Abstract
CD4+ T cells use the chemokine receptor CCR7 to home to and migrate within lymphoid tissue, where T-cell activation takes place. Using primary T-cell receptor (TCR)–transgenic (tg) CD4+ T cells, we explored the effect of CCR7 ligands, in particular CCL21, on T-cell activation. We found that the presence of CCL21 during early time points strongly increased in vitro T-cell proliferation after TCR stimulation, correlating with increased expression of early activation markers. CCL21 costimulation resulted in increased Ras- and Rac-GTP formation and enhanced phosphorylation of Akt, MEK, and ERK but not p38 or JNK. Kinase-dead PI3KδD910A/D910A or PI3Kγ-deficient TCR-tg CD4+ T cells showed similar responsiveness to CCL21 costimulation as control CD4+ T cells. Conversely, deficiency in the Rac guanine exchange factor DOCK2 significantly impaired CCL21-mediated costimulation in TCR-tg CD4+ T cells, concomitant with impaired Rac- but not Ras-GTP formation. Using lymph node slices for live monitoring of T-cell behavior and activation, we found that G protein-coupled receptor signaling was required for early CD69 expression but not for Ca2+ signaling. Our data suggest that the presence of CCL21 during early TCR signaling lowers the activation threshold through Ras- and Rac-dependent pathways leading to increased ERK phosphorylation.
Introduction
Naive T cells continuously traffic to secondary lymphoid organs, including peripheral lymph nodes (PLNs), where they screen antigen-presenting cells (APCs), in particular dendritic cells (DCs), for the presence of specific peptide Ag presented on MHC (pMHC) complexes.1 In proinflammatory conditions, DCs expressing cognate pMHC and costimulatory signals, such as B7 molecules, induce efficient T-cell activation through the T-cell receptor (TCR) and CD28.2 This leads to an early signaling response characterized by activation of multiple signaling pathways, including tyrosine kinase cascades, sustained increase in intracellular Ca2+, and activation of phosphoinositide-3-kinase (PI3K), small GTPases of the Ras and Rho family, mitogen-activated protein kinase, nuclear factor of activated T-cell, and nuclear factor-κB. Activated T cells subsequently increase surface expression of early activation markers, such as CD69 and CD25, produce interleukin-2 (IL-2), expand clonally, and differentiate into effector cells.
Direct observations of lymphocytes and DCs presenting cognate pMHC complexes in explanted PLNs or live mice using 2-photon microscopy have uncovered a dynamic range of cellular interactions within lymphoid tissue. In some settings, T cells almost immediately arrest on encountering DCs.3 Alternatively, T cells were observed to continue to migrate during the first several hours after entry into lymphoid tissue along the stromal network formed by fibroblastic reticular cells (FRCs), where they underwent brief serial contacts with DCs.4 This first phase of high motility is reminiscent of naive T-cell migration in the absence of pMHC-loaded DCs.4,5 Of note, even during continuous motility during initial DC encounters, T cells integrate TCR-derived signals, as they gradually increase CD44 and CD69 surface levels.6 Differences in T-cell deceleration in distinct models are probably the result of variations in total Ag load and TCR-MHC affinity, which influence the time T cells require to reach a threshold for efficient arrest and formation of long-lasting contacts with DCs.6-8
FRCs in the T-cell area of PLN express the homeostatic chemokines CCL19 and CCL21.9,10 Their G-protein coupled receptor (GPCR) CCR7 is highly expressed on naive T cells and contributes to random motility during DC scanning in vitro and in vivo.11-16 TCR signaling events are thus spatiotemporally tightly connected to chemokine receptor signaling, suggesting a potential crosstalk between both pathways during early T-cell activation. Indeed, chemokines have been shown to contribute to T-cell activation in at least 2 ways. First, in vitro assays showed that CCL19 and CCL21 indirectly contribute to lymphocyte activation by allowing efficient screening of rare Ag-bearing DCs.11,12 DC-bound CCL21 was also found to sensitize CD4+ T cells to pMHC complexes on neighboring DCs, correlating with efficient DC scanning of the leading edge of polarized T cells.17 These observations support a role for CCR7 ligands in promoting efficient encounters with other cell types present in lymph nodes, such as DCs or B cells, through increased motility and scanning.
Independent of their chemoattractant and guiding activities, chemokines also act directly as costimulatory factors and consequently modulate the outcome of an immune response.18-20 CCL5 costimulates Jurkat T cells by recruiting its receptor CCR5 and Gq/11 to the immunologic synapse without inducing migration.21 Similarly, CXCL12 and CCL21 increase anti-CD3-induced T-cell activation, suggesting that migration-independent chemokine receptor- and TCR-triggered signals are combined for optimal T-cell activation.19,22 Thus far, the underlying molecular mechanisms of chemokine-mediated costimulation and intracellular integrators acting both downstream TCR and chemokine receptors remain incompletely described. In addition, it is not known whether chemokines costimulate T cells in a lymphoid environment.
Chemokine-induced cell migration, scanning of Ag-bearing DCs, and subsequent formation of a stable immunologic synapse require cytoskeletal reorganizations in T cells.23,24 The small GTPase Rac is a key modulator of F-actin polymerization in cells and is important for cell migration.25 The hematopoietic lineage-restricted guanine exchange factor (GEF) DOCK2 catalyzes GDP-GTP exchange of Rac downstream chemokine receptors. Accordingly, DOCK2-deficient lymphocytes migrate poorly to homeostatic chemokines in chemotaxis assays, 2-dimensional surfaces, and within lymph nodes.26-28 DOCK2 also acts downstream the TCR to promote Rac-GTP formation. In the absence of DOCK2, clustering of TCR and lipid rafts, and to a lesser extent ERK phosphorylation, are impaired, resulting in decreased DC-induced T-cell proliferation.29 Furthermore, PI3K isoforms are involved in DOCK2-independent chemokine-induced lymphocyte migration.30,31 PI3Kγ has also been reported to contribute to T-cell proliferation in some32,33 but not all studies.31 PI3Kδ is a major class IA isoform expressed in immune cells and is activated by tyrosine kinases downstream T- and B-cell antigen receptors.34,35 In vitro, B cells expressing a kinase-dead PI3Kδ mutant, p110D910A/D910A, show decreased migration toward CXCL13, whereas T-cell migration to CCR7 ligands appears not to be affected.36 Thus, similar to DOCK2, PI3K isoforms act downstream TCR and chemokine receptors, making them potential transmitters of chemokine-mediated costimulation.
