Abstract
Endothelial sialomucin CD34 functions as an L-selectin ligand mediating lymphocyte extravasation only when properly glycosylated to express a sulfated carbohydrate epitope, 6-sulfo sialyl Lewis x (6-sulfo SLex). It is thought that multivalent 6-sulfo SLex expression promotes high-affinity binding to L-selectin by enhancing avidity. However, the reported low amount of 6-sulfo SLex in total human CD34 is inconsistent with this model and prompted us to re-evaluate CD34 glycosylation. We separated CD34 into 2 glycoforms, the L-selectin–binding and nonbinding glycoforms, L-B-CD34 and L-NB-CD34, respectively, and analyzed released O- and N-glycans from both forms. L-B-CD34 is relatively minor compared with L-NB-CD34 and represented less than 10% of total tonsillar CD34. MECA-79, a mAb to sulfated core-1 O-glycans, bound exclusively to L-B-CD34 and this form contained all sulfated and fucosylated O-glycans. 6-Sulfo SLex epitopes occur on core-2 and extended core-1 O-glycans with approximately 20% of total L-B-CD34 O-glycans expressing 6-sulfo SLex. N-glycans containing potential 6-sulfo SLex epitopes were also present in L-B-CD34, but their removal did not abolish binding to L-selectin. Thus, a minor glycoform of CD34 carries relatively abundant 6-sulfo SLex epitopes on O-glycans that are important for its recognition by L-selectin.
Introduction
Leukocyte traffic from blood circulation into the lymphoid organs and inflamed tissues is part of the normal immunologic defense system against invading organisms. Lymphocytes continuously patrol between peripheral blood circulation and secondary lymphoid organs searching for foreign antigens. Lymphocyte homing to secondary lymphoid tissues occurs in high endothelial venules (HEVs) present only in secondary lymphoid organs. Lymphocytes first bind transiently (tether) to high endothelial cells lining the inner surface of HEVs, and start to roll along endothelium. Rolling is followed by lymphocyte activation and firm adhesion mediated by protein-protein interactions involving integrins and their counterreceptors. As a final step, lymphocytes migrate through the endothelium into the lymphoid tissues. Initial lymphocyte tethering and rolling is mediated by molecular interactions between L-selectin on the surface of lymphocytes and L-selectin counterreceptors present on endothelium.1 Several candidate L-selectin ligands have been identified in the HEVs of secondary lymphoid tissues, including GlyCAM-1 (Sgp50),2,3 CD34 (Sgp90),2,4 podocalyxin-like protein,5 Sgp200,6 MadCAM-1,7 endomucin,8,9 endoglycan,10 and nepmucin.11 Many of these endothelial sialomucins express sulfated carbohydrate structures on O-glycans of which 6-sulfo sialyl Lewis x epitope (6-sulfo SLex, NeuAcα2-3Galβ1-4(Fucα1-3)(SO3-6)GlcNAcβ1-) has been shown to contribute to recognition by L-selectin.12-17 The evidence that sulfated glycans are important for L-selectin recognition is based partly on the observation that a monoclonal antibody MECA-79 binds to a group of endothelial mucins called peripheral lymph node vascular addressin (PNAd) on lymph node HEVs and inhibits lymphocyte homing to lymph nodes in mice.18,19 MECA-79 recognizes a 6-sulfated N-acetyllactosamine present on extended O-glycan core-1 structure (Galβ1-4(SO3-6)GlcNAcβ1-3Galβ1-3GalNAc) but does not require sialic acid or fucose residues for binding.20 Therefore, 6-sulfo SLex and MECA-79 epitopes are partly overlapping. Although MECA-79 binds 6-sulfo SLex epitope only when present on extended core-1 O-glycans, 6-sulfo SLex expressed on core-2 O-glycans can also be bound by L-selectin during lymphocyte homing in mice.20,21 Interestingly, a recent study with mice deficient in core-1 extension and core-2 branching enzymes indicated that 6-sulfo SLex on N-glycans could contribute to L-selectin–dependent lymphocyte homing in mice.22 These findings suggest that the O-glycan core structure presenting 6-sulfo SLex may not be required for L-selectin recognition.
L-selectin also binds to P-selectin glycoprotein ligand-1 (PSGL-1) present on the surface of leukocytes, mediating leukocyte-leukocyte interactions at inflammatory sites.23 L-selectin recognizes sulfated tyrosines and a core-2–based O-glycan carrying a nonsulfated SLex epitope, both present at the extreme N-terminus of PSGL-1. Earlier data have indicated that SLex expressed on core-2 O-glycans on a PSGL-1 model glycosulfopeptide or on CHO cell transfectants with PSGL-1 exhibited stronger binding to L-selectin than glycosulfopeptide or CHO cell transfectants containing SLex on extended core-1 O-glycans, suggesting that core-2–based O-glycans have a major role for L-selectin binding.24,25 It has been shown that L-selectin binds PSGL-1 with a different mechanism than 6-sulfo SLex.26 L-selectin recognizes sulfated tyrosines and other nearby peptide components in addition to core-2–based O-glycan with SLex at the extreme N-terminus of PSGL-1.
