allogeneic, mature moDCs. The candidate Tregs were in contact (□) or separated in transwell inserts (▩) from the responder T cells, compared with control MLRs containing no candidate Tregs (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of quadruplicate means ± SEM, n = 2 independent experiments; P value indicated on each graph). (B) Neutralizing anti–TGF-β and anti–IL-10 antibodies, or their respective isotype-matched, nonreactive antibodies (mouse and rat IgG1), were added to MLRs containing CD4+CD25bright candidate Tregs added 1:1 with new allogeneic responder T cells stimulated by mature moDCs, autologous to the candidate Tregs. After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with control MLRs containing no candidate Tregs (■) and control MLRs containing candidate Tregs without neutralizing antibodies (□; average of triplicate means ± SEM, n = 2 independent experiments; P values indicated on graph).
Suppression of T-cell proliferation by CD4+CD25brightFoxp3+ Tregs involves both contact-dependent and contact-independent mechanisms. (A) Autologous T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright candidate Tregs. These were then added 1:1 with either (A left panel) new allogeneic responder T cells stimulated by freshly thawed, mature moDCs, autologous to the candidate Tregs, or (A right panel) new responder T cells, autologous to the candidate Tregs, stimulated by new allogeneic, mature moDCs. The candidate Tregs were in contact (□) or separated in transwell inserts (▩) from the responder T cells, compared with control MLRs containing no candidate Tregs (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of quadruplicate means ± SEM, n = 2 independent experiments; P value indicated on each graph). (B) Neutralizing anti–TGF-β and anti–IL-10 antibodies, or their respective isotype-matched, nonreactive antibodies (mouse and rat IgG1), were added to MLRs containing CD4+CD25bright candidate Tregs added 1:1 with new allogeneic responder T cells stimulated by mature moDCs, autologous to the candidate Tregs. After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with control MLRs containing no candidate Tregs (■) and control MLRs containing candidate Tregs without neutralizing antibodies (□; average of triplicate means ± SEM, n = 2 independent experiments; P values indicated on graph).
Discussion
These results clearly define the role of IDO-expressing, mature, human moDCs in expanding Tregs in a physiologically and clinically relevant autologous system, free of any exogenous cytokines or allogeneic stimulation. MoDCs up-regulate the immune-inhibitory enzyme IDO with maturation and expand functionally active, naturally occurring, and adaptive autologous Tregs by a mechanism that depends on IDO. The priming of resting T cells with autologous, IDO-expressing, mature moDCs in the absence of exogenous cytokines or heterologous serum results in the expansion of a subpopulation of CD4+CD25bright T cells that coexpress Foxp3 and lack CD127, consistent with a Treg phenotype.11,12 Importantly, these candidate Tregs exert significant dose-dependent suppression of both allogeneic and autologous T cells stimulated de novo by mature, activated moDCs and inhibit the generation of antigen-specific CTLs in vitro. Autologous priming in the presence of the IDO inhibitor, l-1MT, significantly abrogates Treg expansion.
Despite the emphasis on their immunogenicity, DCs also play an integral role in immune modulation.6 By processing and presenting self-antigens, steady-state DCs induce and maintain tolerance.3-5 The immunoregulatory activity of DCs, however, is not restricted to steady-state conditions or immature DC populations. Mature DCs can induce Tregs to curb immune responses.28,39,40 Furthermore, mature human moDCs,36,41 including a CD123+CCR6+ subset,21 as well as Langerhans cells treated with IFN-γ,42 can up-regulate IDO. Increased IDO activity in turn decreases T-cell proliferation by catabolism of tryptophan, which T cells cannot synthesize de novo and upon which T-cell expansion depends.21,41,42 A direct connection between mature human moDCs and the generation of autologous Tregs, free of exogenous cytokine support and allogeneic stimulation, but dependent on IDO, has not been previously established.