Here, we report a detailed analysis of the costimulatory effect of chemokines expressed in the T-cell area of secondary lymphoid organs. Using in vitro proliferation assays of various TCR tg mouse lymphocytes, we identify CCL21 as having the greatest costimulatory activity during early TCR signaling, in particular at suboptimal stimulation. We performed a biochemical and genetic analysis of signaling molecules underlying the costimulatory activity, focusing on pathways known to be triggered by both receptor classes. Our data suggest a model in which CCL21-mediated interstitial cell motility and TCR signaling are integrated, in part via DOCK2, during the early, promigratory phase of T-cell–DC encounters, thus lowering the activation threshold in lymphoid tissue.
Methods
Mice
Four- to 12-week-old control or p110γ-deficient and p110δD910A/D910A– knock-in mice backcrossed to the OT-II TCR tg C57BL/6 background and 2B4 TCR tg DOCK2-deficient mice on a B10.BR background were described before.29,37 Female Marylin (anti–H-Y peptide called DBY) TCR-transgenic Rag2−/−CD45.1+/+ mice were obtained from the CDTA. C57BL/6, BALB/c, and DO11.10 mice were bred at the Theodor Kocher Institute or purchased from Harla. All experiments were performed in accordance with approval from the Swiss Kanton of Bern Veterinary Office and French veterinary animal experimentation regulations.
Reagents
All antibodies and other reagents are listed in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Proliferation assays
Primary T cells were purified from spleen and PLN single-cell suspensions via negative selection using antibody-coated magnetic beads (> 90% purity; Dynal). Isolated T cells (5 × 105 cells/mL) were either plated on 96-well plates coated with anti-CD3ϵ monoclonal antibodies (mAbs) or stimulated with soluble anti-CD3ϵ mAb and cultured in RPMI 1640/10% fetal calf serum/standard supplements, in the presence or absence of anti-CD28 mAb or chemokines (100 nM final concentration). [3H]Thymidine was added for the last 16 hours of a 48- or 72-hour culture, followed by quantification in a scintillation counter. Where indicated, T cells were pretreated with 400 ng/mL pertussis toxin (PTX; 30 minutes at 37°C, 7% CO2), washed twice, and cultured as described. For activation of TCR-transgenic CD4+ T cells, isolated T cells (4 × 106 cells/mL) were cultured for 48 or 72 hours with irradiated congenic spleen cells (5 × 106 cells/mL) in the presence or absence of 100 nM chemokine and indicated concentrations of agonist peptide. [3H]Thymidine incorporation was determined as detailed earlier in this paragraph.
In some experiments, T cells were loaded with carboxyfluorescein succinimidyl ester (CFSE) (1 μM, 30 minutes at 37°C, 7% CO2) before activation and analyzed for dye dilution by flow cytometry (FACScalibur, BD Biosciences). From obtained histograms, a proliferation index was calculated as follows. The percentage of divided cells in the presence of anti-CD3ϵ mAb alone (without chemokines), or, in case of APC-triggered proliferation, at a peptide concentration of 0.1 μg/mL without chemokines, was arbitrarily normalized to “1.” Other percentages were adjusted accordingly, with their ratio to “1” being the proliferation index. For Figure 2A, the cpm value of 0.1 μg/mL without chemokines was normalized to “1” and the other cpm values expressed as proliferation index accordingly.
Flow cytometry
TCR Tg DO11.10 T cells were activated by chicken or turkey OVA323-339-pulsed irradiated splenocytes in the presence or absence of CCL21 (100 nM) for indicated times and stained for CD25 and CD69. For measurement of intracellular IL-2, DO11.10 T cells were restimulated with ionomycin/phorbol myristate acetate in the presence of brefeldin A for 3 hours and labeled with mAbs against Thy1.2, KJI-26, and IL-2 following the manufacturer's instructions (BD Biosciences PharMingen).
Immunoblotting
Isolated T cells (5 × 107 cells/mL) were incubated with anti-CD3ϵ mAb (5 μg/mL; 4°C, 20 minutes) after overnight incubation in RPMI 1640/0.5% fatty acid free bovine serum albumin. Cells were stimulated by crosslinking of primary antibody with goat anti–hamster-IgG Ab (G94-56, 20 μg/mL) at 37°C for indicated times, in the presence or absence of CCL21 (100 nM). For quantification, all blots were normalized to the loading control. Fold increase over background was calculated using ImageJ software or according to the manufacturer's instructions (LI-COR Biosciences). Additional information is available in the supplemental data.
Time-lapse imaging
Splenocytes were washed in Hanks Balanced Salt Solution and 4 × 105 cells were left to settle to glass coverslips for 15 minutes at 37°C. In parallel, 5 × 105 T cells prepared from peripheral and mesenteric lymph nodes were incubated for 5 minutes at 37°C with 0.5 μM 5-chloromethylfluorescein diacetate (CMFDA). When indicated, T cells were treated with 1 μg/mL CCL21 for 5 minutes at 37°C before adding to the splenocytes layer. Imaging was performed as described16 on an inverted microscope in a heating chamber (37°C). Images were acquired every 10 seconds during 10 to 15 minutes using MetaFluor software (Molecular Devices). Cell displacements were analyzed with Imaris software (Bitplane).
PLN slice preparation and video imaging
PLN slice preparation was performed as previously described16 with some modifications (supplemental data). The preparation was perfused at a rate of 1 mL/minute with RPMI without phenol red medium bubbled with a mixture of 95% O2 and 5% CO2. A single section located 20 to 30 μm from the surface of slice was acquired every 30 seconds. Five minutes after beginning of image acquisition, slices were perfused with oxygenated RPMI medium containing DBY peptide at indicated concentrations. Fura-2-loaded T cells were alternatively excited at 350 and 380 nm and emission at 510 nm was used for analysis of Ca2+ responses using MetaFluor software. Ca2+ values were represented as the fluorescence intensity ratio at 340/380 nm. T cells were considered responsive when the amplitude of their responses reached at least twice that of the background. When several Ca2+ traces were averaged, the rising phases of the traces were synchronized. T-cell motility was analyzed with Imaris software.
Measurement of CD69 expression on T cells activated within PLN slices
PLN slices overlaid with CMFDA-loaded T cells were treated with indicated concentrations of DBY peptide at 37°C, 6% CO2. After 2 hours, slices were washed and mechanically dissociated using 30-G needles to obtain single-cell suspensions. Cells were stained with phycoerythrin (PE)–labeled anti-CD69 and PE-Cy5-labeled anti-CD45.1 for analysis on a FACScan (BD Biosciences).
Statistical analysis
Data were analyzed using Prism software (GraphPad Software). Student t test was used for statistical analysis, unless indicated otherwise. Significance was set at P less than .05.