The interaction of human L-selectin with endothelial glycoprotein ligands on human lymphoid tissues is still poorly understood. MECA-79 and 6-Sulfo SLex epitopes are expressed on HEVs of human lymphoid tissues, and MECA-79 has been shown to partially inhibit lymphocyte binding to HEVs or to purified PNAd.19,27,28 Two MECA-79–reactive sialomucins, CD3429 and podocalyxin-like protein,5 which were isolated from human tonsils, have been shown to support L-selectin–dependent lymphocyte rolling in vitro. Moreover, endomucin9 and several MECA-79–independent candidate L-selectin ligands10,28,30 have also been isolated from human lymphoid tissues and endothelial cells. Because the binding affinity of L-selectin to monovalent 6-sulfo SLex is relatively weak, it is commonly thought that multivalent epitope expression on individual mucins leads to high-affinity binding due to enhanced avidity. However, when partial glycan analysis was carried out with CD34 purified from human tonsils,31 only 1 O-glycan containing a potential 6-sulfo SLex epitope was found and it represented approximately 1% of the total O-glycan pool and sulfated N-glycans were not detected on human CD34. It is possible that the inconsistency of the multivalency model with the low abundance of 6-sulfo SLex epitopes on human CD34 results from the analysis of crude tonsil CD34, which may be heterogeneous in glycosylation and largely contains nonfunctional glycoforms that do not bind to L-selectin. To address this key question we isolated L-selectin binding glycoforms of human tonsillar CD34 from nonbinding forms, released O- and N-glycans from both forms of CD34, and analyzed free glycans using mass spectrometry and exoglycosidases. The results show that less than 10% of crude tonsillar CD34 bound to L-selectin. All sulfated and fucosylated O-glycans were present exclusively in L-selectin binding form of CD34 recognized by MECA-79. 6-Sulfo SLex epitopes were identified on several O-glycans. Some N-glycans also carried a potential 6-sulfo SLex epitope. Our studies provide new information about the N- and O-glycans of CD34 that are required for L-selectin binding and indicate the importance of 6-sulfo SLex epitopes on O-glycans.
Methods
Purification of CD34 from human tonsils
The study protocol was reviewed and approved by the Research Ethics Board at the Department of Otolaryngology–Head and Neck Surgery, Helsinki University Central Hospital. Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Tonsils were surgically removed under general anesthesia from pediatric patients, freshly frozen, and stored at −80°C until use. CD34 was purified from tonsil tissue homogenates essentially as described by Puri et al.29 Further details are described in supplemental document 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
L-selectin affinity chromatography
Recombinant soluble L-selectin-IgG chimera (L-sel-Ig) was expressed on HEK 293 cells and purified from the conditioned cell culture medium using Protein A Sepharose 4 Fast Flow (Amersham Biosciences AB) affinity chromatography.24 L-sel-Ig was immobilized into Protein A Sepharose at high density (∼ 10 mg/mL). The L-selectin column (∼ 1 mL, 0.5 × 5.5 cm) was equilibrated with buffer containing low salt concentration (20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% n-octyl-β-d-glucopyranoside [OG]) at + 4°C. Concentrated CD34 samples were applied into the column in a total volume of 200 μL equilibration buffer and 0.5-mL fractions were collected at a flow rate of approximately 0.3 mL/minutes at +4°C. Fractions 1 to 10 were eluted with equilibration buffer and tightly bound material was eluted by replacing divalent cations by 5 mM EDTA in buffer (fractions 11-20). Fractions from each run were screened for the presence of CD34 using dot blot analysis. Fractions containing a detectable amount of CD34 were further analyzed by Western blotting. Fractions 2 to 4 containing the majority of L-selectin–nonbinding CD34 (L-NB-CD34) and fractions 13 to 16 containing L-selectin–bound CD34 (L-B-CD34) were pooled together and concentrated using a Microcon Ultracel YM-50 centrifugal filter devices (Millipore) at +4°C to the final volumes of 500 μL (L-NB-CD34) and 85 μL (L-B-CD34). Relative amount of L-B-CD34 versus L-NB-CD34 was estimated by Western blotting from serially diluted samples. Calibration of the L-selectin column was carried out using synthetic radiolabeled glycosulfopeptide-6 (GSP-6) and glycopeptide-6 (GP-6; 1000-2000 cpm, ∼ 1 pmol) modeled after N-terminus of PSGL-1.24
Western blotting and dot blot analysis
Samples were separated by SDS–polyacrylamide gel electrophoresis (PAGE) under reducing conditions on 8% or 8% to 16% precast Precise polyacrylamide mini gels (Pierce) using Tris-HEPES-SDS running buffer and transferred onto nitrocellulose membrane (BioRad Laboratories). Membranes were blocked overnight at room temperature in 5% nonfat dry milk solution in TBS (10 mM Tris, pH 7.5, containing 150 mM NaCl). After washing, membranes were incubated for 1 hour at room temperature (RT) with mAbs QBend10 (Serotec) or MECA-79 (BD Biosciences Pharmingen; 2 μg/mL), washed, and subsequently incubated for 1 hour at RT with peroxidase-conjugated goat anti–mouse IgG (H+L) or goat anti–rat IgG + IgM (H+L; Jackson ImmunoResearch Laboratories; 1:40 000), respectively. After washing, membranes were incubated for 3 minutes with SuperSignal West Pico chemiluminescent substrate (Pierce) and exposed to a film (Amersham Hyperfilm ECL; GE Healthcare). Antibodies were diluted in TBS containing 1% BSA and 0.05% Tween-20, and membranes were washed using 10 mM Tris, pH 7.5, containing 300 mM NaCl and 0.05% Tween-20. Dot blot analysis was carried out by pipetting sample directly into the nitrocellulose membrane in 2-μL aliquots and drying using a hair dryer between aliquots. Immunodetection was performed as in Western blotting using anti-CD34 mAb 581 (2 μg/mL; ImmunoTools) and peroxidase-conjugated goat anti–mouse IgG.
