For patients on warfarin therapy, an international normalized ratio (INR) recall interval not exceeding 4 weeks has traditionally been recommended. Less frequent INR monitoring may be feasible in stable patients. We sought to identify patients with stable INRs (defined as having INR values exclusively within the INR range) and comparator patients (defined as at least one INR outside the INR range) in a retrospective, longitudinal cohort study. Occurrences of thromboembolism, bleeding, and death were compared between groups. Multivariate logistic regression models were used to identify independent predictors of stable INR control. There were 2504 stable and 3569 comparator patients. The combined rates of bleeding and thromboembolism were significantly lower in stable patients. Independent predictors of stable INR control were age older than 70 years and the absence of comorbid heart failure and diabetes. Stable patients were significantly less likely to have target INR of 3.0 or higher or chronic diseases. We hypothesize that many patients demonstrating stable INR control could be safely treated with INR recall intervals greater than the traditional 4 weeks.
Introduction
Warfarin is effective for the primary and secondary prevention of both arterial and venous thromboembolic disorders. Its variable dose response and narrow therapeutic index mandate periodic monitoring of the international normalized ratio (INR).1 Target INR ranges of 2.0 to 3.0 or 2.5 to 3.5 have been recommended for most indications because INR values in these ranges are associated with the best combination of thrombosis reduction and bleeding avoidance.1 Although multiple studies have addressed the optimum target intensity of anticoagulation, few studies have addressed the optimal testing frequency. Current guidelines suggest a time interval not exceeding 4 weeks between INR determinations.1,2 However, this recommendation is not evidence based, having evolved instead from regional differences in routine clinical practice and expert opinion.3
More frequent INR testing has been suggested as a means to increase time in the therapeutic range, especially among patients who self-monitor warfarin using point-of-care technology.1,4,5 Although more frequent testing may increase the proportion of time within the therapeutic INR range in some patients, it is not likely to benefit those patients who demonstrate long-term INR stability as demonstrated by minimal INR deviation and longitudinal warfarin dose stability. Hypothetically, less frequent INR monitoring may be possible for such patients. Supporting evidence comes from the United Kingdom where anticoagulation providers routinely allow INR recall intervals in stable patients up to 90 days.6 Recent evidence suggests that longer INR recall intervals may also be associated with improved INR control,7,8 which has in turn been associated with reduced risk for anticoagulation therapy-related adverse events.9,10
Our objectives were to identify a subgroup of patients with very stable (ie, all INR values in the therapeutic range) INR control, to compare the risk of anticoagulation therapy-related adverse events in such patients to the corresponding risk in patients without exclusively therapeutic INR control, and to describe patient characteristics associated with long-term INR stability.
Methods
Study design and setting
The study was a retrospective, longitudinal cohort study conducted at Kaiser Permanente Colorado (KPCO), an integrated health care delivery system that provides services to more than 480 000 members in the Denver-Boulder metropolitan area. Anticoagulation services at KPCO are provided by a centralized Clinical Pharmacy Anticoagulation Service (CPAS).9 Working collaboratively with the referring physician and using standardized dosing algorithms,11 CPAS clinical pharmacists initiate, adjust, and refill anticoagulant medications and order relevant laboratory tests. Dosing algorithms used during the study specified a maximum INR recall interval of 6 weeks. Integrated, electronic medical, pharmacy, and laboratory records system and CPAS database (Dawn-AC; 4S Systems Ltd) were used to identify patients, treatments, and outcomes for this study. Approval to conduct this study was obtained from the KPCO Institutional Review Board.
Patients
Patients with a duration of warfarin therapy in excess of 90 days, at least one INR determination during the study time frame (January 2000 through December 2005), an age of greater than 18 years, and warfarin therapy continuing throughout a 6-month observation period were included in the study.
