Abstract
Non-Hodgkin lymphoma (NHL) incidence is greatly increased after kidney transplantation. NHL risk was investigated in a nationwide cohort of 8164 kidney transplant recipients registered on the Australia and New Zealand Dialysis and Transplant Registry. NHL diagnoses were ascertained using linkage with national cancer registry records. Multivariate Poisson regression was used to compute incidence rate ratios (IRRs) with 95% confidence intervals (CIs) comparing risk by transplant function, and risk factors for early (< 2 years) and late (≥ 2 years) NHL during the first transplantation. NHL occurred in 133 patients. Incidence was strikingly lower after transplant failure and cessation of immunosuppression than during transplant function (IRR, 0.25; 95% CI, 0.08-0.80; P = .019). Early NHL (n = 27) was associated with Epstein-Barr virus (EBV) seronegativity at transplantation (IRR, 4.66; 95% CI, 2.10-10.36, P < .001) and receipt of T cell–depleting antibodies (IRR, 2.39; 95% CI, 1.08-5.30; P = .031). Late NHL (n = 79) was associated with increasing year of age (IRR, 1.02; 95% CI, 1.01-1.04; P = .006), increasing time since transplantation (P < .001), and current use of calcineurin inhibitors (IRR, 3.13; 95% CI, 1.53-6.39; P = .002). These findings support 2 mechanisms of lymphomagenesis, one predominantly of primary EBV infection in the context of intense immunosuppression, and another of dysregulated lymphoid proliferation in a prolonged immunosuppressed state.
Introduction
Non-Hodgkin lymphoma (NHL) occurs at markedly increased rates after solid organ transplantation and represents the most serious form of posttransplant lymphoproliferative disorder (PTLD). Most cases, particularly those arising shortly after transplantation, are associated with infection by the herpesvirus Epstein-Barr virus (EBV) in the setting of impaired T-cell function brought about by iatrogenic immunosuppression.1 Although several large cohort studies of PTLD have been conducted,2-9 there has been considerable inconsistency in the assessment of potentially relevant exposures, such as the duration of immunosuppression and the use of specific immunosuppressive agents
Herein, we report an examination of incidence and risk factors for NHL in Australian kidney transplant recipients on the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Particular focus is on the comparison of risk factors for cases arising early (< 2 years) and late (≥ 2 years) after transplantation, because they appear to differ clinically and pathologically.10-13
Methods
Study population
The study population and method of cancer ascertainment have been described in detail previously.14,15 Briefly, the ANZDATA Registry contains comprehensive information on all patients who commence chronic dialysis or receive a kidney transplant in Australia and New Zealand, as collected by biannual surveys of patients' treating dialysis or transplant units.16 For this analysis, the study population was restricted to patients registered on the ANZDATA Registry in Australia who received their first transplant between January 1, 1982, and September 30, 2003 (n = 8173). Patients with NHL before transplantation were excluded (n = 9); patients were not excluded based on a history of other types of cancer.
Cancer data collection
NHL diagnoses were ascertained through data linkage with the Australian National Cancer Statistics Clearing House with the use of an established probabilistic matching technique.17 Full details of the data linkage algorithm used have been documented previously.14 The National Cancer Statistics Clearing House compiles information on all diagnoses of primary invasive cancer, excepting non–melanoma skin cancer, as notified by statute to each of the 8 state and territory cancer registries of Australia.18 Cancer data were available from January 1, 1982, until December 31, 2001, 2002, or 2003, depending on the jurisdiction of cancer registration.
For each case, the date of diagnosis, and topography and morphology coding (ICDO-3; ICD10) were obtained. Monomorphic PTLDs are classified as per the current World Health Organization guidelines for the classification of NHL.1 Therefore, all cases designated as NHL were included (ICDO-3 9670-9729, 9820-9837, 9940, 9948, 9591, and 9596). Cases of lymphoma for which the precise morphology was unspecified (ICDO-3 9590; n = 11) were included only if NHL was indicated by the corresponding ICD10 code (ICD10 C82-C85). Cases of multiple myeloma and plasmacytoma (ICDO-3 9731-9734) were not included. Polymorphic PTLD (ICDO-3 9970/1, lymphoproliferative disorder) were not included because they are not routinely registered by Australian cancer registries.