Glycan removal and purification
Glycan removal from CD34 was conducted essentially as described in Weitzhandler et al.32 Purified CD34 samples (L-B-CD34 and L-NB-CD34) were separated by SDS-PAGE under reducing conditions on 8% precast polyacrylamide mini gels (Pierce) and transferred onto Immobilon-P PVDF membrane (Millipore). Small amounts of the CD34 samples were run in neighboring wells on both sides of the main samples and immunostained using mAbs QBend10 or MECA-79. Using immunostained CD34 as a marker, the main CD34 samples were excised from membrane for sequential N-glycan and O-glycan removal. CD34 on PVDF membrane was incubated for 51 hours at 37°C with 5 mU recombinant peptide N-glycosidase F (PNGase F; EC 3.5.1.52; Roche Diagnostics) in a total volume of 100 μL of 50 mM Tris-HCl, pH 7.5, containing 1% OG. Supernatant containing free N-glycans was removed and membrane was washed with water. After PNGase F treatment, O-glycans were removed by reductive β-elimination by incubating membrane for 40 hours at 40°C in a total volume of 200 μL of 0.1 M NaOH containing 1 M NaBH4. The reaction was terminated by addition of glacial acetic acid to the sample on ice. Supernatant containing free O-glycan alditols was removed and the membrane was washed with water.
Free N-glycans and O-glycan alditols were purified using solid-phase extraction on Sep-Pak Vac 1cc C18 cartridges (Waters) and Extract-Clean SPE Carbograph 150-mg cartridges (Alltech). Supernatant containing N-glycans was purified using Sep-Pak C18 equilibrated sequentially with acetonitrile and ultrapure water. N-glycans eluted from the Sep-Pak and supernatant containing O-glycan alditols from reductive β-elimination were further purified using Carbograph cartridges as described in Zhang et al,33 except neutral and acidic oligosaccharides were eluted in 1 batch using 40% (vol/vol) acetonitrile containing 0.1% trifluoroacetic acid. Eluates were dried in vacuum.
To study the role of N-glycans of CD34 for binding to L-selectin, N-glycans were removed by incubating CD34 with 5 mU PNGase F in a reaction volume of 55 μL of 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl and 1% OG at 37°C for 46 hours. The reaction was terminated by heating the sample at 90°C for 10 minutes before chromatography on immobilized L-selectin.
Mass spectrometry
Matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) was performed with a Bruker Ultraflex TOF/TOF instrument (Bruker Daltonics). Negatively charged oligosaccharides were analyzed in linear negative ion mode using 2,4,6-trihydroxyacetophenone (THAP; Fluka Chemie) as a matrix essentially as described in Papac et al.34 Briefly, 0.3 μL sample dissolved in ultrapure water was mixed with 0.3 μL THAP (3 mg/mL in acetonitrile/20 mM aqueous diammonium citrate, 1:1 vol/vol) on a polished stainless steel target plate and immediately dried under reduced pressure. Neutral oligosaccharides were analyzed in reflector positive ion mode using 2,5-dihydroxybenzoic acid (DHB; Sigma-Aldrich) matrix dissolved in water at concentration 10 mg/mL. Sample (0.3-1 μL) and matrix (0.3-1 μL) were applied to the target plate and dried using a hair dryer. The negative-ion spectra were externally calibrated with [M-H]− signals from NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4Glc and NeuAcα2-3Galβ1-4GlcNAcβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)Galβ1-4Glc. The positive-ion spectra were externally calibrated with [M+Na]+ signals from Dextran-1000 (Sigma-Aldrich).
Results
Purification of CD34 from human tonsils
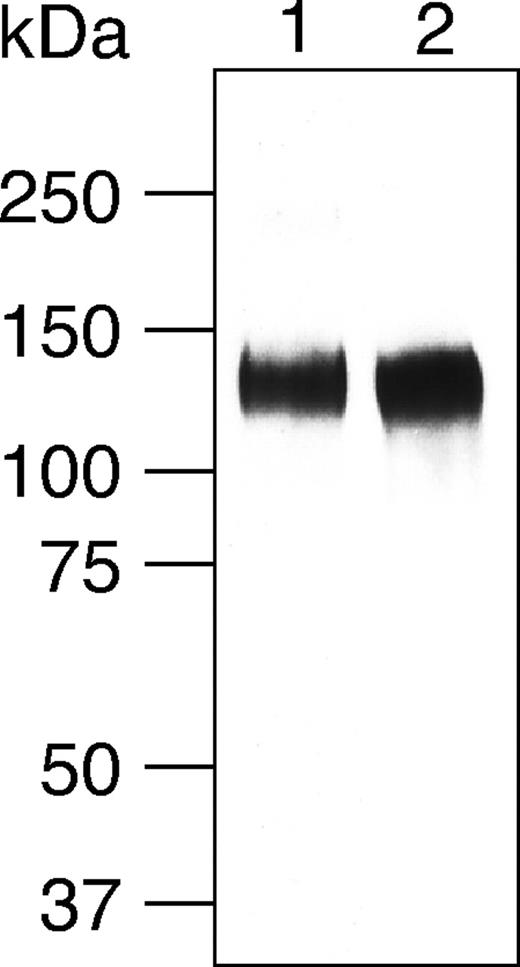
CD34 was purified from detergent extracts prepared from tonsils of 4 different donors using a 2-step affinity chromatography procedure. First, wheat germ agglutinin (WGA) affinity chromatography, which binds primarily sialylated glycoproteins, was used to separate glycoproteins from nonglycosylated proteins. In the second step, the WGA-bound material was passed through an immobilized anti-CD34 mAb (4H11) column. Eluted material was analyzed by SDS-PAGE after fluorescent glycoprotein gel staining as well as by Western blotting after immunostaining using anti-CD34 mAb (QBend10) and mAb to a sulfated carbohydrate epitope (MECA-79). Western blot analysis of purified CD34 showed a single band at molecular weight approximately 125 kDa recognized by both QBend10 and MECA-79 mAbs (Figure 1). Fluorescent glycoprotein gel staining of purified CD34 also showed a single band at approximately 125 kDa (not shown).
