Response
We thank Shimabukuro-Vornhagen et al for their comments on our recent study where we developed a simple and low-cost protocol using allogeneic CD40-activated B cells to induce and expand highly efficient human alloantigen-specific CD4highCD25+Foxp3+ regulatory T cells (Tregs) from naive CD4+CD25− T cells in large scale.1 First, we have to clarify that we did not use autologous CD40-activated B cells but we used allogeneic cells to induce and expand Tregs instead. Second, the CD40-activated B cells used in our system are live cells but not the irradiated peptide-pulsed cells as others used.2,3 Third, the ratio of B to T cells for induction and expansion of CD4highCD25+ Tregs is 1:10 in our system but not 1:4 as others used to induce antigen-specific T and cytotoxic T cells.2,3 Indeed, several reports have demonstrated that weekly stimulation with antigen-presenting cells (APCs) such as dendritic cells (DCs) is an effective way to induce and expand Tregs in vitro and in vivo.4-9 Similar to our report,1 Jonuleit et al demonstrated that allogeneic immature DCs induced Tregs from naive CD4 T cells at a 1:10 ratio of DC:T cells.6 Therefore, it is not surprising that CD40-activated B cells, as one of the APCs,2,3 can induce and expand Tregs, in particularly under weekly stimulation.
Although the process of cryopreservation and thawing would undoubtedly affect the absolute number of live CD40-activated B cells (the recovery rate of live B cells preserved in liquid nitrogen for 6 months is approximately 80% in our system), it does not affect their function. As shown in Table 1, there are no significant differences in the induction, expansion, and suppressive ability of CD4highCD25+ Tregs induced by frozen versus fresh CD40-activated B cells.
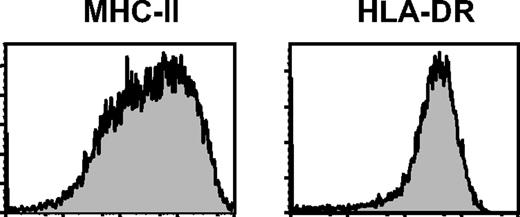
Another question raised by von Bergwelt-Baildon et al was the phenotype of CD40-activated B cells. We think the difference of the major histocompatibility complex (MHC-II) expression in B cells resulted from the different antibodies used. In our study, we determined MHC–II expression in these B cells with fluorescein isothiocyanate (FITC)–anti-human MHC-II antibody, which reacts with all MHC class II molecular HLA-DR, DP, and most DQ antigens (BD Biosciences, San Jose, CA). In contrast, von Bergwelt-Baildon et al only examined the HLA-DR expression in these B cells. We further checked the MHC-II and HLA-DR expressions in CD40-activated B cells (Figure 1). Consistent with our previous report,1 more than 1 peak of MHC-II expressions were observed in these B cells, whereas only 1 peak of HLA-DR expression in the B cells was found (Figure 1).
MHC-II and HLR-DR expressions in human CD40-activated B cells.Expression of MHC-II and HLR-DR on the CD40-activated B cells cultured for 8 days. Data shown here are representatives of B cells from 4 different healthy adult donors.
MHC-II and HLR-DR expressions in human CD40-activated B cells.Expression of MHC-II and HLR-DR on the CD40-activated B cells cultured for 8 days. Data shown here are representatives of B cells from 4 different healthy adult donors.
More evidence of CD40-activated B cells as the tolerogenic cells was also found in our recent study (Zheng J. and Tu W. manuscript submitted). In this study, we demonstrated that allogeneic CD40-activated B cells induced novel CD8highCD25+ cells from naive CD8+ T cells. These CD8highCD25+ T cells were alloantigen-specific Tregs with relatively poor alloantigen-specific cytotoxicity.
Taken together, the process of cryopreservation and thawing does not affect the function of CD40-activated B cells to induce and expand alloantigen-specific Tregs, and the strength of the activation to T cells by CD40-activated B cells is critical for determining whether B cells act as the stimulatory or tolerogenic cells.
Authorship
Acknowledgments: This work was supported in part by Seed Funding for Basic Research, HKU/URC (W.T.); GRF, Research Grants Council of Hong Kong, Hong Kong SAR, China (W.T.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wenwei Tu, MD, PhD, Department of Paediatrics and Adolescent Medicine, LKS Faculty of Medicine, the University of Hong Kong, Hong Kong SAR, PR China; e-mail: wwtu@hkucc.hku.hk.
References
National Institutes of Health