We have confirmed that mature moDCs express IDO,29,36,41 despite their potent immunogenicity. In fact, mature moDCs express even more IDO protein and exert more IDO-mediated immune suppression than do immature moDCs. This may seem counterintuitive, given the tolerogenic role often ascribed to immature or steady-state DCs. The presence of an immunosuppressive enzyme like IDO in mature moDCs, however, underscores a recurring theme in immune system modulation during inflammation or infection, which is the elicitation of counter-regulatory responses to prevent unrestrained T-cell activation.
Our data demonstrate a novel IDO-based mechanism by which mature, human moDCs expand primarily natural as well as adaptive Tregs in an entirely autologous culture system without exogenous IL-2 supplementation. The phenotypic identification of Tregs is challenging due to the lack of specific markers and, in humans, due to the absence of a clearly discrete population identifiable by flow cytometry as exists in mice.13 Our studies nevertheless indicate that naturally occurring CD4+CD25brightFoxp3+ Tregs are preferentially expanded by IDO-expressing, mature moDCs in autologous priming cultures. Supporting this conclusion is the demonstration that both the expansion of the Treg population and its mode of suppression are primarily contact dependent.13 Whether natural Tregs have a survival advantage in our system over other autoreactive T cells, due to decreased susceptibility to tryptophan deprivation and kynurenine catabolite toxicity, requires further investigation.
The induction of adaptive Tregs from CD4+CD25neg T cells by mature DCs has been demonstrated in other studies,28,39,40 although our data importantly demonstrate an IDO-based mechanism in their primary expansion and confirm their suppressive activity. Treg expansion persists in autologous priming cultures initiated with CD4+CD25neg T cells. Restoration of T-cell proliferation is incomplete in the suppression assays with transwell inserts, and the addition of an anti–TGF-β–neutralizing antibody reduces suppression. These findings indicate a soluble, contact-independent mode of suppression by adaptive Tregs generated by mature moDCs.
The prevailing hypothesis of the interplay between DCs, IDO, and Tregs is that Tregs express CTL antigen 4, which interacts with CD80 and/or CD86 on DCs to trigger the up-regulation of IDO.22,43 This in turn results in IDO-mediated tryptophan catabolism and suppression of T-cell and NK-cell activity.35,44-47 This model of immune regulation presupposes functional plasticity whereby IDO-competent DCs become IDO positive (tolerogenic) or remain IDO negative (immunogenic), depending on the influence of Tregs. Conversely, recent evidence suggests that IDO-positive DCs might induce or activate Tregs. IFN-γ is a universal inducer of IDO, and IDO activity in IFN-γ–treated, mouse splenic DCs supports the conversion of CD4+CD25neg T cells into Foxp3-expressing Tregs.48 In addition, a subset of IDO-expressing pDCs in mouse tumor-draining lymph nodes directly activates resting Tregs.49 Tryptophan catabolism by acute myeloid leukemia cells in humans has been implicated in the differentiation of allogeneic CD4+CD25neg T cells into CD4+CD25+Foxp3+ Tregs.50 LPS-treated human DCs expressing IDO have also been shown to expand allogeneic CD4+CD25+ Tregs in the presence of exogenous IL-2.29 Allogeneic Treg expansion by IDO-expressing pDCs has also been described.30,31 Aside from our results, however, there are no published data using a purely autologous culture system without exogenous cytokines or heterologous serum, demonstrating that mature human DCs by an IDO-dependent mechanism actively induce the expansion of autologous Tregs that can provide a self-reinforcing network of suppressor T cells. We conclude that this IDO-dependent, DC-based expansion of Tregs provides an additional level of immune regulation beyond mere tryptophan deprivation, which should have important physiologic relevance because it develops in an autologous system without exogenous IL-2 and thus approximates what occurs in vivo.
In the cytofluorographic studies to detect IDO, we used a polyclonal antibody, which is specific for the IDO1 gene product and does not cross-react with the product of a newly discovered IDO gene, IDO2.51,52 Although one group has reported that only IDO1 is catabolically active in human moDCs,38 the immunoregulatory role of IDO2 has yet to be fully defined. The participation of both IDO proteins in immune suppression driven by IDO-expressing DCs remains a possibility that warrants further study.