Western blotting analysis of purified CD34 from human tonsils. Affinity-purified human CD34 was separated under reducing conditions on a 8% SDS-PAGE gel, transferred onto the nitrocellulose filter, and immunostained using anti-CD34 mAb QBend10 (lane 1) and MECA-79 mAb (lane 2).
Western blotting analysis of purified CD34 from human tonsils. Affinity-purified human CD34 was separated under reducing conditions on a 8% SDS-PAGE gel, transferred onto the nitrocellulose filter, and immunostained using anti-CD34 mAb QBend10 (lane 1) and MECA-79 mAb (lane 2).
A minor proportion of tonsillar CD34 binds to immobilized L-selectin: L-selectin–bound form of CD34 is MECA-79 positive
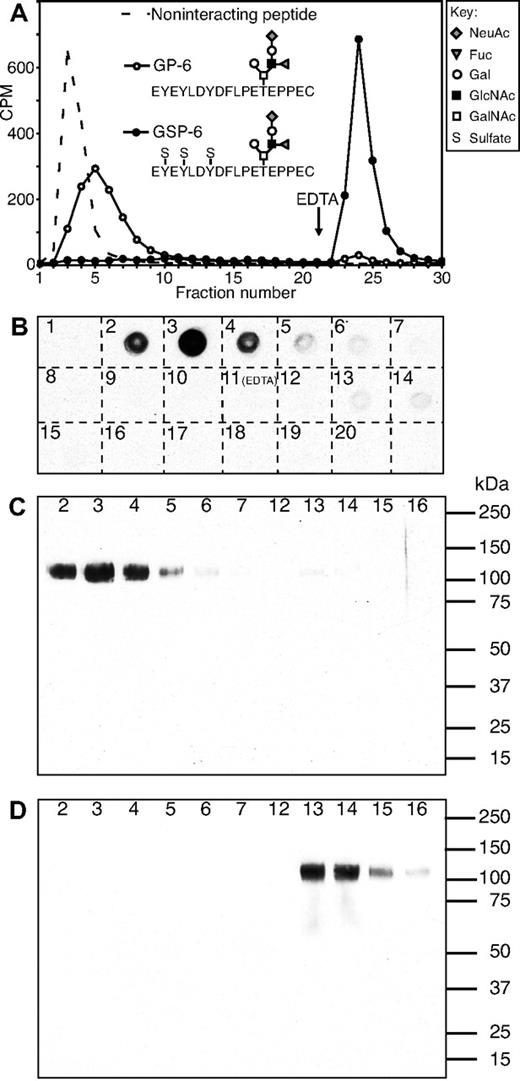
L-selectin Ig chimera (L-sel-Ig) was immobilized on Protein A Sepharose at high density (∼ 10 mg/mL). To increase the sensitivity of binding, chromatography on immobilized L-sel-Ig was performed at a low salt (50 mM NaCl) concentration, because reduced salt concentration below physiologic levels increases the binding affinity of selectins to their ligands.24,35 Glyco(sulfo)peptides GSP-6 and GP-6, modeled after the N-terminus of human PSGL-1, were used to calibrate the column (Figure 2A). Sulfated GSP-6 binds to L-sel-Ig with relatively high affinity (Kd ∼ 5 μM under physiologic conditions),24 whereas nonsulfated GP-6 binds to L-sel-Ig with approximately 11-fold weaker affinity (A.L., Ville Parviainen, Elina Ahola, Nisse Kalkkinen, and R.D.C., manuscript in preparation). In the present experiment, GSP-6 bound tightly to immobilized L-sel-Ig in the presence of Ca2+ and Mg2+ and was eluted out of the column by replacing divalent cations with EDTA in low-salt buffer (Figure 2A). Under these conditions, nonsulfated GP-6 showed only weak interaction to immobilized L-sel-Ig and eluted out of the column in the presence of Ca2+ and Mg2+ as a broad peak before fraction 10 (Figure 2A).
Small proportion of CD34 purified from human tonsils binds to L-selectin and is MECA-79 positive. (A) Calibration of L-selectin affinity column using radiolabeled synthetic glyco(sulfo)peptides modeled after N-terminus of human PSGL-1. Fractions 1 to 20 were collected using 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% OG, and fractions 21 to 30 were eluted with 5 mM EDTA in 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1% OG. (B) Purified tonsillar CD34 was separated in L-selectin affinity chromatography and fractions were analyzed by dot blot analysis using anti-CD34 mAb 581 (numbers in figure refer to fraction numbers). Note that fractions 1 to 10 were collected with 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% OG, and fractions 11 to 20 were eluted with 5 mM EDTA in 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1% OG. (C-D) Western blotting analysis of fractions from L-selectin affinity chromatography of CD34 (numbers refer to the same fraction numbers as in panel B). Immunostaining was performed using anti-CD34 mAb QBend10 (C) and MECA-79 mAb (D).
Small proportion of CD34 purified from human tonsils binds to L-selectin and is MECA-79 positive. (A) Calibration of L-selectin affinity column using radiolabeled synthetic glyco(sulfo)peptides modeled after N-terminus of human PSGL-1. Fractions 1 to 20 were collected using 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% OG, and fractions 21 to 30 were eluted with 5 mM EDTA in 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1% OG. (B) Purified tonsillar CD34 was separated in L-selectin affinity chromatography and fractions were analyzed by dot blot analysis using anti-CD34 mAb 581 (numbers in figure refer to fraction numbers). Note that fractions 1 to 10 were collected with 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% OG, and fractions 11 to 20 were eluted with 5 mM EDTA in 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1% OG. (C-D) Western blotting analysis of fractions from L-selectin affinity chromatography of CD34 (numbers refer to the same fraction numbers as in panel B). Immunostaining was performed using anti-CD34 mAb QBend10 (C) and MECA-79 mAb (D).