Results
Homeostatic chemokines act as costimulatory factors during mAb- or APC-induced T-cell activation
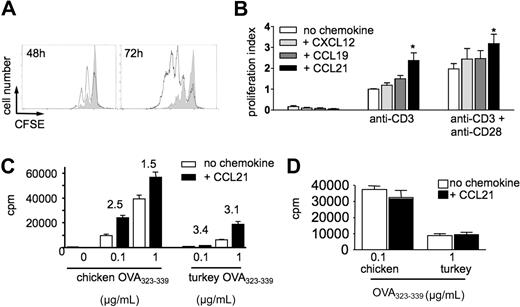
The lymphoid tissue-expressed chemokines CCL21 and CXCL12 have been shown to act as costimulatory factors during T-cell activation.17,19,22 In CFSE dilution experiments, we confirmed that CCL21 enhanced proliferation of T cells activated with plate-bound anti-CD3ϵ mAb alone or in combination with anti-CD28 mAb (Figure 1A-B). CXCL12 and CCL19 were not effective, although CCL19 showed a nonsignificant tendency to increase proliferation on plates coated with anti-CD3ϵ mAb alone (data not shown). Similar results were obtained when stimulating with soluble anti-CD3ϵ mAb (data not shown). No increased proliferation was observed in the presence of human CCL2 or heat-inactivated CCL21 (data not shown). Thus, CCR7 ligands, especially CCL21, possess higher costimulatory potency than the CXCR4 ligand CXCL12, reflecting receptor expression levels on murine naive T cells. In the conditions used here, chemokines alone did not induce T-cell proliferation (Figure 1B), whereas both CCL19 and CCL21 slightly increased the percentage of live (7-amino-actinomycin D-negative) T cells after 3 days of culture from 18.4% plus or minus 1.1% to 27% plus or minus 1% and 27.4% plus or minus 0.7%, respectively. For subsequent experiments, we focused on CCL21, which is expressed by FRCs at 100-fold higher levels than CCL19 and therefore probably physiologically more relevant.38
Homeostatic chemokines function as costimulatory factors during Ab- and peptide-induced T-cell activation. (A) Flow cytometry histogram of CFSE-labeled T-cell division after 48 and 72 hours of activation with anti-CD3ϵ mAb in the presence (bold line) or absence (gray fill) of 100 nM CCL21. Data are representative from 1 of 6 independent experiments. (B) Proliferation in the presence and absence of homeostatic chemokines. CFSE-labeled T cells were stimulated with plate-bound anti-CD3ϵ mAb with or without anti-CD28 mAb in the presence or absence of CCL21, CCL19, or CXCL12 (100 nM). Proliferation was determined after 48 hours by fluorescence-activated cell sorter and normalized to fold increase compared with cells cultured with anti-CD3ϵ mAb (proliferation index). The presence of chemokines increases the percentage of cells having undergone cell divisions within 48 hours. Data are pooled from 4 to 6 independent experiments. Statistical significance was determined using analysis of variance comparison of anti-CD3 or anti-CD3/CD28–stimulated T-cell proliferation with or without chemokines. *P < .05 compared with “no chemokine.” (C) DO11.10 T cells were cocultured with chicken or turkey OVA323-339-loaded congenic splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined by 3H-thymidine-incorporation after 48 hours. Numbers indicate fold increase of proliferation in the presence of CCL21. *P < .05 compared with “no chemokine.” (D) Prior activation as in panel C; DO11.10 T cells were incubated with PTX. Data are pooled from 2 independent experiments.
Homeostatic chemokines function as costimulatory factors during Ab- and peptide-induced T-cell activation. (A) Flow cytometry histogram of CFSE-labeled T-cell division after 48 and 72 hours of activation with anti-CD3ϵ mAb in the presence (bold line) or absence (gray fill) of 100 nM CCL21. Data are representative from 1 of 6 independent experiments. (B) Proliferation in the presence and absence of homeostatic chemokines. CFSE-labeled T cells were stimulated with plate-bound anti-CD3ϵ mAb with or without anti-CD28 mAb in the presence or absence of CCL21, CCL19, or CXCL12 (100 nM). Proliferation was determined after 48 hours by fluorescence-activated cell sorter and normalized to fold increase compared with cells cultured with anti-CD3ϵ mAb (proliferation index). The presence of chemokines increases the percentage of cells having undergone cell divisions within 48 hours. Data are pooled from 4 to 6 independent experiments. Statistical significance was determined using analysis of variance comparison of anti-CD3 or anti-CD3/CD28–stimulated T-cell proliferation with or without chemokines. *P < .05 compared with “no chemokine.” (C) DO11.10 T cells were cocultured with chicken or turkey OVA323-339-loaded congenic splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined by 3H-thymidine-incorporation after 48 hours. Numbers indicate fold increase of proliferation in the presence of CCL21. *P < .05 compared with “no chemokine.” (D) Prior activation as in panel C; DO11.10 T cells were incubated with PTX. Data are pooled from 2 independent experiments.
To evaluate the costimulatory potential of CCL21 during physiologic TCR activation, we activated DO11.10 TCR tg CD4+ T cells with either chicken or turkey OVA323-339 peptide-pulsed irradiated splenocytes as APCs. Proliferation induced by both peptides was increased in the presence of CCL21 (Figure 1C). The relative increase in the presence of CCL21 was particularly noticeable at low peptide concentrations and with the low affinity turkey OVA323-339 peptide, representing suboptimal T-cell activation conditions (Figure 1C). In line with previous observations,17 inhibition of Gαi signaling by preincubation of T cells with PTX completely blocked the costimulatory effect of CCL21, indicating that CCL21 acted directly on T cells (Figure 1D).
CCL19 and CCL21 have been reported to induce cell motility under certain experimental conditions, thus increasing the likelihood of T-cell–DC encounters.11,12 We examined whether motility, rather than direct signaling, was involved in CCL21-mediated costimulation. Using time-lapse videomicroscopy, we followed individual CD4+ T cells coincubated with splenocytes on glass coverslips during 10-minute observation periods in the presence or absence of CCL21. In the absence of CCL21, very few T cells displayed a motile behavior. CCL21 did not increase the percentage of motile T cells under these conditions, although cells were more elongated (supplemental Figure 1; supplemental Videos 1-2). Taken together, our data suggest that, in the conditions used here, CCL21 acts mainly as a costimulatory factor independent of motility.