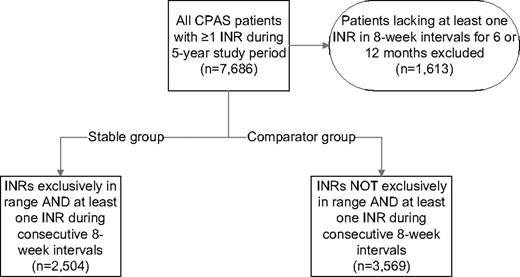
Stable patients were defined as having all INR values within the strictly defined therapeutic reference interval for the first identifiable continuous 6-month period (ie, 100% INR control). Comparator patients were those who did not have any continuous 6-month period where all INR values were within the therapeutic range. To ensure a minimal standard for compliance with ongoing INR monitoring, both stable and comparator patients had to have at least one INR determination every 8 weeks during the respective observation periods. The process for defining the study cohorts is depicted in Figure 1.
Data collection
Variables collected for analysis included the primary warfarin indication (atrial fibrillation, venous thromboembolism, heart valve disorder, other), age at start of the observation period, sex, INR target, duration of warfarin therapy, and INR values. Patient-specific factors that could influence the risk for anticoagulant-related complications were also recorded: diabetes mellitus, hypertension, heart failure, prior venous thrombosis, hemorrhage or stroke, cancer, and estrogen therapy. Risk factors were considered present when a coded assessment for a given factor was identified in the 180 days prior to the start of the observation period. Estrogen therapy was defined as a prescription for a systemic estrogen-containing product sold within 90 days prior to the start of the observation period. A validated measure of patient acuity, the chronic disease score (CDS), was calculated for each patient using ambulatory prescription drug data from the observation period.12 Chronic disease scores can range from 0 to 35, with increasing scores indicating an increasing burden of chronic diseases under treatment. Use of the CDS allows for the accounting of each patient's risk of mortality and future health care use.12,13
The first occurrence of anticoagulant-related complications (thromboembolism, bleeding, and death) was determined as previously described.14 Briefly, specific complications requiring admission to the emergency department or hospital were sought using ICD-9 discharge diagnostic codes (available upon request) within KPCO electronic administrative databases. All events were subsequently confirmed through independent review of the patient's electronic medical record by 2 investigators. Events were scored using a modified Naranjo scale to quantify the relationship of the adverse event with warfarin therapy.15 A third reviewer was employed to resolve disagreements.
Thromboembolic complications were defined as any deep vein thrombosis, pulmonary embolism, cerebral vascular accident, transient ischemic attack, systemic embolism, or heart valve thrombosis. Bleeding complications included episodes such as intracranial bleeding, gastrointestinal hemorrhage, hematoma, hemoptysis, epistaxis, and hematuria. All bleeding episodes resulting in admission to the emergency department or hospital were included regardless of severity. Fatal events were assessed for direct relationship to bleeding or thromboembolism using the medical record and/or a death certificate.
Statistical analysis
Data analyses were performed using SAS 9.1.3 statistical software. Patient characteristics were reported as means and standard deviations for interval-level variables (eg, age, warfarin dose, length of warfarin therapy) and percentages for categoric variables (eg, sex, target INR, occurrence of anticoagulation therapy-related complications). Associations between categoric variables were assessed using the chi-square test and continuous variables were compared using the independent samples t test or Wilcoxon rank sum test (depending on the distribution of the data). Patient characteristics and risk factors were entered into multivariate logistic regression models to identify variables that independently predict INR stability. The alpha was set at .05.
Results
Records from 7686 patients were screened; of these, 6073 patients had a period where an INR was measured every 8 weeks for at least 6 months. The stable group was composed of 2504 patients with INR values within the desired reference interval on all determinations and the comparator group of 3569 patients with at least one INR outside the desired reference interval (Figure 1).