Purified tonsillar CD34 was chromatographed on immobilized L-sel-Ig under the same conditions as the calibration was performed. Fractions 1 to 10 (0.5 mL each) were collected using Ca2+– and Mg2+–containing buffer, and fractions 11 to 20 were collected using buffer containing EDTA. First, small aliquots of each fraction were used for screening for the presence of CD34 by dot blot analysis using anti-CD34 mAb (clone 581). Dot blot analysis showed that the majority of CD34 did not bind to immobilized L-sel-Ig and eluted within fractions 2 to 6 (Figure 2B). A very small, but detectable, amount of CD34 was contained in fractions 13 to 15 eluted with EDTA. Fractions 2 to 7 and 12 to 16 were further analyzed by Western blotting using anti-CD34 mAb (QBend10), which verified the result obtained in dot blot analysis (Figure 2C). Fractions 2 to 7 and 12 to 16 were also analyzed by Western blotting using MECA-79 mAb. The results indicated that MECA-79–reactive CD34 can be detected only in the L-selectin–bound fractions 13 to 16, and that the L-selectin–nonbinding fractions 2 to 6 were MECA-79 negative (Figure 2D). This result is clearly in agreement with earlier results indicating that the MECA-79 epitope is important for L-selectin binding. L-selectin–nonbinding CD34 (L-NB-CD34, fractions 2-4) and L-selectin–bound CD34 (L-B-CD34, fractions 13-16) were pooled separately and concentrated to a small volume. Western blotting analysis of serially diluted samples using anti-CD34 mAb (QBend10) showed that approximately 7% to 8% of total tonsillar CD34 bound to L-selectin (not shown).
L-selectin–bound form of CD34 contains sulfated and fucosylated O- and N-glycans
Samples of L-selectin–nonbinding (L-NB-CD34) and –bound (L-B-CD34) CD34 were run on an SDS-PAGE gel and blotted onto the PVDF membrane. A portion of membrane corresponding to the size of CD34 was cut out for glycan isolation. N- and O-glycans were sequentially removed from membrane-bound CD34 samples by PNGase F and reductive β-elimination, respectively. Free N-glycans and O-glycan alditols were purified and analyzed by negative-ion MALDI-TOF mass spectrometry. MALDI-TOF mass spectrum of O-glycan alditols released from L-NB-CD34 showed molecular ion signals derived from simple core-1– and core-2–based O-glycans capped with 1 or 2 sialic acid residues (observed at m/z 675.0, 966.2, 1040.3 and 1331.4; Figure 3A). Signals from fucosylated and/or sulfated O-glycan alditols were not observed in the spectrum. The O-glycan alditols released from L-B-CD34 showed a very complex glycan composition in comparison with glycans released from L-NB-CD34 (Figure 3B; Table 1). In addition to the simple core-1– and core-2–based O-glycan alditols present also in L-NB-CD34, the majority of other O-glycan alditols were fucosylated and/or sulfated. Lower mass region of the spectrum contained a nonsialylated but sulfated O-glycan alditol at m/z 829.1 and its fucosylated derivative at m/z 975.2. Monosialylated and disialylated derivatives of the 2 species were also present (at m/z 1120.2, m/z 1266.3, m/z 1411.3, and m/z 1557.3). Higher mass region of the spectrum contained monosialylated and sulfated O-glycan alditol at m/z 1485.3 and its monofucosylated derivative at m/z 1631.3. Other high-mass O-glycans were derivatives of disialylated O-glycan alditol (at m/z 1696.3), containing 1 sulfate (m/z 1776.2) or 1 fucose (m/z 1842.3), or combination of 1 sulfate and 1 or 2 fucose residues (at m/z 1922.2 and m/z 2068.5, respectively).
Negative ion MALDI-TOF mass spectrometric profiles of O-glycan alditols and N-glycans released from L-selectin–bound (L-B-CD34) and –nonbinding (L-NB-CD34) forms of CD34. O-glycan alditols released from L-NB-CD34 (A) and L-B-CD34 (B). N-glycans released from L-NB-CD34 (C) and L-B-CD34 (D). Based on further structural analysis of O-glycan alditols, only the most abundant isomeric structures are shown, but other isomeric structures may also be present (see supplemental document 1 for details). Panel E summarizes O-glycan structures containing 6-sulfo SLex epitope identified in the present study from L-B-CD34. Molecular ions ([M-H]−) in panel E refer to original nondigested O-glycan alditols of panel B. Carbohydrate symbol representation shown is according to nomenclature suggested by the Consortium for Functional Glycomics (http://www.functionalglycomics.org). All molecular ions are in [M-H]− form.
Negative ion MALDI-TOF mass spectrometric profiles of O-glycan alditols and N-glycans released from L-selectin–bound (L-B-CD34) and –nonbinding (L-NB-CD34) forms of CD34. O-glycan alditols released from L-NB-CD34 (A) and L-B-CD34 (B). N-glycans released from L-NB-CD34 (C) and L-B-CD34 (D). Based on further structural analysis of O-glycan alditols, only the most abundant isomeric structures are shown, but other isomeric structures may also be present (see supplemental document 1 for details). Panel E summarizes O-glycan structures containing 6-sulfo SLex epitope identified in the present study from L-B-CD34. Molecular ions ([M-H]−) in panel E refer to original nondigested O-glycan alditols of panel B. Carbohydrate symbol representation shown is according to nomenclature suggested by the Consortium for Functional Glycomics (http://www.functionalglycomics.org). All molecular ions are in [M-H]− form.