CCL21-mediated costimulation is required during an early time window
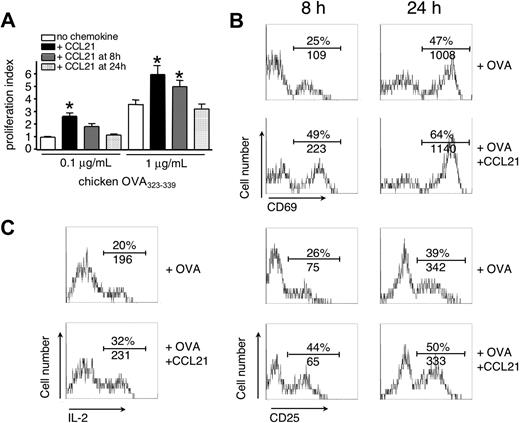
In lymphoid tissue containing few pMHC complexes, T-cell activation starts with an early phase of continuous motility most probably codependent on CCL21, followed by stable T-cell–DC interactions.4,5,8,39 We thus examined the effect of CCL21 on T-cell activation triggered by low and high pMHC levels during early or late time points in vitro. Addition of CCL21 to DO11.10 TCR tg CD4+ T cells at 8 or 24 hours after activation led to a gradual decrease in pMHC-stimulated proliferation, indicating that for efficient costimulation, presence of CCL21 is required within an early time frame of T-cell activation (Figure 2A). Similarly, addition of neutralizing anti-CCL21 Abs to the T-cell culture only blocked chemokine-induced costimulation when added from the start but not when added 8 hours after activation (data not shown).
CCL21 costimulation is effective during early T-cell activation. DO11.10 T cells were cocultured with OVA323-339-loaded congenic splenocytes in the presence or absence of CCL21 (100 nM). (A) CCL21 was added at indicated times, and proliferation of T cells was analyzed after 48 hours. Statistical significance was determined using analysis of variance comparison of peptide-stimulated T-cell proliferation without or with CCL21 added at indicated times. *P < .05 compared with “no chemokine.” (B) Up-regulation of early activation markers CD69 and CD25 on CD4+ KJ1-26+ DO11.10 T cells 8 and 24 hours after Ag-specific T-cell activation in the presence or absence of CCL21 (100 nM). One representative experiment of 3 is shown. (C) IL-2 production of DO11.10 T cells after 24 hours as determined by intracellular staining. One representative experiment of 2 is shown. In panels B and C, numbers indicate percentage of positive cells and mean fluorescence intensity (MFI), respectively.
CCL21 costimulation is effective during early T-cell activation. DO11.10 T cells were cocultured with OVA323-339-loaded congenic splenocytes in the presence or absence of CCL21 (100 nM). (A) CCL21 was added at indicated times, and proliferation of T cells was analyzed after 48 hours. Statistical significance was determined using analysis of variance comparison of peptide-stimulated T-cell proliferation without or with CCL21 added at indicated times. *P < .05 compared with “no chemokine.” (B) Up-regulation of early activation markers CD69 and CD25 on CD4+ KJ1-26+ DO11.10 T cells 8 and 24 hours after Ag-specific T-cell activation in the presence or absence of CCL21 (100 nM). One representative experiment of 3 is shown. (C) IL-2 production of DO11.10 T cells after 24 hours as determined by intracellular staining. One representative experiment of 2 is shown. In panels B and C, numbers indicate percentage of positive cells and mean fluorescence intensity (MFI), respectively.
In line with a priming effect of CCL21, the percentage of T cells expressing the early activation marker CD69 was doubled as soon as 8 hours after activation in the presence of CCL21, with cells expressing higher mean CD69 levels (Figure 2B). We also observed an increase in the percentage of CD25+ DO11.10 CD4+ T cells 8 hours after activation (Figure 2B), paralleled by enhanced IL-2 production 24 hours after antigen-specific stimulation (Figure 2C). Taken together, T-cell costimulation through CCL21 is effective during an early time window and directly increases the percentage and expression levels of early activation markers.
To examine whether CCR7-mediated costimulation was a general phenomenon, we stimulated peripheral blood human T cells with anti-CD3 mAbs in the presence or absence of CCL19 or CCL21. Addition of CCL19 accelerated the initiation of TCR-triggered Ca2+ increase but did not affect the amplitude of the response (supplemental Figure 2A). Furthermore, simultaneous activation of TCR and CCR7 resulted in a marked increase in cells expressing CD69, with CCL19 being more potent than CCL21, in contrast to mouse lymphocytes (supplemental Figure 2B). Altogether, these results support a conserved role for CCR7 during T-cell activation.
Signal transduction analysis in primary T cells after TCR and CCR7 costimulation
To explore the biochemical basis of CCL21-induced costimulation, we investigated signaling events activated downstream of both TCR and chemokine receptors. The TCR on primary mouse T cells was cross-linked for various times in the presence or absence of CCL21 and lysates analyzed by quantitative Western blotting. As shown in Figure 3A (left panel), simultaneous activation of TCR and CCR7 resulted in additive Rac-GTP formation up to 2 minutes (supplemental Table 1). Unexpectedly, early Ras-GTP formation was mainly mediated by CCR7 at early time points, whereas at 5 minutes, Ras-GTP was only observed in CCR7 and TCR-costimulated cells (2.2- ± 0.7-fold increase over added values of single stimulation; mean ± SEM). This was paralleled by increased phosphorylation of MEK1/2 when both anti-CD3 and CCL21 were combined and by synergistic phosphorylation of its downstream target ERK1/2 (2.4- ± 1.3-fold and 2- ± 0.1-fold increase over added values of single stimulation at 5 and 10 minutes, respectively). Flow cytometric analysis uncovered that both percentage of pERK+ cells as well as cellular pERK levels were strongly increased when CCL21 and TCR signaling coincided (Figure 3B).
Biochemical analysis of CCL21 costimulation. Primary mouse T cells were stimulated with anti-CD3 crosslinking, in the presence or absence of CCL21 (100 nM), for indicated times, and the activation of early signaling molecules was analyzed by immunoblotting. (A) Immunoblots of Rac2-GTP, Ras-GTP, and phosphorylated MEK1/2, ERK1/2, JNK, and Akt after costimulation of TCR and/or CCL21. For loading controls, blots were stripped and probed for total protein, or alternatively, a separate gel with lysates was analyzed. (B) Flow cytometric analysis of phosphorylated ERK1/2 formation in nonstimulated primary mouse T cells (dark gray fill) or after 5 minutes of stimulation with CCL21 (100 nM; dashed line), TCR-crosslinking (light gray fill), and TCR-crosslinking in the presence of 100 nM CCL21 (bold line). One representative experiment of 2 is shown.
Biochemical analysis of CCL21 costimulation. Primary mouse T cells were stimulated with anti-CD3 crosslinking, in the presence or absence of CCL21 (100 nM), for indicated times, and the activation of early signaling molecules was analyzed by immunoblotting. (A) Immunoblots of Rac2-GTP, Ras-GTP, and phosphorylated MEK1/2, ERK1/2, JNK, and Akt after costimulation of TCR and/or CCL21. For loading controls, blots were stripped and probed for total protein, or alternatively, a separate gel with lysates was analyzed. (B) Flow cytometric analysis of phosphorylated ERK1/2 formation in nonstimulated primary mouse T cells (dark gray fill) or after 5 minutes of stimulation with CCL21 (100 nM; dashed line), TCR-crosslinking (light gray fill), and TCR-crosslinking in the presence of 100 nM CCL21 (bold line). One representative experiment of 2 is shown.