Baseline characteristics of stable patients and comparators are presented in Table 1. Stable group patients were older than comparator group patients and more likely to have had a target INR of 2.5 and to have been receiving warfarin for atrial fibrillation, but less likely to have had a target INR of 3.0 or higher, to have been receiving warfarin for heart valve replacement, to have comorbid diabetes, heart failure, or prior venous thrombosis, or to be receiving concurrent estrogen therapy. The mean chronic disease score was also lower in stable group patients. Differences in duration of warfarin therapy between groups prior to inclusion in the study were not statistically significant. The mean proportion of INR values in the therapeutic range for the comparator group was 46.9% (standard deviation [SD] = 22.0). The stable group had a lower mean number of INRs measured per patient, 6.7 (SD = 1.3) during the observation period compared with 10.7 (SD = 4.5) per patient for comparators (P < .001).
Rates of anticoagulation therapy–related adverse events (thromboembolism, bleeding, and death) are summarized in Table 2. Compared with stable group patients, the rate of overall mortality was higher in the comparator group (P < .01); however, the difference in anticoagulation therapy–related mortality rate was not statistically significant. The rate of anticoagulation-related bleeding complications was higher in the comparator group compared with their stable counterparts (P < .05). Compared with stable group patients, the combined complication rates of bleeding or thromboembolism occurred at a higher rate in the comparator group (P < .001). Patients in the comparator group were more likely than the stable group to require coadministration of heparin or low-molecular-weight heparin (P < .001).
Table 3 summarizes patient characteristics predictive of stable status. Significant predictors of stable group status were age older than 70 years (odds ratio [OR] = 1.54; 95% confidence interval [CI], 1.38-1.72) and the absence of comorbid diabetes (OR = 1.87; 95% CI, 1.3-2.67), heart failure (OR = 1.43; 95% CI, 1.16-1.76), or concurrent estrogen therapy (OR = 1.32; 95% CI, 1.09-1.60). Stable patients were significantly less likely to have a target INR of 3.0 or higher (OR = 0.48; 95% CI, 0.38-0.61) and increasing chronic disease scores (OR = 0.96; 95% CI, 0.94-0.98).
Discussion
In this large retrospective cohort study, we identified 2504 patients with very stable long-term INR control. We identified that age older than 70 years and the absence of comorbid diabetes or heart failure independently predicted this INR stability. Patients with a target INR of 3.0 or higher and those with a greater burden of chronic diseases were less likely to have such long-term INR stability. On average, the proportion of comparator patients' INRs in the therapeutic range was 46.9%, whereas stable patients' INRs were 100% therapeutic. The seemingly suboptimal INR control reflects the absence of these very stable patients from the comparator group. The time in therapeutic range for all patients managed by CPAS is typically about 64%.9 Although other investigations have examined predictors of very poor INR control,16,,–19 to our knowledge this study is the first to assemble a large cohort of anticoagulated patients and carefully evaluate them for predictors of INR stability.
Our findings are important as patients with long-term stable INR control may be adequately treated with less frequent INR monitoring, perhaps as infrequently as every 8 weeks. Extending the INR recall interval in such patients is likely to reduce costs and increase convenience (and therefore perhaps adherence) without impacting the risk for bleeding or thrombosis.
The most surprising observation in our analysis was that age older than 70 years predicted long-term INR stability. This observation is somewhat counterintuitive and should be confirmed in additional studies. This finding argues against innate INR variability associated with advancing age. The possibility that younger patients were more likely to have been receiving warfarin for heart valve indications was explored posthoc by comparing the proportion of patients 70 years or older in both groups on warfarin for this indication. In the stable group, 5.0% had a heart valve indication compared with 7.5% in the comparator group (P = .09). An interaction term for age and warfarin indication was tested in the predictive model but was not significant.