General population NHL incidence rates were obtained by 5-year age group, sex, calendar year, and state or territory, for each year since 1982.
Ethical approval was obtained from all relevant institutional ethics committees. The requirement for informed consent from patients was waived because the researchers received only de-identified data.
Statistical analysis
NHL incidence by transplant function.
For each patient, person-years (PYs) of follow-up were accumulated from the date of first transplantation until the date of first NHL diagnosis, death, last contact, or the latest date for which cancer data were available, whichever occurred first. The incidence of NHL was compared for periods of transplant function and dialysis after transplant failure, reflective of the presumed receipt or nonreceipt of immunosuppressive agents,19 thereby allowing analysis of the effect of currency of immunosuppression. For this analysis only, PYs and incident cases diagnosed during the first 3 months of each period of transplant function or dialysis were disregarded (n = 1 case during transplant function and n = 7 during dialysis), because cases arising within this time were probably present in the preceding period. For comparison purposes, NHL incidence was also examined during the period of dialysis before transplantation for all patients on the ANZDATA Registry first dialyzed between January 1, 1982, and September 30, 2003 (n = 23764), irrespective of subsequent transplantation.
Multivariate Poisson regression was used to compute incidence rate ratios (IRRs) with 95% confidence intervals (CIs) comparing NHL incidence during periods of dialysis after transplant failure and second or higher-order transplantation to that during the first functioning transplant, adjusted for current age (time dependent; single years), sex, and the cumulative duration of transplant function (time dependent; years). Standardized incidence ratios (SIRs) with 95% CIs were also calculated for each period by comparing the number of observed cases with that expected based on the application of 5-year age-, sex-, calendar year-, and state or territory-specific general population incidence rates.
NHL incidence and risk factors during the first functioning transplant.
NHL incidence and risk factors were examined in detail for the first functioning transplant. For this analysis, follow-up was censored at first transplant failure and the reinstitution of dialysis; follow-up during the first 3 months was not excluded. The cumulative incidence at 2, 5, and 10 years after transplantation was examined for descriptive purposes based on the Kaplan-Meier failure estimate. SIRs with 95% CIs were calculated by current age (time dependent; < 20, 20-49, 50-69, ≥ 70 years) to examine risk among pediatric (< 20 years) and adult patients relative to the general population. SIRs were also calculated by time since transplantation (time dependent; < 1, 1-1.99, 2-4.99, 5-9.99, 10-14.99, and ≥ 15 years after transplantation). Because of the possibility that statistical comparison of SIRs over time since transplantation may be confounded by age, multivariate Poisson regression was also used to determine IRRs with 95% CIs, adjusted for current year of age and sex.
Risk factor analyses were conducted separately for early (< 2 years) and late (≥ 2 years) NHL. Two years was chosen to delineate early and late cases based on the distribution of cases and the well-documented increase in risk in the 1 to 2 years after transplantation.2,5,7,8 Risk factor data, including patient demographics and medical and immunosuppressive history, were obtained or derived from data held on the ANZDATA Registry. Demographic factors assessed included current age (time dependent; single years), sex, self-reported race (White, Nonwhite), and country of birth (Australia or New Zealand, Europe, Asia, other or unknown). Data were available on the receipt of individual immunosuppressive agents at transplantation and at 1, 2, 3, 6, 12, 24, 36, and 60 months after transplantation, and at 5-yearly intervals thereafter. With the use of this information, a time-dependent variable was constructed to represent the current receipt of each of the major classes of immunosuppressive agents, including the calcineurin inhibitors (CNIs) cyclosporine and tacrolimus, and the antiproliferative agents azathioprine and mycophenolate. Of the newer immunosuppressive agents, only sirolimus was considered and only for early NHL, because of insufficient PYs beyond this time. Receipt of the steroid prednisolone was not examined, given its near universal (95%) use within the cohort. Combination therapy (CNI ± antiproliferative agents) was examined for descriptive purposes. The blood levels of individual immunosuppressive agents were not available. Patients for whom the type of immunosuppressive agent was unknown at 2 or more consecutive time points (n = 335 patients; n = 2 with NHL) were excluded from risk factor analyses.