Conversely, the phosphorylation of JNK (Figure 3A), p38, and Raf (supplemental Table 1) did not increase during chemokine-mediated costimulation. We also analyzed PI3K activity by quantifying phosphorylation of its downstream target Akt. CCL21 triggered robust Akt phosphorylation, which was less pronounced in CD3-crosslinked T cells at the early time points measured here. Simultaneous TCR and CCR7 activation by CCL21 had an additive effect on Akt phosphorylation (Figure 3A). In summary, our data suggest a rapid and pronounced activation of Rac, Ras, and MEK1/2 on simultaneous TCR and CCR7 triggering, resulting in enhanced ERK1/2 phosphorylation.
PI3K activity is not required for CCL21-mediated costimulation
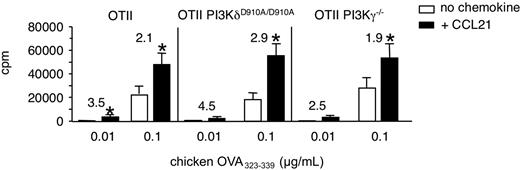
We wanted to examine whether PI3K activity had an effect on baseline and CCL21-enhanced T-cell proliferation, focusing on the lymphocyte-enriched PI3Kγ and PI3Kδ isoforms. To this end, we compared the proliferation of control, PI3KδD910A/D910A, or PI3Kγ−/− OT-II TCR-tg CD4+ T cells in response to increasing concentrations of chicken OVA323-339. Similar to our previous results with DO11.10 T cells, OT-II TCR tg T cells proliferated more in the presence of CCL21, in particular at low peptide concentrations (Figure 4). Similarly, PI3KδD910A/D910A and PI3Kγ-deficient OT-II TCR tg T cells OT-II cells showed comparable antigen-induced and CCL21-costimulated proliferation (Figure 4). Furthermore, pretreatment of human lymphocytes with the pan-PI3K inhibitor Wortmannin had no effect on chemokine-induced enhancement of T-cell activation as measured by Ca2+ and CD69 responses (supplemental Figure 2A; and data not shown). Similar results were obtained with murine T lymphocytes (data not shown). In summary, these data suggest that PI3K activity downstream TCR or CKR is not involved in CCL21-costimulated proliferation, although we did not assess effector differentiation in these assays.
CCL21-mediated costimulation in the absence of PI3Kγ- or PI3Kδ-activity. OT-II TCR-tg CD4+ T cells were cocultured with chicken OVA323-339-pulsed irradiated congenic splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined as described in “Proliferation assays.” Proliferation of control, PI3Kγ-deficient, and PI3KδD910A/D910A OT-II TCR-tg CD4+ T cells after 72 hours is shown. Numbers indicate fold increase of proliferation in the presence of CCL21. Data are pooled from 4 independent experiments. *P < .05 compared with “no chemokine.”
CCL21-mediated costimulation in the absence of PI3Kγ- or PI3Kδ-activity. OT-II TCR-tg CD4+ T cells were cocultured with chicken OVA323-339-pulsed irradiated congenic splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined as described in “Proliferation assays.” Proliferation of control, PI3Kγ-deficient, and PI3KδD910A/D910A OT-II TCR-tg CD4+ T cells after 72 hours is shown. Numbers indicate fold increase of proliferation in the presence of CCL21. Data are pooled from 4 independent experiments. *P < .05 compared with “no chemokine.”
Decreased costimulatory effect of CCL21 in the absence of DOCK2
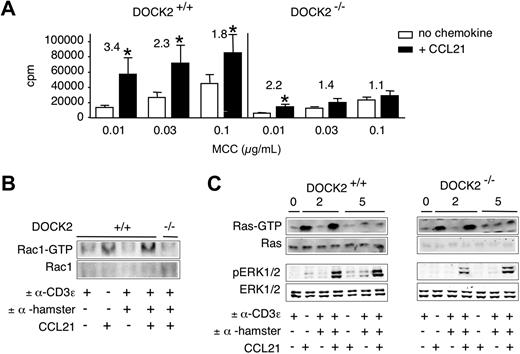
T cells lacking the RacGEF DOCK2 show reduced Rac-GTP formation and ERK phosphorylation after TCR stimulation, whereas most other signaling pathways remain intact.29 Given the strong synergistic effect of simultaneous TCR and CCR7 stimulation on ERK activation and the involvement of DOCK2 downstream both receptors, we examined the costimulatory effect of CCL21 in control and DOCK2−/− 2B4 TCR-tg CD4+ T cells activated by Ag-pulsed splenocytes. Control 2B4 TCR tg cells responded with increased proliferation to the presence of CCL21. In agreement with previous results,29 proliferation of DOCK2−/− 2B4 TCR-tg T cells was strongly reduced at all peptide concentrations examined (Figure 5A). Importantly, the costimulatory effect of CCL21 was significantly reduced in the absence of DOCK2. This was particularly noticeable comparing the costimulatory effect of CCL21 at similar baseline proliferation (eg, 0.03 μg/mL MCC peptide in control cells vs 0.1 μg/mL MCC peptide in DOCK2−/− 2B4 TCR tg CD4+ T cells). In addition, CCL21-stimulated CD69 and CD25 expression was reduced in DOCK2-deficient cells (supplemental Figure 3A). Similarly, pharmacologic inhibition of the Rac effector Pak1 using a novel, highly specific compound40 strongly reduced CCL21-induced proliferation and CD69 expression (supplemental Figure 4; and data not shown).
CCL21-mediated costimulation and signaling pathways in DOCK2-deficient T cells. (A) 2B4 TCR-tg CD4+ T cells were cocultured with MCC88-103-pulsed irradiated splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined as described in “Proliferation assays.” Proliferation of TCR-tg DOCK2+/− and DOCK2−/− 2B4 TCR-tg T cells after 48 hours is shown. Numbers indicate fold increase of proliferation in the presence of CCL21. Data are pooled from 3 independent experiments. *P < .05 compared with “no chemokine.” (B) Control and DOCK2−/− 2B4 TCR-tg T cells were stimulated as in Figure 3 and analyzed for Rac-GTP formation. One representative experiment of 2 is shown. (C) Control and DOCK2−/− 2B4 TCR-tg T cells were stimulated as in Figure 3 and analyzed for phosphorylation of ERK1/2 and Ras-GTP formation. One representative experiment of 3 is shown.