The O-glycan structures shown in Figure 3B were elucidated by further structural analysis using exoglycosidases and MALDI-TOF MS as described in supplemental document 1 and supplemental Figure 1. In summary, digestion with α2,3-specific neuraminidase showed that all sialic acid residues were α2,3 linked, except a sialic acid residue in disialylated core-1 alditol at m/z 966.2. Our data based on the unique specificity of α3/4-fucosidases toward sulfated glycans suggested that the majority of fucosylated and sulfated O-glycan alditols contained 6-sulfo SLex epitope and not isomeric 6′-sulfo SLex (NeuAcα2-3(SO3-6)Galβ1-4(Fucα1-3)GlcNAcβ1-). Our results also strongly suggest that 6-sulfo SLex is expressed more commonly on an extended core-1 backbone in low-mass O-glycans and on an extended core-2 backbone in high-mass O-glycans. In summary, we were able to identify 5 different O-glycan alditols containing a potential 6-sulfo SLex epitope from L-B-CD34 (Figure 3E).
Negative ion MALDI-TOF mass spectrum of PNGase F released N-glycans from L-selectin–nonbinding CD34 (L-NB-CD34) showed molecular ion signals derived from complex type N-glycans capped with 1, 2, 3, or 4 sialic acid residues (Figure 3C). The majority of N-glycans were monofucosylated, which is consistent with earlier N-glycan analysis of CD34.31 One difucosylated species was observed (m/z 2222.5), likely representing a biantennary monosialylated N-glycan. Signals from sulfated N-glycans were not observed in the spectrum. The N-glycan profile of L-B-CD34 showed a very complex glycan composition in comparison with N-glycans of L-NB-CD34 (Figure 3D; Table 1). The spectrum of N-glycans of L-B-CD34 showed the presence of several sulfated N-glycans, of which 2 were also fucosylated (signals at m/z 2302.5 and m/z 2594.5) and therefore carry potential sulfo SLex epitopes. We were able to detect signals from sulfated N-glycans only in biantennary, nonextended N-glycans, which is consistent with N-glycan structures from murine endothelial CD34.22 Due to the small amount of material, N-glycans were not analyzed any further.
PNGase F digestion of tonsillar CD34 does not affect the ability of MECA-79–reactive CD34 to bind to L-selectin
Tonsillar CD34 was digested with PNGase F and chromatographed on immobilized L-sel-Ig column using low-salt conditions. Equivalent amount of nontreated CD34 was also chromatographed on immobilized L-sel-Ig under the same conditions. Fractions 3 to 10 and 13 to 15 were analyzed by Western blotting using MECA-79 mAb. Fractions 3 to 10 were MECA-79 negative and fractions 13 and 14 were MECA-79 positive in nontreated and in PNGase F–digested samples. Figure 4 shows Western blotting analysis of peak fractions 3 (L-NB-CD34) and 13 (L-B-CD34). MECA-79 staining showed that the molecular weight of the digested CD34 was reduced by approximately 20 kDa, which demonstrates that N-glycan removal was successful. QBend10 mAb recognized CD34 only in nondigested sample. QBend10 was still able to recognize CD34 in the control sample that was heated but not treated with PNGase F (not shown), demonstrating that the QBend10 binding epitope of CD34 was lost upon PNGase F treatment. In summary, PNGase F digestion of CD34 did not affect the ability of MECA-79–reactive CD34 to bind to L-selectin.
PNGase F–treated CD34 binds to L-selectin. PNGase F–digested and –nondigested CD34 was separated on L-selectin affinity chromatography, and fractions 3 (L-NB-CD34) and 13 (L-B-CD34) from both runs were analyzed on a 8% SDS-PAGE gel under reducing conditions, transferred onto the nitrocellulose filter, and immunostained using MECA-79 mAb.
PNGase F–treated CD34 binds to L-selectin. PNGase F–digested and –nondigested CD34 was separated on L-selectin affinity chromatography, and fractions 3 (L-NB-CD34) and 13 (L-B-CD34) from both runs were analyzed on a 8% SDS-PAGE gel under reducing conditions, transferred onto the nitrocellulose filter, and immunostained using MECA-79 mAb.
Discussion
Sulfated sialyl Lewis x determinants are commonly expressed on HEV sialomucins.13,14,36 Studies with monoclonal antibodies against sulfated carbohydrate epitopes have indicated that 6-sulfo SLex is commonly expressed in HEVs, whereas isomeric 6′-sulfo SLex is not.13,14 Genetic deletion of 2 of the 6-sulfotransferases that direct the synthesis of 6-sulfo SLex epitope on HEV sialomucins resulted in impaired lymphocyte homing to lymph nodes, indicating that 6-sulfo SLex has a major role for L-selectin binding.37,38 In vitro evidence has also indicated that L-selectin binds to 6-sulfo SLex with higher affinity than to isomeric 6′-sulfo SLex.39,40 6-Sulfo SLex epitope can be expressed on core-2–type or extended core-1–type O-glycans on HEV sialomucins, and both types of O-glycans have been shown to contribute to L-selectin–dependent lymphocyte homing in mice.20,21 Structural analysis of O-glycans of murine GlyCAM-1 has revealed that approximately 25% of total O-glycans on GlyCAM-1 are modified with 6-sulfo SLex.37 Although a small amount of sulfate was present on galactose residues, it was found mainly on the core-1 galactose and the 6′-sulfo SLex epitope was not identified on O-glycans of GlyCAM-1. The major O-glycan with 6-sulfo SLex was a small core-2–based O-glycan with 6-sulfo SLex on the core-2 branch. Only a small amount of 6-sulfo SLex was found on extended core-1 branch or on both branches on O-glycans of GlyCAM-1.