Other immunosuppression-related factors examined were duration of immunosuppression, approximated by time since transplantation (time dependent; < 1, 1-1.99 years for early NHL; 2-4.99, 5-9.99, 10-14.99, ≥ 15 years for late NHL); the receipt of T cell–depleting antibodies, specifically the polyclonal agents antithymocyte globulin and antilymphocyte globulin, and the monoclonal agent muromonab-CD3 (time dependent from time of receipt, given for either induction immunosuppression or for the treatment of acute rejection); donor source (living-related, cadaveric/living-unrelated); the number of human leukocyte antigen mismatches between the recipient and the donor (0-1, 2-3, 4-6); and the receipt of a different organ transplant in addition to a kidney, either at the time of, or after, kidney transplantation (time-dependent, yes or no).
Data on EBV-antibody IgG serostatus at the time of transplantation were available for 3559 patients (44%). A summary variable was constructed to represent potential primary infection. Patients who were EBV negative at transplantation were considered to be at risk of primary infection. Given the high seroprevalence of EBV, it is likely that most patients with unknown EBV serostatus at transplantation would have been EBV positive. Thus, patients who were either EBV positive or of unknown EBV serostatus at transplantation were considered not to be at risk of primary infection. Data on the EBV serostatus of the donor, available for 558 patients (15%) with known EBV status, were examined for descriptive purposes only. Patient CMV IgG serostatus at the time of transplantation (positive, negative, unknown) was also examined, as was history of infection with HCV (time dependent; positive, negative, unknown).
Other factors examined included history of another type of cancer before transplantation (yes or no, excluding non–melanoma skin cancer), cause of primary renal disease leading to renal failure (diabetic nephropathy, primary or secondary glomerulonephritis, hypertensive or arteriopathic, other or unknown), the number of years of dialysis before first transplantation (< 1, 1-1.99, ≥ 2), and smoking history (never, former, current) and diabetes comorbidity (none, type 1, type 2) at entry onto the ANZDATA Registry.
Poisson regression modeling was used to determine IRRs with 95% CIs for each risk factor. All variables with univariate 2-sided statistical significance (P < .10) were considered in multivariate analysis. The final multivariate models were determined with the use of a forwards stepwise approach, retaining only those covariates with 2-sided statistical significance (P < .05) after adjustment for each other and for current year of age, sex, time since transplantation, current receipt of immunosuppressive agents, receipt of T cell–depleting antibodies, and EBV serostatus at transplantation, which were included a priori. On the basis of previous reports, statistical interaction was assessed between the receipt of CNI and T cell–depleting antibodies,7 as well as between concomitant EBV- and CMV-negative status at transplantation.20,21 For all variables, missing data were analyzed as unknown, unless otherwise stated.
It has been argued that histologically benign PTLD can act in a biologically malignant manner in the immunosuppressed host.22 This, together with the likelihood that polymorphic and monomorphic PTLD are essentially one disease presenting at different stages of evolution,23 has led to the suggestion that epidemiologic analyses should not be restricted to malignant disease.22 Cases of lymphoproliferative disease are notified to the ANZDATA Registry; therefore, sensitivity analyses were conducted to determine the effect on risk factor analyses of the inclusion of these cases (11 early, 4 late) in addition to the cases of NHL ascertained from the cancer registry.
All analyses were performed with the use of Stata version 10 (StataCorp LP).