In summary, our results demonstrate a mechanism by which mature, rather than immature, IDO-expressing, human moDCs expand naturally occurring and adaptive Tregs in a completely autologous system without exogenous cytokines. These Tregs suppress autologous T-cell proliferation, as well as T-cell responses to DCs presenting either strong stimuli like alloantigens or a self-differentiation tumor antigen like WT1. This IDO-dependent process should therefore provide a means of terminating otherwise unchecked cellular immunity after a physiologic immune response. Where prolonged responses against infections or tumors are desirable, IDO activity exerted by mature, activated DCs provides a rational target to enhance immunity. Inhibition of this counter-regulatory pathway should therefore prove useful in sustaining responses stimulated by DC-based immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Christophe Antczak of the High-Throughput Drug Screening Facility, MSKCC, for his technical expertise and assistance with the HPLC assays. We thank Dr Francesca Orlandi of the Immune Monitoring Facility, Ludwig Center for Cancer Immunotherapy, MSKCC, for her assistance with the IFN-γ assays. We gratefully acknowledge the various contributions of members of the Laboratory of Cellular Immunobiology to the development of this work. We thank the nurses and physicians of the Allogeneic Bone Marrow Transplantation Service, as well as the Blood Donor Room and Cytotherapy Laboratory staffs at MSKCC, for assistance with sample procurement and processing. We also thank the patients and healthy volunteers who provided samples for research.
This work was supported by a Young Investigator Award from the American Society of Clinical Oncology (to D.J.C.); a Mortimer J. Lacher Fellowship from the Lymphoma Foundation (to D.J.C.); R01 CA112431 (to D.H.M.), R01 CA83070 (to J.W.Y.), R01 CA118974 (to J.W.Y.), R21 CA119528 (to J.W.Y.), and P01 CA23766 (to J.W.Y.) from the National Cancer Institute, National Institutes of Health; and William H. Goodwin and Alice Goodwin of the Commonwealth Foundation for Cancer Research through the Experimental Therapeutics Center of MSKCC (to J.W.Y.).
National Institutes of Health
Authorship
Contribution: D.J.C. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; M.R. and E.R. designed and performed experiments, analyzed and interpreted data, and made comments on the manuscript; J.G. assisted with experiments and made comments on the manuscript; J.Y. performed experiments; D.H.M. contributed to the intellectual design of the overall study, provided reagents, and assisted in completion of the manuscript; and J.W.Y. designed the overall study, supervised performance of experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: D.H.M. has intellectual property interests in the therapeutic use of IDO and IDO inhibitors, and receives consulting income and research support from NewLink Genetics Inc. The remaining authors declare no competing financial interests.
Correspondence: David J. Chung, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: chungd1@mskcc.org.

![Figure 2. IDO-expressing, mature moDCs prime autologous T cells to suppress allogeneic T-cell responses. (A) T cells were cultured twice for 6 days, each with fresh autologous, mature moDCs, with or without the IDO inhibitor, dl-1MT. These primed T cells (Auto) from either the untreated or dl-1MT–treated group were harvested, washed, and added to separate allogeneic MLRs composed of fresh autologous moDCs with new allogeneic responder T cells (Allo), but no dl-1MT. MoDC to new allogeneic responder T-cell ratio was 1:30; and candidate suppressor T-cell (Auto) to responder allogeneic T-cell (Allo) ratios were 1:10 (□), 1:2 (▩), and 1:1 (▤). Controls were MLRs without suppressor cells (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of triplicate means ± SEM, n = 6 independent experiments; P = NS). (B) T-cell viability was determined by trypan blue exclusion and was compared in autologous priming cultures with or without dl-1MT or l-1MT. T-cell counts were determined at day 0 and after 6 days of culture (average of triplicate means ± SEM, n = 6 independent experiments; P = NS).