CCL21-mediated costimulation and signaling pathways in DOCK2-deficient T cells. (A) 2B4 TCR-tg CD4+ T cells were cocultured with MCC88-103-pulsed irradiated splenocytes in the presence or absence of CCL21 (100 nM). T-cell activation was determined as described in “Proliferation assays.” Proliferation of TCR-tg DOCK2+/− and DOCK2−/− 2B4 TCR-tg T cells after 48 hours is shown. Numbers indicate fold increase of proliferation in the presence of CCL21. Data are pooled from 3 independent experiments. *P < .05 compared with “no chemokine.” (B) Control and DOCK2−/− 2B4 TCR-tg T cells were stimulated as in Figure 3 and analyzed for Rac-GTP formation. One representative experiment of 2 is shown. (C) Control and DOCK2−/− 2B4 TCR-tg T cells were stimulated as in Figure 3 and analyzed for phosphorylation of ERK1/2 and Ras-GTP formation. One representative experiment of 3 is shown.
Next, we determined whether the decreased costimulatory effect in DOCK2−/− T cells correlated with impaired intracellular signaling. We performed anti-CD3ϵ crosslinking experiments in the presence and absence of CCL21. As reported,29 DOCK2−/− 2B4 TCR-tg T cells did not show any detectable Rac-GTP even when TCR and CCR7 were simultaneously activated (Figure 5B). In contrast, both wild-type and DOCK2−/− T cells showed normal Ras-GTP formation after activation of TCR and CCR7, or CCR7 alone (Figure 5C). Simultaneous activation of TCR and CCR7 in both control and DOCK2−/− 2B4 TCR-tg T cells synergistically increased ERK phosphorylation (Figure 5C; supplemental Table 1). However, absolute ERK phosphorylation levels at 2 minutes were reduced in DOCK2−/− T cells by 72% plus or minus 5% (mean ± SD), with a recovery 5 minutes after stimulation (Figure 5C). CCL21 elicited similar Akt phosphorylation in both control and DOCK2−/− T cells, indicating normal PI3K function (supplemental Figure 3B). Taken together, these data suggest that DOCK2/Rac contributes to efficient ERK activation downstream both TCR and CCR7 at early time points.
Optimal CD4+ T-cell activation inside lymphoid tissue requires GPCR signaling
Our in vitro results indicated that CCR7 ligands act as costimulatory factors during CD4+ T-cell activation. We next performed experiments to study the influence of the chemokine-rich lymphoid microenvironment on T-cell responses, using PLN slices. One of the advantages of this system is the possibility to interfere acutely with the molecular composition of the tissue. PLN slices (320 μm thickness), containing previously overlaid Marylin TCR tg CD4+ T cells, were perfused with the cognate DBY peptide during the imaging experiment. This experimental setting enabled us to measure the initiation of Ca2+ responses of T cells after their antigen encounter. Large concentrations of the antigenic peptide (100-1000 nM) triggered a strong increase in the intracellular Ca2+ concentration, which was associated with a reduction in the T-cell velocity (supplemental Figure 5; supplemental Video 3). The perfused peptide probably binds rapidly to MHC molecules expressed by resident DCs that form a dense network within the T-cell zone.41 One to 2 hours after the first Ca2+ response, a significant proportion of Marylin CD4+ T cells showed increased CD69 levels (Figure 6, supplemental Figure 4).
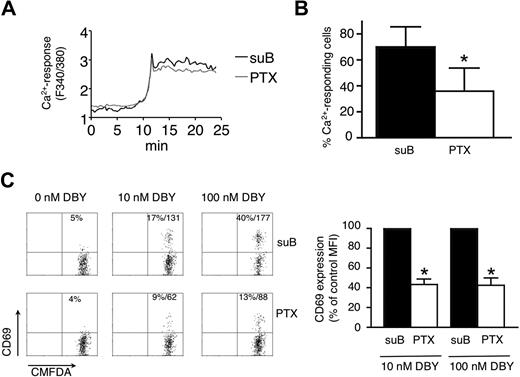
Antigen-induced T-cell responses measured in PLN slices are decreased by PTX. (A) Average Ca2+ responses of Marylin TCR-tg CD4+ T cells incubated with suB or PTX and stimulated with DBY peptide. Fura-2-loaded T cells were incubated for 10 minutes with 100 ng/mL suB (black line) or PTX (gray line), washed, and overlaid on PLN slices. Time-lapse imaging was started 2 hours after suB or PTX treatment. After several minutes of imaging, the preparation was perfused with a solution containing 100 nM DBY peptide. Ca2+-signals of responding T cells were synchronized at the rising phases of the response. (B) Percentage of Ca2+-responding CD4+ T cells induced by DBY peptide (100 nM). Mean plus or minus SD of 3 independent experiments in which more than 50 cells were analyzed per experiment. *P < .05. (C) CD69 expression measured by flow cytometry on T cells activated within PLN slices. Marylin CD4+ TCR-tg T cells were incubated with 100 ng/mL of suB or PTX, labeled with CMFDA and overlaid on PLN slices. Two hours after DBY peptide treatment, slices were mechanically dissociated and recovered cells stained with anti–CD69-PE and anti–CD45.1-PE-Cy5 Abs. Results in the left panel are representative of 3 independent experiments and show percentage and MFI of CD69+ cells. The right panel shows combined MFI of CD69 expression after normalization to the value of suB-treated T cells. *P < .05.
Antigen-induced T-cell responses measured in PLN slices are decreased by PTX. (A) Average Ca2+ responses of Marylin TCR-tg CD4+ T cells incubated with suB or PTX and stimulated with DBY peptide. Fura-2-loaded T cells were incubated for 10 minutes with 100 ng/mL suB (black line) or PTX (gray line), washed, and overlaid on PLN slices. Time-lapse imaging was started 2 hours after suB or PTX treatment. After several minutes of imaging, the preparation was perfused with a solution containing 100 nM DBY peptide. Ca2+-signals of responding T cells were synchronized at the rising phases of the response. (B) Percentage of Ca2+-responding CD4+ T cells induced by DBY peptide (100 nM). Mean plus or minus SD of 3 independent experiments in which more than 50 cells were analyzed per experiment. *P < .05. (C) CD69 expression measured by flow cytometry on T cells activated within PLN slices. Marylin CD4+ TCR-tg T cells were incubated with 100 ng/mL of suB or PTX, labeled with CMFDA and overlaid on PLN slices. Two hours after DBY peptide treatment, slices were mechanically dissociated and recovered cells stained with anti–CD69-PE and anti–CD45.1-PE-Cy5 Abs. Results in the left panel are representative of 3 independent experiments and show percentage and MFI of CD69+ cells. The right panel shows combined MFI of CD69 expression after normalization to the value of suB-treated T cells. *P < .05.