Results
The study population consisted of 8164 eligible patients (4827 males, 3337 females). As summarized in Table 1, most patients (88%) were of White race. The median age at first transplantation was 43 years; 710 patients (9%) patients were younger than 20 years at transplantation. Of the 3559 patients with known EBV IgG serostatus at transplantation, 891 (25%) were EBV negative and were therefore considered to be at risk of primary infection. Of the EBV-negative patients, 33 (4%) were known to have received a kidney from an EBV-negative donor. CMV IgG serostatus at transplantation was known for 5010 patients (61%), of whom one-third were CMV negative. Most patients (81%) had at some stage received combination therapy with CNI and antiproliferative agents, and 1829 (22%) were administered T cell–depleting antibodies (48% antithymocyte globulin or antilymphocyte globulin, 59% muromonab-CD3). Antibodies were mostly (63%) administered for the treatment of acute rejection, at a median of 12 days after transplantation. First transplant failure with resumption of dialysis occurred in 2074 patients (25%) patients, and 800 (10%) underwent retransplantation. Three or more transplants were received by 82 patients (1%).
NHL incidence by transplant function
In total, 133 cases of NHL were identified. After the exclusion of follow-up and incident cancers during the first 3 months of each period of transplant function and reinstitution of dialysis, 125 cases were identified; most cases (107; 86%) occurred during the first functioning transplant, and 15 (12%) occurred during a higher-order transplant. As shown in Table 2, after adjustment for current year of age, sex, and the cumulative duration of transplant function, incidence of NHL during dialysis subsequent to transplant failure was significantly lower than during the first functioning transplant (IRR, 0.25; 95% CI, 0.08-0.80), returning to a rate (51 per 100000 PYs; 95% CI, 16-158 per 100000 PYs) similar to that seen during dialysis before transplantation (56 per 100000 PYs; 95% CI, 39-79 per 100000 PYs). Risk on retransplantation was not significantly different from that during the first functioning transplant (IRR, 1.50; 95% CI, 0.87-2.60).
NHL incidence and risk factors during the first functioning transplantation
During the first functioning transplant 108 cases of NHL were identified, with 27 cases diagnosed during the first 2 years and 81 cases thereafter. The median age at transplantation was 44 years (interquartile range, 19-64 years) for patients diagnosed with early NHL, and 47 years (interquartile range, 20-63 years) for patients with late NHL. Of patients with early NHL, 10 (37%) were EBV negative at transplantation compared with 5 (6%) of patients with late NHL (χ2 = 16.13; P < .001). All patients who were EBV negative at transplantation and who developed NHL had received a kidney from an EBV-positive or EBV-unknown donor. Diffuse large B-cell lymphomas comprised almost half of both early and late cases, and only a few cases were of T-cell origin, although data on the precise morphology of cases were unspecified for a large proportion (38%) of cases. A greater proportion of early cases were nodal; only 3 (14%) of 22 early cases for which topography was specified involved extranodal sites compared with 26 (38%) late cases (see Table 3).
The cumulative incidence of NHL during the first functioning transplant was 0.4%, 0.7%, and 2.2% at 2, 5, and 10 years, respectively. Relative risk varied substantially with age. The SIR was greatest for patients aged younger than 20 years (SIR, 55.27; 95% CI, 17.95-128.98) and declined significantly with increasing age (P < .001); the SIR was 18.55 (95% CI, 13.43-24.99) for patients aged 20 to 49 years, 6.43 (95% CI, 4.75-8.29) for patients aged 50 to 69 years, and 5.56 (95% CI, 2.24-11.46) for patients aged 70 years or older.
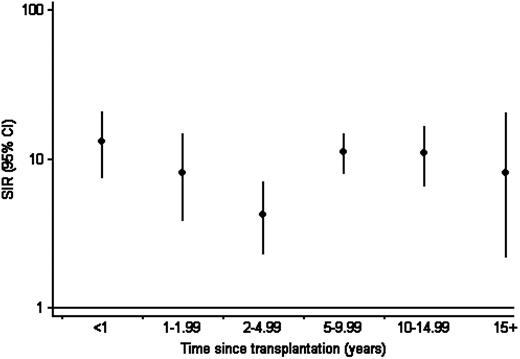
Incidence was bimodally distributed by time since transplantation; in analyses adjusted for current year of age and sex, incidence was significantly lower during the period 2 to 4.99 years after transplantation (IRR, 0.40; 95% CI, 0.21-0.76; P = .005) than during the first 2 years. Incidence during the period 5 to 9.99 years (IRR, 1.23; 95% CI, 0.76-2.00; P = .399) and more than 10 years (IRR, 1.32; 95% CI, 0.75-2.31; P = .322) was not significantly different from that during the first 2 years. As shown in Figure 1, compared with the Australian general population, relative risk was 10-fold higher during the first 2 years (SIR, 10.66; 95% CI, 7.03-15.51). The SIR was 4.24 (95% CI, 2.32-7.11) during the period 5 to 9.99 years, and from 10 years it was 10.31 (95% CI, 6.61-15.34).