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430002.jpeg?Expires=1769108435&Signature=Zk7autXd0VM0MFXoOMbEyk9Nm-QjEeboFhNKl4sEHbbonYSDDaLwfomQyFM2SLcO8-ej~rfzoE-sBsHiMfaPHgU72C-kKvMIY46PoiUyc0khrg9joEwTuqHyAa~Ye7VBBEpxSGHly1JxYaH8cFgRjHDhS-uBuFoFUKD5Xkw3znCQFeQCPiI-PrYv6NE-gK~eDSjJJWJyiYwHrk~GzFO4L4PpZHf52-BVvRMghYgrMW4GNuIhqNQHK2~Jld2Jn5HjkRMHZNjlVcRo~sbsH1xhQ86jyza3b3Rxmo7EykFHFKnrDbTaX4xi6r8Bfzj040R-NJmUueLuX~JZ2s-CXabeHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Suppression of both autologous and allogeneic T-cell responses segregates with the CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs. (A) T cells harvested after two 6-day rounds of priming with autologous, mature moDCs were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups, and (B) assessed for Foxp3 expression postsort. Data are from 1 representative experiment of 4. (C-D) Cytofluorographically sorted CD4+CD25bright, CD4+CD25int, and CD4+CD25neg T cells were added to allogeneic MLRs composed of either fresh autologous moDCs and new allogeneic T cells (C) or fresh autologous T cells with new allogeneic moDCs (D). MoDC to responder T-cell ratio was 1:30 throughout, and candidate suppressor T-cell to responder T-cell ratios were 1:25 (□), 1:5 (▩), and 1:1 (▤). Controls included MLRs without suppressor cells (Ctrl#1, ■) and moDCs cultured with candidate suppressor cells without responder T cells (Ctrl#2, ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430005.jpeg?Expires=1769108435&Signature=Tzs~wIKG0NJ4WPclQ4kNkvfcaoOzfnfmKVLULiFTe4eYXt1VP7P2TZyMtQcOlqyKMOd~Bg4IlxnxGNCULgJikZdhtvsChBKGdZAq1hn4IVOW~ABQg0OnQPTnDIAFoIwO0ZjSSHzfjW5FVVpDrQKsSvM0A~2A4CUwl3RlH1m5y-o04~xAQ2Hz5xKTnzRkvelk5n7Ae2aqSwm51gp8IfBt0zckYsubM~Srm4U8Pqmr7yq2dhYQxny4hRD5We-9dX5ZnIu9jawmOwLNt0nSxSkWz3SEQjnzY9UhVWc0w871v3TJfKSyMB5OkZf16q0R7yFP~5A7cv~lNs9qDVyfKN1KWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Suppression of T-cell proliferation by CD4+CD25brightFoxp3+ Tregs involves both contact-dependent and contact-independent mechanisms. (A) Autologous T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright candidate Tregs. These were then added 1:1 with either (A left panel) new allogeneic responder T cells stimulated by freshly thawed, mature moDCs, autologous to the candidate Tregs, or (A right panel) new responder T cells, autologous to the candidate Tregs, stimulated by new allogeneic, mature moDCs. The candidate Tregs were in contact (□) or separated in transwell inserts (▩) from the responder T cells, compared with control MLRs containing no candidate Tregs (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of quadruplicate means ± SEM, n = 2 independent experiments; P value indicated on each graph). (B) Neutralizing anti–TGF-β and anti–IL-10 antibodies, or their respective isotype-matched, nonreactive antibodies (mouse and rat IgG1), were added to MLRs containing CD4+CD25bright candidate Tregs added 1:1 with new allogeneic responder T cells stimulated by mature moDCs, autologous to the candidate Tregs. After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with control MLRs containing no candidate Tregs (■) and control MLRs containing candidate Tregs without neutralizing antibodies (□; average of triplicate means ± SEM, n = 2 independent experiments; P values indicated on graph).