Next, we investigated whether GPCR ligands are involved in T-cell activation leading to increased CD69 surface levels. Marylin TCR-tg CD4+ T cells pretreated or not with PTX for 15 minutes were overlaid on PLN slices. Our previous experiments revealed that the blocking effect of PTX is only complete after 2 hours, allowing efficient migration of both control and inhibitor-treated lymphocytes into the slice. After 2 hours of PTX treatment, interstitial T-cell motility was significantly impaired compared with that of cells treated with the B subunit (suB) of the toxin that does not possess a catalytic activity and was used as a control.16
Addition of 100 nM specific peptide to slices induced rapid induction of Ca2+ flux in both control and PTX-treated populations (supplemental Video 3). On average, the mean delay between the perfusion of the peptide and the initiation of the Ca2+ response was 12.3 plus or minus 4.1 minutes for suB-treated cells and 12.0 plus or minus 1.9 minutes (n = 4) for PTX-treated cells. Moreover, the amplitude of Ca2+-flux was not affected by PTX, indicating that early TCR signaling is intact in PTX-treated lymphocytes, although the percentage of responding cells was significantly decreased (Figure 6A-B). Accordingly, the percentage of CD69+ cells after peptide perfusion was reduced in PTX-treated CD4+ T cells compared with suB-treated lymphocytes. Notably, the mean expression levels of CD69 in activated T cells were reduced by approximately 60% after PTX treatment at 10 and 100 nM DBY peptide (Figure 6C). These data support the notion that GPCR ligands contribute to efficient CD4+ T-cell activation inside the lymphoid microenvironment containing chemokines and other promigratory factors.
Discussion
The aim of this study was to examine whether CCL21 and other homeostatic chemokines present in the lymph node paracortex have a direct effect on TCR-induced intracellular signaling leading to T-cell activation. Our data suggest that the presence of CCL21 results in higher T-cell proliferation, with the costimulatory action of CCL21 being more pronounced at suboptimal activation. We provide evidence that the presence of CCL21 during the early stages of CD4+ T-cell activation leads to a selective and synergistic increase in ERK but not JNK or p38 phosphorylation, concomitant with increased expression of the early activation markers CD69 and CD25. Furthermore, we provide evidence that CCL21-triggered costimulation correlates with increased and prolonged Ras- and Rac-GTP levels. The latter was mediated by the RacGEF DOCK2 activated downstream of CCR7 and TCR, whereas PI3K activity was not required for costimulation in our system. Finally, observation of T-cell activation in PLN slices supports a costimulatory function of GPCR ligands in the paracortex.
Chemokines have previously been shown to participate in T-cell activation. For example, CCL5 induces recruitment of its ligand CCR5 and coupling with Gq/11 proteins at the interface between APC and T cells, enhancing conjugate stability and proliferation.21 This mechanism probably does not underlie the CCL21-stimulated costimulation described here, as the increased proliferation we observed was pertussis toxin sensitive, and hence Gαi-dependent, and because CCR7 is not thought to accumulate at the immunologic synapse.21 Furthermore, CCL21 also increased Ab-elicited proliferation, in the absence of adhesive ligands. It is nonetheless conceivable that CCR7-triggered integrin avidity contributes to more stable T-cell–DC interactions and therefore influences the outcome of an immune response.42 In addition, T-cell adhesion leads to potentiation of TCR signaling through ERK activation.43 The participation of adhesion in chemokine-induced T-cell costimulation deserves further investigations.
Similar to the Ras-GTP formation downstream of CCR7 reported here, CXCL12- and PI3K-dependent signals trigger a physical interaction between CXCR4 and the TCR, which promotes Ras-GTP formation and ERK phosphorylation.44-46 The fact that CCR7 and TCR do not colocalize at the T-cell–APC interface21 and the refractiveness of CCL21 costimulation to PI3K inhibition argue against a physical interaction of CCR7 and TCR. Although the precise mechanisms by which CCR7- and TCR-derived signals are integrated for synergistic Ras activation remain unknown, we identified a role for the RacGEF DOCK2 in integrating TCR- and CCR7-triggered Rac activation. DOCK2-mediated Rac-GTP formation correlated with increased and prolonged ERK phosphorylation, possibly via the Rac-effector Pak, which can phosphorylate Raf and MEK.47 Further support for a downstream role for Pak1 is provided by pharmacologic inhibition, which largely phenocopies the DOCK2 deficiency. Although we did not detect increased Raf phosphorylation after costimulation with CCL21, levels of phosphorylated MEK1/2 were increased in TCR- and CCR7-stimulated CD4+ T cells. Thus, both Ras- and Rac-GTP are efficiently formed after costimulation with homeostatic chemokines and, via activation of MEK1/2, mediate ERK phosphorylation (Figure 7). A digital all-or-nothing, highly amplified ERK phosphorylation is a central feature of T-cell activation in vitro48,49 and may help explain the narrow peptide concentration range between no T-cell proliferation and a full proliferative response observed in vivo.8
Proposed model for integration of TCR and CCR7-triggered signals. During an early promigratory phase of an ongoing antigenic response, CD4+ T cells undergo sequential encounters with pMHC-presenting DCs (left panel). During this period, T cells are exposed to TCR- and CCR7 (in addition to other GPCR)-derived signals, both of which activate PI3K, DOCK2-Rac, and Ras. Active Rac- and Ras-GTP contribute to enhanced MEK-ERK activation leading to up-regulation of early activation markers, such as CD69, and rapid production of IL-2 (right panel).
Proposed model for integration of TCR and CCR7-triggered signals. During an early promigratory phase of an ongoing antigenic response, CD4+ T cells undergo sequential encounters with pMHC-presenting DCs (left panel). During this period, T cells are exposed to TCR- and CCR7 (in addition to other GPCR)-derived signals, both of which activate PI3K, DOCK2-Rac, and Ras. Active Rac- and Ras-GTP contribute to enhanced MEK-ERK activation leading to up-regulation of early activation markers, such as CD69, and rapid production of IL-2 (right panel).
Although DOCK2 deficiency leads to delayed ERK phosphorylation downstream of TCR and CCR7, we still observed a significant chemokine-dependent costimulatory effect on ERK phosphorylation in these cells, potentially mediated by Ras-GTP. In contrast, the proliferation of DOCK2-deficient T cells was strongly impaired. These observations suggest additional roles for DOCK2 and Rac during TCR signal transduction, including TCR and lipid raft polarization.29,50 Lymphocytes also express the RacGEF Vav1, which acts downstream both TCR and chemokine receptors.51,52 Vav1-deficiency leads to decreased Rac and ERK activation,51,53-55 among other defects. As most Rac-GTP formation downstream the TCR is mediated by DOCK2,29 defective activation of Rac in Vav-1–deficient T cells may be linked because of its adapter function, rather than its GEF activity. Alternatively, Vav-induced Rac activation may depend on preceding DOCK2-mediated F-actin formation.