Standardized incidence ratio for non-Hodgkin lymphoma by time since transplantation. Shows incidence during the first functioning transplant by time since transplantation, relative to that in the Australian general population, standardized by 5-year age, sex, calendar year, and state or territory.
Standardized incidence ratio for non-Hodgkin lymphoma by time since transplantation. Shows incidence during the first functioning transplant by time since transplantation, relative to that in the Australian general population, standardized by 5-year age, sex, calendar year, and state or territory.
In multivariate analysis, early NHL (Table 4) was significantly associated with EBV-negative status at transplantation (IRR, 4.66; 95% CI, 2.10-10.36) and with the receipt of T cell–depleting antibodies (IRR, 2.39; 95% CI, 1.08-5.30). No statistically significant difference in risk was observed between patients receiving only monoclonal (IRR, 2.81; 95% CI, 1.17-6.78) or polyclonal (IRR, 1.11; 95% CI, 0.26- 4.82; Pdiff = .248) agents in univariate analysis, or whether given for prophylaxis (IRR, 3.09; 95% CI, 1.04-9.18) or rejection (IRR, 2.15; 95% CI, 0.84-5.46; Pdiff = .576). There were insufficient cases with which to assess statistical interaction between receipt of T cell–depleting antibodies and CNI. No significant interaction was observed between concomitant EBV and CMV seronegative status at transplantation and risk of NHL in univariate analysis (IRR, 1.45; 95% CI, 0.21-9.94; P = .707).
Risk of late NHL (Table 4) was significantly associated with increasing year of age (IRR, 1.02; 95% CI, 1.01-1.04), increasing time since transplantation (P < .001), and with the current receipt of CNI (IRR, 3.13; 95% CI, 1.53-6.39). When agents were modeled individually, the IRR was 5.15 (95% CI, 0.63-41.98; P = .126) for receipt of tacrolimus and 3.30 (95% CI, 1.62-6.72; P = .001) for cyclosporine. No increase in risk was observed with receipt of antiproliferative agents (IRR, 1.14; 95% CI, 0.63-2.07); when agents were modeled individually, the IRR was 0.19 (95% CI, 0.02-1.47; P = .110) for receipt of mycophenolate and 1.23 (95% CI, 0.68-2.24; P = .489) for azathioprine.
Sensitivity analyses, in which cases of lymphoproliferative disease notified to ANZDATA were also included, strengthened but did not substantially alter the observed associations (data not shown).
Discussion
Risk of NHL in this Australian cohort of kidney transplant recipients was strongly related to the current receipt of immunosuppression and reverted quickly to pretransplantation level on transplant failure, when immunosuppression is usually ceased. Relative risk remained substantially elevated after 10 years of continuous transplant function and was comparable with that observed during the first 2 years. Early NHL was independently associated with EBV-seronegative status at transplantation and with the receipt of T cell–depleting antibodies. Late NHL was associated with increasing age, with increasing duration of continuous immunosuppression, and with the receipt of CNI, but not with antiproliferative agents. These findings provide evidence in support of 2 mechanisms of lymphomagenesis after transplantation, one driven predominantly by primary EBV infection in the context of profound immunosuppression and another by prolonged immunosuppression, aging, and, probably, exposure to specific immunosuppressive agents.