In the present study, we identified 5 different O-glycans containing a potential 6-sulfo SLex epitope from L-selectin–bound glycoforms of CD34 (Figure 3E). We found that low mass O-glycans contain 6-sulfo SLex epitope on an extended core-1 backbone structure, whereas high-mass O-glycans carry 6-sulfo SLex more commonly on the extended core-2 backbone. Based on mass spectrometric comparison of signal intensities of O-glycan alditols containing 6-sulfo SLex suggests that high-mass O-glycans contain approximately 2-fold more 6-sulfo SLex than low-mass O-glycans. Sialylated core-2–based O-glycan with 6-sulfo SLex, which was a major 6-sulfo SLex–containing O-glycan in GlyCAM-1, is a minor component among 6-sulfo SLex–containing O-glycans on CD34 (signal at m/z 1557.3 in Figure 3B). Our data based on the unique specificity of α3/4-fucosidases toward sulfated glycan structures also suggest that O-glycans containing 6′-sulfo SLex epitopes, if present, are very minor in comparison with 6-sulfo SLex–containing O-glycans in CD34. This is in agreement with the results from immunohistochemical staining of human lymphoid tissues using monoclonal antibodies to sulfated carbohydrate epitopes.14 Moreover, we did not detect biantennary O-glycans containing 6-sulfo SLex epitopes on both core-1 and core-2 branches in CD34. Our results also show that the majority of MECA-79 epitopes can be found in the low-mass O-glycans, whereas sulfated high-mass O-glycans containing an extended core-2 structure are not recognized by MECA-79. MECA-79 is able to block lymphocyte binding to human HEVs only partially.27,28 O-glycoprotease–resistant L-selectin ligands that are not recognized by MECA-79 have been suggested to have a role in lymphocyte homing.28 Our finding that most of 6-sulfo SLex epitopes are expressed on extended core-2 O-glycans could explain in part the partial function-blocking activity of MECA-79 in inhibiting lymphocyte binding to HEVs. It is likely that the 6-sulfo SLex epitopes on extended core-2 O-glycans are recognized by L-selectin, but not by MECA-79. MECA-79 can inhibit only L-selectin binding to 6-sulfo SLex on extended core-1 O-glycans.
Earlier partial glycan analysis of total CD34 isolated from human tonsils indicated that only 1 O-glycan was found to contain a potential 6-sulfo SLex epitope and it represented approximately 1% of the total O-glycan pool of CD34.31 The low abundance of 6-sulfo SLex epitopes in total tonsil CD34 is in agreement with our present data showing that more than 90% of tonsil CD34 does not bind to L-selectin and does not carry 6-sulfo SLex. L-B-CD34 that represents less than 10% of total tonsillar CD34 carries relatively high abundance of 6-sulfo SLex epitopes. Among 5 different O-glycans with 6-sulfo SLex identified from L-B-CD34, 1 O-glycan alditol has the same mass as previously identified O-glycan alditol (m/z 1922.2, Figure 3E). Our detailed structural analysis indicated that the major signal at m/z 1922.2 is derived from O-glycan alditol with 6-sulfo SLex on extended core-2 branch, and not on extended core-1 branch, as suggested earlier based on the presence of MECA-79 epitope.
MALDI-TOF MS analysis of N-glycans from L-selectin–bound form of CD34 (L-B-CD34) revealed the presence of several sulfated biantennary N-glycans. Some of them were also fucosylated and thus may carry 6-sulfo SLex epitope. 6-Sulfo SLex has been identified from N-glycans of murine CD34.22 N-glycans carrying the epitope were shown to contribute to L-selectin–dependent lymphocyte homing in mice when extended core-1– and core-2–type O-glycans were not expressed.22 In an attempt to explore the role of N-glycans of CD34 for binding to L-selectin, we studied the ability of CD34 to bind to L-selectin after removing its N-glycans. Enzymatic removal of N-glycans from CD34 was successful, because the molecular weight of CD34 decreased approximately 20 kDa after PNGase F treatment. PNGase F–treated CD34 bound to L-selectin equivalently to nondigested CD34, based on MECA-79 binding to CD34 from eluted fractions of L-selectin affinity chromatography (Figure 4). All MECA-79–reactive CD34 remained in L-selectin–bound fractions and MECA-79–reactive CD34 was not detected from nonbinding fractions. These data suggest that N-glycans of CD34 do not have a major contribution for binding to L-selectin, at least in an in vitro assay, but they may have a minor contribution for binding. However, our assay using immobilized L-selectin does not rule out a possibility that N-glycans of CD34 may have an important role for binding to L-selectin under flow conditions. Removal of N-glycans from CD34 may also unmask some O-glycans that are not otherwise accessible for L-selectin binding. Our finding that 6-sulfo SLex epitopes are much more commonly found on O-glycans than on N-glycans of L-B-CD34 support the major role for 6-sulfo SLex–containing O-glycans for binding to L-selectin. Moreover, CD34 contains only 9 potential N-glycosylation sites and approximately 70 extracellular O-glycan attachment sites, of which more than 30 are predicted to be glycosylated according to prediction results from NetOGlyc 3.1 Server (Technical University of Denmark, http://www.cbs.dtu.dk). Based on mass spectrometric identification and comparison of signal intensities of O-glycan alditols derived from L-B-CD34 (Figure 3B), we estimate that approximately 20% of total O-glycans of L-B-CD34 contain 6-sulfo SLex epitope.