Our results are likely to be valid. The data set used to complete this study is robust and has been used previously in health records and data extraction research.9,14 The large number of patients included in our analysis increases the generalizability of our results and reduces the likelihood that unmeasured bias may have influenced them. Real-world patients with a variety of indications for warfarin and therapeutic INR targets were included. Clinical events were comprehensively collected and described, and INR determinations were performed by a single laboratory and systematically captured in an integrated electronic medical record. All clinical events were independently assessed for causality by 2 expert reviewers. The long-term stable cohort was carefully established using a definition for stability (ie, 100% of INR values within the strict INR range) more rigorous than that used by most anticoagulation providers in routine practice. For example, had INR results within 0.2 of the upper and lower limits of the specified INR range qualified as “in-range” (as is common clinical practice in North America), the number of patients with long-term INR stability would have been substantially larger.20 Most patients observed in our study had been on warfarin therapy for several years. Although differences were not statistically significant between groups, individuals with long-term stability tended to have been on warfarin longer than comparator patients. Potential adherence and survivor biases were minimized by the fact that both groups observed in our study could appropriately be termed “prevalent” warfarin users.
This study does have important limitations. It is retrospective and relies upon extraction of data from administrative databases and medical records. Not all variables likely to enter into clinical decision making were collected. The observational study design also precludes definitive establishment of cause and effect relationships between study variables and outcomes. Retrospective database analysis is particularly prone to missing clinical events if care is delivered outside participating institutions. However, given that KPCO patients are either seen within an affiliated hospital or the costs of care are billed to KPCO when care is provided at nonaffiliated hospitals, it is likely that the vast majority of clinically important events were captured. As patients are provided with comprehensive care by our anticoagulation service, we are confident that all pertinent laboratory values were captured. Our study was conducted within an integrated health care delivery system with a specialized anticoagulation service using standardized warfarin dosing protocols and, thus, the observed results may not directly translate to other health care settings.
We would like to have estimated the actual proportion of anticoagulated patients within KPCO with exclusively therapeutic INR control. As not all patients managed by CPAS met initial eligibility criteria, this was not possible. However, of 7686 anticoagulated patients with at least one measured INR during the 5-year study period, we were able to identify 2504 patients (33%) who had at least 6 months of INR values within their desired therapeutic range. Other researchers have reported that approximately 37% of patients with atrial fibrillation managed in community settings are within the therapeutic INR range 75% or more of the time.7 Irrespective of the actual proportion, our data suggest that a substantial number of patients would be adequately treated with INR recall intervals in excess of 4 weeks. More frequent INR monitoring would of course be necessary in the presence of new comorbidities or new medications affecting the INR.
In conclusion, our work supports the hypothesis that a subgroup of anticoagulated patients with therapeutically stable INR values over 6 months can be identified. In general, these patients will tend to be older, with a target INR less than 3.0, and without significant chronic disease burden. Patients with such stable INR control experience significantly fewer anticoagulation therapy-related complications. We agree with others who have suggested that INR recall intervals should be individually tailored based on recent INR control rather than being fixed at minimum frequency such as 4 weeks.7 We acknowledge that our findings need to be validated in future prospective evaluations. Specifically, we suggest a prospective randomized study that will enroll chronically anticoagulated patients and, after a period of INR stability, gradually increase the interval between INR determinations up to 8 weeks and possibly as long as 12 weeks in patients with stable INR values and baseline characteristics predictive of long-term INR stability.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.M.W., T.D., and N.P.C. designed the research and extracted information from medical records; T.D. performed the statistical analysis; D.M.W., T.D., N.P.C., M.A.C., D.A.G., W.A., and E.M.H. interpreted the analysis and revised the paper; M.A.C. wrote the initial draft of the paper; and T.T. and C.M. extracted information from medical records and reviewed the paper.
Conflict-of-interest disclosure: E.M.H. reports serving as an advisor to Boehringer Ingelheim, Bristol-Myers Squibb, Sanofi-Aventis, and The Medicines Company, and participating in clinical symposia sponsored by Bayer and Bristol-Myers Squibb. D.A.G. reports serving as a consultant to Roche Diagnostics. The remaining authors declare no competing financial interests.
A complete list of WARPED Consortium members appears in the supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Correspondence: Daniel M. Witt, PharmD, FCCP, BCPS, CACP; 16601 E Centretech Pkwy, Aurora, CO 80011; e-mail: dan.m.witt@kp.org.