The rapid and complete reversal of NHL risk on cessation of immunosuppression strongly supports a direct role of immunosuppression24 and shows the important contribution of current functional immunity to lymphomagenesis. This finding accords with the regression of some, primarily polymorphic, lesions shortly after the reduction of immunosuppression, both in transplanted25,26 and other populations, such as those immunosuppressed with methotrexate for the treatment of autoimmune disease.27 It is also consistent with the regression of Kaposi sarcoma, another herpesvirus-related cancer, on cessation of immunosuppression.28,29
The increased risk of NHL early after transplantation has been well described. In this study, relative risk at 5 and 10 years after transplantation was comparable with that observed during the first 2 years. This bimodal distribution, noted previously for physician-notified PTLD in the ANZDATA cohort,7 contrasts with that reported for other registry-based30,31 and large-scale cohort studies with similar follow-up,2,5 in which relative risk declined after the first year after transplantation. Emerging evidence from clinic-based studies suggests that late NHL differs pathologically and clinically from early disease, being less frequently associated with EBV, and less amenable to treatment.10-13 The possibility of a distinct causative mechanism for early and late NHL is supported in this study by several observations.
A near 5-fold risk of early NHL was observed with EBV-negative status at transplantation; receipt of T cell–depleting antibodies was associated with a 2-fold risk. In the immunosuppressed state, patients undergoing primary EBV infection are unable to mount an adequate EBV-specific immune response, lacking the requisite EBV-specific CD8+ T cells and producing few anti-EBNA antibodies.32 This may be exacerbated in patients receiving T cell–depleting antibodies, the effect of which is greatest within the first 3 months of receipt.33,34 Restoration of functional immunity over time, particularly EBV-specific immunity, may therefore explain the lack of association between primary EBV infection and late NHL. This may also account for the observation in clinic-based data that the EBV genome is less frequently detected in late cases.11,13,35
The risk profile for late NHL differed starkly from that for early NHL. Risk increased with increasing age, as noted in the Collaborative Transplant Study cohort for cases arising after the first year.5 Age is a strong risk factor for NHL in the general population,36 and this may reflect age-related loss of immunosurveillance brought about by senescence of the immune system.37,38 Risk also increased with duration of immunosuppression, even after adjustment for cohort aging, suggesting a role for prolonged, continuous T-cell immunosuppression and immune dysregulation. Chronic antigenic stimulation, resulting from antigenic differences with the transplanted organ or from repeated infection, has been postulated to play a role, as has infection with an as yet undescribed virus.26,29,37 However, neither of these hypotheses could be directly evaluated in the present study.
Late NHL was also associated with the current receipt of CNI. However, a direct carcinogenic effect of these agents is difficult to establish because of the interrelationship between T-cell suppression and control over EBV infection. Both cyclosporine and tacrolimus inhibit T-cell activation, including that of EBV-specific CD8+ T cells.39,40 In addition, cyclosporine enhances the expression of interleukin-6, which promotes the growth of EBV-transformed B cells in vitro.41,42 Prior epidemiologic data are inconclusive. No increase in risk of PTLD was observed with use of cyclosporine or cyclosporine-based regimens in the United States Renal Data System or Collaborative Transplant Study cohorts.5,6 However, those studies examined NHL risk in relation to agents received for maintenance immunosuppression on hospital discharge rather than on current receipt. However, receipt of mycophenolate, a potent suppressor of B-cell proliferation,43 was associated with significantly reduced risk of NHL in 2 prior cohorts.3,6 In the present study, no increase in NHL risk was observed with receipt of antiproliferative agents.
This study had several strengths, including the use of population-based registers of both transplant recipients and incident cancers. Although several large cohort studies of PTLD have been conducted,2-9 few have involved population-based registers of transplant recipients,4,7,8 and none have used cancer registry-based lymphoma diagnoses. The large cohort size and the long period of follow-up enabled the examination of risk during periods of higher-order transplantation and dialysis, as well as of risk factors for early and late cases. Data on the receipt of individual immunosuppressive agents were also sufficiently detailed so as to allow time-dependent examination, which is important, given the changes in the availability and use of immunosuppressive agents over time and the practice of switching between treatment regimens.44
There were also some limitations. It is possible that some misclassification may have occurred because of the recognized difficulty of accurate PTLD diagnosis and classification, as well as secular changes in diagnostic criteria and classification of lymphoid neoplasms. Thus, it is also possible that improvements in diagnosis of lymphoma over time may have contributed to the pattern we observed with increasing time since transplantation. Some have argued that analyses should include all cases of PTLD.22 Australian national cancer registration is restricted to malignant disease; however, the risk profile of early and late NHL was not altered in this study by the inclusion of a small number of physician-notified and presumably polymorphic lymphoproliferative lesions notified to ANZDATA.