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430007.jpeg?Expires=1769108435&Signature=r1drwx2-RmwX5rnYS37qiLH039NkBFiosL03Oca2VIh2xdacWiIO31b-hvU~9uUpPYvdm0AzroAwWvOAQoiaT6gXYx9ZaVOaG2Rh4RsFYKDNfej4hZeDD~0HsKLxMn--dRr2tl3FFaCMcz8nH9~OY5hNiz0L29MYpZp39Ev~iE3B3NJL4X3BKgCREB0vDn6602LMn7BO30d83zXknZVp1~MijSl6aCcix0DmHQJtDTqeDIYEgomI~GJ7P-TydfL1aXAMUHDuH5GS79cX02gMSIzOHk6vBaftw0liBba-TD8xI-kncXVIm7IQsiKCOxBNuPTpLm26I-1J-bI9-2inwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. IDO-expressing, mature moDCs prime autologous T cells to suppress allogeneic T-cell responses. (A) T cells were cultured twice for 6 days, each with fresh autologous, mature moDCs, with or without the IDO inhibitor, dl-1MT. These primed T cells (Auto) from either the untreated or dl-1MT–treated group were harvested, washed, and added to separate allogeneic MLRs composed of fresh autologous moDCs with new allogeneic responder T cells (Allo), but no dl-1MT. MoDC to new allogeneic responder T-cell ratio was 1:30; and candidate suppressor T-cell (Auto) to responder allogeneic T-cell (Allo) ratios were 1:10 (□), 1:2 (▩), and 1:1 (▤). Controls were MLRs without suppressor cells (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of triplicate means ± SEM, n = 6 independent experiments; P = NS). (B) T-cell viability was determined by trypan blue exclusion and was compared in autologous priming cultures with or without dl-1MT or l-1MT. T-cell counts were determined at day 0 and after 6 days of culture (average of triplicate means ± SEM, n = 6 independent experiments; P = NS).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430002.jpeg?Expires=1769108436&Signature=ZKqnaZaCZgXeEwR~2yNJNHVpTvaj6TK~sQdpZ2T35rBeXis0vYfmLamDUdQ3Wn-iSjhCtDsZLpb9Lv7bVuB9JBw6IzdbVFZ9cedIP8AGP8wO6R1XJyYm3~RblQ0fNullMwpvYP056xJ5DI-0s2tSVRHsnhSDvfd567qH7R4Jg7cDHWoBvan41SFBS~WiRsYS5K8j4mF2UXekEyLMfs~jh76Mc-kzfOphuuBhkFVfPtp9SHyOGcOHF0rmHQUdQb5fjXm-SxJvYvlac1mlTjASGYMMW60T-Jbd9smJp2Nk5nj2K2CKDo~DYZZmG28TWfVTFFX~xAklO1HoDiiTIxKFnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Suppression of both autologous and allogeneic T-cell responses segregates with the CD4+CD25brightFoxp3+ Tregs expanded by autologous, IDO-expressing, mature moDCs. (A) T cells harvested after two 6-day rounds of priming with autologous, mature moDCs were sorted cytofluorographically into CD4+CD25bright, CD4+CD25int, and CD4+CD25neg subgroups, and (B) assessed for Foxp3 expression postsort. Data are from 1 representative experiment of 4. (C-D) Cytofluorographically sorted CD4+CD25bright, CD4+CD25int, and CD4+CD25neg T cells were added to allogeneic MLRs composed of either fresh autologous moDCs and new allogeneic T cells (C) or fresh autologous T cells with new allogeneic moDCs (D). MoDC to responder T-cell ratio was 1:30 throughout, and candidate suppressor T-cell to responder T-cell ratios were 1:25 (□), 1:5 (▩), and 1:1 (▤). Controls included MLRs without suppressor cells (Ctrl#1, ■) and moDCs cultured with candidate suppressor cells without responder T cells (Ctrl#2, ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430005.jpeg?Expires=1769108436&Signature=SsqEZBrlJvqQK6JU-4mMFyHgcw4~gqjd8oMPkM8DfTizy9j3LsrmBE38~RP5n6Y6Pt1B0YxmXV1nScinL0B6SLnyha7SRv6MJygKbCYwJnP6bjnbUaJLjekitsfSclOE68ox0f6uxckmhC7qsVlOZlQ788YxD7QsRW0MkXYxNO9Q7oIt-yH1Vx5cvsyLGbziV32y5ch5P3FxTEsBwySVWKKu6hsbfKkx3DYisaHyoD23N9dFpgA7p8L3lfAKfCyZpo1xXBfKOE9u7JZQhgQkFVa5zW1kEprkUkHodAQdNnUz0bIx6W97O-gCKLcXAm-0pzxH8Y7Fdsp385cJipDGtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).