Deficiency in Rac2 inhibits Th1 differentiation,56 and DOCK2−/− CD4+ T cells are skewed toward a Th2 phenotype because of inefficient down-regulation of the IL-4Rα chain.57 Conversely, exposure to CCL21 increases Th1 differentiation in vitro through increased IFN-γ production,22 and CCR7 ligands induce IL-12 production in DCs.58 Taken together, these observations indicate that the CCL21-rich paracortex favors Th1 differentiation through DOCK2-Rac activity. During infections, expression of homeostatic chemokines is strongly decreased,59,60 implying a change not only in streptolysin O architecture but also in lymphocyte differentiation pathways. Secondary challenges in mice with low lymphoid chemokine levels may thus show a preferential Th2 differentiation, although other cytokines are probably involved in decision-making.
We were unable to detect any defects in activation of PI3Kγ-deficient T cells or an impairment of CCL21-mediated costimulation in our experimental system. The discrepancy to other published reports32,33 is unclear at the moment but could be the result of different transgenic models. Although PI3Kδ activity is required for full ERK phosphorylation downstream TCR signaling,61,62 expression of inactive PI3Kδ did not reduce CCL21-mediated costimulation in the experimental model used here. PI3K activity thus appears dispensable for CCL21-mediated costimulation in vitro. It will be important to investigate the function of PI3K during T-cell activation under more physiologic conditions, in particular for the generation of cytokine-producing effector T cells. This is especially relevant as directional migration of PI3Kγ-deficient T cells is affected in the paracortex,28 and PI3K δD910A/ D910A T cells show defective raft recruitment when stimulated with surrogate APCs in vitro.62
As CCL21 contributes to some extent to the exploratory behavior of T cells in lymphoid tissue,13-16 it is experimentally difficult to dissect a costimulatory effect of chemokines from their indirect facilitation of efficient scanning of rare DCs. Besides a direct signaling effect, the promigratory functions of CCR7 ligands and other Gαi-dependent factors probably influence T-cell responses by increasing the chances for T cells to encounter and interact with APCs. Using the PLN slice system, we were able to synchronize T-cell activation in a physiologic environment while following motility, Ca2+ responses, and CD69 up-regulation as functional readout. Although CCL21 is abundant inside lymphoid tissue, other GPCR ligands contribute to DOCK2-dependent interstitial T-cell migration13,15,28 and may thus add to T-cell activation in vivo. We therefore used the general Gαi inhibitor PTX, which reduced the percentage of T cells showing Ca2+ flux and CD69 up-regulation. This observation may reflect the decreased cellular motility and ability to engage in productive encounters with DCs, although the responding PTX-treated T cells increase their Ca2+ with a delay similar to that of control T cells, indicating that DCs are forming a tight network inside lymphoid tissue. Notably, PTX-treated T cells showed normal Ca2+-flux responses, indicating that Gαi signaling is not generally required for T-cell activation. Nonetheless, PTX exerted an inhibitory effect on the level of CD69 expressed by antigen-stimulated T cells at the early time points measured here, suggesting that GPCR ligands contribute to optimal T-cell activation within lymphoid tissue. Because CD69 levels continuously increase over the first 24 to 48 hours of an immune response, it is possible that PTX-treated cells eventually reach similar activation levels as untreated cells.
Our data support a scenario where, within lymphoid tissue, migrating CD4+ T cells measure and integrate TCR- and GPCR-derived signals, resulting in more efficient CD69 up-regulation and IL-2 production. The physiologic situation is probably more complex, as CCL21 and other promigratory factors may influence T-cell activation both in positive and negative manners. It has been suggested that promigratory and antimigratory signals mediated by CCR7 and TCR, respectively, compete with each other, only allowing efficient T-cell activation to take place when the TCR-triggered stop signal overrides promigratory signals from chemokine gradients.23 How may these seemingly contradictory propositions on the role of CCL21 be reconciled? One potential answer may lie in the ability of T cells to integrate activation signals over time. At low doses of antigen, T cells do not immediately form tight stable contacts with DCs but undergo brief serial interaction with APCs for several hours.8 On repeated encounters with low amounts of pMHC complexes, intracellular promigratory signaling molecules may contribute to reach a lower activation threshold, thus actively participating in T-cell activation. In case T cells do not repeatedly encounter peptide-MHC complexes with a threshold frequency, no signal integration takes place. Homeostatic chemokines may thus serve as tissue “rheostat” for recirculating lymphocytes, informing them on their presence inside lymphoid tissue by increasing their sensitivity to repetitive TCR-derived signals. This may also help prevent unwanted lymphocyte activation in the nonlymphoid tissue to which naive T cells occasionally migrate.63 Simultaneously, promigratory signals may act to “silence noise” by avoiding T-cell adhesion at individual DCs displaying low affinity antigenic peptide. At later time points, full effector T-cell differentiation requires prolonged interactions with DCs, in which cell displacement is suppressed by continuous TCR signaling.23
In conclusion, our data support a role for homeostatic chemokines, in particular CCL21, during CD4+ T-cell costimulation in vitro and a role for GPCRs for T-cell activation in situ. We hypothesize that CCL21 and other GPCR ligands lower the threshold for T-cell activation in the early promigratory phase of T-cell activation by sustaining increased ERK phosphorylation levels downstream Rac and Ras.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Britta Engelhardt (University of Bern) and Dr Alain Trautmann (CNRS and INSERM, Paris) for continuous support, Drs Emilio Hirsch (University of Turin) and Matthias Wymann (University of Basel) for providing PI3Kγ-deficient mice, and Drs Marcus and Silvia Thelen (IRB, Bellinzona) and Michael Huber (MPI, Freiburg) who generously shared reagents and protocols.
This work was supported by the Swiss National Foundation (grants SNF3100A0-107510 and EU-MEXT 25405; J.V.S.). J.R.P. was supported by the Department of Defense Research Program (W81XWH-05-1-0200) and the National Institutes of Health (GM083025).
National Institutes of Health
Authorship
Contribution: K.G., F.A.-B., and Y.T. performed experiments and analyzed results; K.O., B.V., J.R.P., and Y.F. provided genetically modified mouse strains and synthesized compounds; and K.G., E.D., and J.V.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: B.V. is an advisor to Intellikine (San Diego, CA). The remaining authors declare no competing financial interests.
Correspondence: Jens V. Stein, University of Bern, Theodor Kocher Institute, Freiestrasse 1, 3012 Bern, Switzerland; e-mail: jstein@tki.unibe.ch.