L-selectin binds to monovalent 6-sulfo SLex epitope with low affinity, and exhibits a dissociation constant (Kd) in the low millimolar range.12,41 Although the binding affinity of L-selectin to monovalent 6-sulfo SLex is weak, it is commonly thought that multivalent epitope expression on individual mucins leads to high-affinity binding due to enhanced avidity. In fact, direct biochemical binding experiments have been carried out only with recombinant rat L-selectin and purified murine GlyCAM-1. Results show that there is some enhancement in avidity, either due to the peptide determinants or the numbers of sulfated glycans, because L-selectin bound to GlyCAM-1 with a Kd of 108 μM.42 O-glycan analysis of murine GlyCAM-1 has revealed that approximately 25% of total O-glycans on GlyCAM-1 contain a 6-sulfo SLex epitope.37 It is also known that monomeric L-selectin binds to immobilized PSGL-1 with relatively low affinity (Kd = 47 μM) but binding to PSGL-1 is through a different mechanism than binding to 6-sulfo SLex.26 L-selectin recognizes sulfated tyrosines and other nearby peptide components in addition to core-2–based O-glycan with SLex at the extreme N-terminus of PSGL-1. In our present study, we used synthetic glyco(sulfo)peptides, GSP-6 and GP-6, modeled after N-terminus of PSGL-1 to set up elution conditions in L-selectin affinity chromatography, under which GSP-6 was clearly separated from GP-6 that binds to L-selectin with 11-fold lower affinity than GSP-6 (A.L., Ville Parviainen, Elina Ahola, Nisse Kalkkinen, and R.D.C., manuscript in preparation). Under the same conditions, we were able to isolate a small amount of L-selectin–bound CD34 from nonbinding CD34. We estimated that L-B-CD34 represents 7% to 8% of total CD34 isolated from human tonsils. Although the binding affinity of L-selectin to L-B-CD34 has not been determined, based on calibration of L-selectin column, we anticipate that L-selectin binds to CD34 with similar affinity than to PSGL-1.
In summary, we purified CD34 from human tonsils and separated L-selectin–binding (L-B-CD34) and –nonbinding (L-NB-CD34) glycoforms of endothelial CD34. Less than 10% of tonsillar CD34 bound to L-selectin and MECA-79–reactive CD34 occurred exclusively in the L-selectin–bound form of CD34. MALDI-TOF MS revealed that L-B-CD34 contained a complex pattern of sialylated O-glycans, most of which were sulfated and/or fucosylated, whereas L-NB-CD34 contained only simple sialylated O-glycans that were not sulfated or fucosylated. Detailed structural analysis of O-glycans of L-B-CD34 revealed that majority of 6-sulfo SLex epitopes were on extended core-2–type and on small extended core-1–type O-glycans. We also identified monosialylated and disialylated biantennary N-glycans that carry potential 6-sulfo SLex epitope on L-B-CD34. Our results suggest that extended core-2 O-glycans are the major glycan structures that carry 6-sulfo SLex epitope on L-selectin–binding glycoforms of human CD34. These results extend the studies on a key L-selectin ligand in this field and demonstrate that there are significant differences in glycosylation of different CD34 glycoforms that are associated with interactions with L-selectin.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Nisse Kalkkinen (Institute of Biotechnology, University of Helsinki) for kindly providing MALDI-TOF mass spectrometer instrument time throughout this study.
This work was supported in part by grants from the Academy of Finland (no. 107203 [R.R.] and no. 118469 [A.L.]), from the National Institutes of Health (Bethesda, MD; HL085607 [R.D.C.]), by Magnus Ehrnrooth Foundation (Helsinki, Finland [A.L.]), and by Helsinki University Central Hospital Research Funds, Helsinki, Finland (R.R.).
National Institutes of Health
Authorship
Contribution: G.H.M. and J.H., designed and performed experiments, analyzed data, and edited the paper; K.-P.S. designed and performed experiments; R.D.C. provided key reagents and edited the paper; A.M. provided patient samples; R.R. designed experiments, analyzed data, and edited the paper; and A.L. designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Leppänen, Department of Biological and Environmental Sciences, Division of Biochemistry, University of Helsinki, Viikinkaari 5D, 00014, Helsinki, Finland; e-mail: anne.leppanen@helsinki.fi.

![Figure 3. Negative ion MALDI-TOF mass spectrometric profiles of O-glycan alditols and N-glycans released from L-selectin–bound (L-B-CD34) and –nonbinding (L-NB-CD34) forms of CD34. O-glycan alditols released from L-NB-CD34 (A) and L-B-CD34 (B). N-glycans released from L-NB-CD34 (C) and L-B-CD34 (D). Based on further structural analysis of O-glycan alditols, only the most abundant isomeric structures are shown, but other isomeric structures may also be present (see supplemental document 1 for details). Panel E summarizes O-glycan structures containing 6-sulfo SLex epitope identified in the present study from L-B-CD34. Molecular ions ([M-H]−) in panel E refer to original nondigested O-glycan alditols of panel B. Carbohydrate symbol representation shown is according to nomenclature suggested by the Consortium for Functional Glycomics (http://www.functionalglycomics.org). All molecular ions are in [M-H]− form.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/3/10.1182_blood-2009-03-210237/4/m_zh89990937250003.jpeg?Expires=1766236102&Signature=u6jt9b3S-IbtzDfk6CCWVkrKNOv7fQJGOkb8LApRvyk2LzVYWbnP-3~vSWEJoRQkgtCT6MCHSen250E5YSvkzdG3U48L-5WXzBzLUE9hZ-4sUTESqHyI9xpB3V1fbkapevJO8SOKpOzK40a4FtQCnOIbHr46qhp-u5Mk4qsdAsmBuEgqZ4FtHr2kj12emKzbBWvgsmm55gjSqw-Qv~o1s9HKRWQUiadhmZJgICRgxEhBExWQL6xbNZO1SugOcWUEgDFTHihDedNy729j10HyVZgL55jZDbCArlpaxbfiKLyi1CNY88kqEOLVW4ADyaiwLa4~bTCUuRDyO8ISmlT8jA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