). After 4 to 5 days' culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with Ctrl#1 (average of quadruplicate means ± SEM, n = 4 independent experiments; P value(s) indicated on each graph).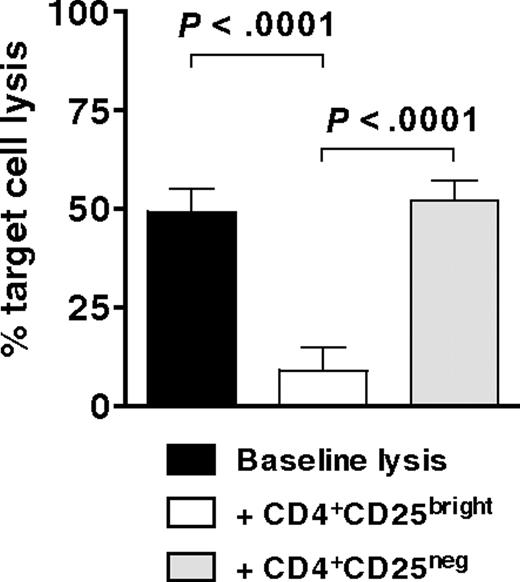
![Figure 7. Suppression of T-cell proliferation by CD4+CD25brightFoxp3+ Tregs involves both contact-dependent and contact-independent mechanisms. (A) Autologous T cells stimulated by IDO-expressing, mature moDCs were sorted cytofluorographically to collect CD4+CD25bright candidate Tregs. These were then added 1:1 with either (A left panel) new allogeneic responder T cells stimulated by freshly thawed, mature moDCs, autologous to the candidate Tregs, or (A right panel) new responder T cells, autologous to the candidate Tregs, stimulated by new allogeneic, mature moDCs. The candidate Tregs were in contact (□) or separated in transwell inserts (▩) from the responder T cells, compared with control MLRs containing no candidate Tregs (■). After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation (average of quadruplicate means ± SEM, n = 2 independent experiments; P value indicated on each graph). (B) Neutralizing anti–TGF-β and anti–IL-10 antibodies, or their respective isotype-matched, nonreactive antibodies (mouse and rat IgG1), were added to MLRs containing CD4+CD25bright candidate Tregs added 1:1 with new allogeneic responder T cells stimulated by mature moDCs, autologous to the candidate Tregs. After 4 to 5 days in culture, responder T-cell proliferation was measured by [3H]TdR incorporation and compared with control MLRs containing no candidate Tregs (■) and control MLRs containing candidate Tregs without neutralizing antibodies (□; average of triplicate means ± SEM, n = 2 independent experiments; P values indicated on graph).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2008-11-191197/4/m_zh89990939430007.jpeg?Expires=1769108436&Signature=M~sN7rX2hNkYT5f9yDaB~Nk1WrnzKWZUGnmHusQgTfre4fWlvdOEtRTVOCza-fDokg88yA0oWmqr1vt8UQiGmpquZMMWTGRtjnsJ0e3gdU-wicEP2It2AQjHGa5zIk3o8TNP1B64r4qTAjxPHFcZr0nHViJp5ZuCzGLZNHCWQibZPaAT0yHjc9yix9H8-1gana7s7-7P1zXHOlzU10zKy4A9rxKxLW7TGUMy21k3r0nPQ5bkHFlS5CdbgcW9aXgsqVMuyPjkZ6gpxdk8bSiFP7F8gfG49udl4Dh~OYikAuOXeGhUf5bnCFTL-cFgMNIMRdIrvMujLjwBXKh6njNaXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)