Risk factor analyses were limited to the information contained on the ANZDATA Registry. Data on EBV IgG serostatus were incomplete; however, because patients with unknown serostatus were categorized together with those who were EBV positive at transplantation, our estimate of the effect of primary infection is probably conservative. No data were available on EBV prophylaxis, EBV viral load, reactivated EBV infection, or EBV detection in tumors. In terms of immunosuppressive agents, we could not consider the effect of drug dose. Because this was an observational study, we cannot exclude the possibility that patient-specific factors taken into account when prescribing drug type and dose may have contributed to the findings. For instance, the diagnosis of reactive plasmacytic hyperplasia or infectious mononucleosis may have resulted in a change in immunosuppression.
To conclude, NHL risk after kidney transplantation is closely related to the currency of immunosuppression and reverts to the pretransplantation level when immunosuppression is ceased, reinforcing the role of functional immunity in lymphomagenesis. This study extends previous clinical observations to provide epidemiologic support for 2 distinct causative mechanisms of NHL. The strong association between EBV-negative status at transplantation, receipt of T cell–depleting antibodies and risk of early NHL confirms the importance of primary EBV infection in the context of profound immunodeficiency. The combined role of age and duration of immunosuppression in late NHL supports a model of unchecked lymphoid proliferation in the setting of prolonged immunosuppression and immune dysregulation. This appears to be exacerbated or mediated by certain immunosuppressive agents. Population-based analysis of biologic host and tumor characteristics may help inform the complex interplay between immune function, lymphocyte proliferation, and lymphoma development in the long-term immunosuppressed, as well as in the immunocompetent.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the dialysis and transplantation units throughout Australia for the dedication and care with which they have regularly submitted the information on which this analysis has been performed, and the ANZDATA Registry staff who have created and maintained the database so accurately. We thank the staff of the state and territory cancer registries for the use of their data. We also thank the Australian Institute of Health and Welfare and the Cancer Council Victoria for assistance in the conduct of this study.
This work was supported by the Cancer Council NSW (New South Wales; RG 47/03); the National Health and Medical Research Council (ID 510346 to C.M.V.; ID 401131 to M.T.v.L.); and the Cancer Institute New South Wales (07/CDF/1-38 to C.M.V.; 06/RSA/1/28 to M.T.v.L). The ANZDATA Registry administrative office is supported by funding from the Australian government Department of Health and Ageing, the New Zealand Ministry of Health, and Kidney Health Australia; data collection costs are borne by contributing renal units.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry.
Authorship
Contribution: M.T.v.L., A.E.G., A.C.W., M.R.E.M., J.H.S., S.P.M., J.A., J.M.K., J.R.C., and C.M.V. designed the research, analyzed and interpreted data, and revised and reviewed the final manuscript; M.T.v.L. and J.A. performed statistical analysis; and M.T.v.L., C.M.V., and A.E.G. were responsible for drafting the manuscript.
Conflict-of-interest disclosure: J.R.C. is on advisory boards and speaker panels for Astellas, Novartis, Wyeth, and Hoffmann la Roche, and he has received research support from the Juvenile Diabetes Foundation International, Novartis, Wyeth, Jannsen-Cilag, and Hoffmann la Roche. A.E.G. is on the advisory board for the Gardasil human papillomavirus vaccine for the Commonwealth Serum Laboratories. The remaining authors declare no competing financial interests.
Correspondence: Claire M. Vajdic, UNSW Cancer Research Centre, Prince of Wales Clinical School, UNSW, Level 1, South Wing, Edmund Blacket Bldg, Prince of Wales Hospital, Randwick NSW 2031, Australia; e-mail: claire.vajdic@unsw.edu.au.