Abstract
Recent multicenter studies have clarified the molecular basis underlying the different von Willebrand disease (VWD) types, all of which are caused by the deficiency and/or abnormality of von Willebrand factor (VWF). These studies have suggested a unifying pathophysiologic concept. The diagnosis of VWD, remains difficult because its clinical and laboratory phenotype is very heterogeneous and may overlap with normal subjects. Stringent criteria are therefore required for a clinically useful diagnosis. In this paper, we delineate a practical approach to the diagnosis and treatment of VWD. Our approach is based on the critical importance of a standardized bleeding history that has been condensed into a final bleeding score and a few widely available laboratory tests, such as VWF ristocetin cofactor activity, VWF antigen and factor VIII. This approach would help identify those subjects who will probably benefit from a diagnosis of VWD. The next step involves performing a trial infusion with desmopressin in all patients who fail to exhibit an enhanced responsiveness to ristocetin. On the basis of these results and through a series of illustrative examples, the clinician will be able to select the best approach for the optimal management of VWD, according to the patient's characteristics and clinical circumstances.
Introduction
The risk assessment and diagnosis of von Willebrand disease (VWD), which is probably the most frequent inherited bleeding disorder,1-3 remain difficult tasks because of the wide spectrum of clinical severity and genetic penetrance of the disease. Furthermore, because the present classification system does not accurately predict a person's response to desmopressin (1-deamino-8-D-arginine vasopressin), which is the principal therapeutic option in most cases, therapeutic choices also become difficult. Therefore, at least for the mildest forms, it remains unclear whether the benefits of a diagnosis outweigh its disadvantages. In other words, a VWD diagnosis should provide clear and univocal prognostic and therapeutic indications that are distinct from the burdens associated with an unjustified genetic disease stigma, anxiety that may be caused by overestimates of a patient's bleeding risk and inappropriate resource expenditures.4,5 Many good reviews are available, including a very recent one focusing on the pathophysiology of VWD.6-12 The recent publication of the results from several national and international multicenter studies have shed new light on the clinical characteristics, laboratory phenotype, and the genetic and molecular abnormalities of VWD, particularly for those cases that are mainly caused by partial quantitative defects of von Willebrand factor (VWF), which represent the majority of patients.13-16 These new data allow a sounder approach to VWD, and the conclusions have been partially embedded in the recent U.S. evidence-based guidelines.17 However, for many diagnostic and therapeutic aspects, no evidence-based approaches are available, with the result that an ample margin is still left to the experience of the individual clinician. Since the early 1970s, our department has had the unique opportunity to serve as a tertiary hemostasis center for a large pediatric and adult population. In this paper, we aim to present our approach to VWD management and to discuss this approach in the context of available evidence
A unified concept of the different VWD types based on its molecular pathophysiology
VWF is a nonenzymatic, multifunctional, and multimeric protein that acts as a bridging molecule between platelets and the vessel wall18,19 and as a carrier for plasma FVIII.
The main quantitative (type 1 and 3) or qualitative (type 2) forms of VWD20 can be predicted by appreciating the impacts that the different mutations in one or both VWF alleles may exert on the complex processes of VWF synthesis, assembly, and secretion.
Type 3 VWD, which is characterized by the presence of only trace amounts of VWF in plasma and platelets, is caused by compound heterozygous or homozygous frameshift or nonsense mutations or deletions that result in a null allele.21 Accordingly, type 3 VWD is a recessive disease with a very low expected prevalence (1-2 cases/million). Carriers of a single null allele, such as the parents of type 3 patients, have VWF levels approximately 50 IU/dL, which is usually not sufficient to cause a significant bleeding tendency.22
In the simplest case of type 1 VWD, a single missense mutation codes for an abnormal subunit that hampers the proper dimerization process. If both alleles produce the same number of subunits, which dimerize randomly, then wild-type homodimers would represent 25% of the total VWF synthesized. If only these wild-type homodimers contribute to multimer assembly, then the circulating level of VWF can be predicted to be 25% of normal, or approximately 25 IU/dL. Thus, a qualitative defect at the molecular level can appear as a purely quantitative defect at the plasma level. In such cases, the ratio between VWF levels measured either in terms of the antigen (VWF:Ag) or function (measured as ristocetin cofactor activity, VWF:RCo, a test that mimics the physiologic interaction between VWF and platelet GpIb) is approximately equal to unity, and the multimeric pattern is usually normal or exhibits minor abnormalities that are not usually detectable using standard methodologies.23 In more complex cases of type 1 VWD, variable penetrance and expressivity within the same family are the result of compound heterozygosity, such as the coinheritance of a missense mutation with a null mutation.24 In other cases, increased clearance of the secreted mutant multimers by still unknown mechanisms has been found. The consequent limited exposure to ADAMTS13-mediated proteolysis may allow the persistence of unusually large multimers, as suggested for VWD Vicenza.20
Patients with type 2 VWD, which represent 10% to 20% of all cases, usually have mutations that impair either specific functional domains or multimer assembly. Characteristically, they present a greatly reduced VWF:RCo/VWF:Ag ratio. In the more frequent type 2 (type 2A), impaired multimerization or increased susceptibility to ADAMTS13 causes a selective loss of the intermediate and large multimers, which is easily detectable with standard multimeric techniques and results in diminished activity of the binding domains for GpIb (and possibly for IIb/IIIa). In type 2B, gain-of-function mutations in the A2 domain cause an increased affinity of VWF for platelet GpIb, together with a lack of high-molecular-weight multimers. The multimeric pattern is normal in the rarer subtypes, which are caused by mutations of functional domains that selectively impair VWF:RCo activity (type 2M) or reduce the FVIII carrier activity (type 2N).
VWD diagnosis
Case 1
A 32-year-old man is evaluated because of bleeding after a tooth extraction, which requires suturing and the administration of tranexamic acid. He has a history of mild epistaxis that never required treatment. Laboratory evaluation discloses a VWF:Ag and VWF:RCo approximately 40 IU/dL.
Does the patient have a significant bleeding history?
The diagnosis of any bleeding disease should be prompted by the presence of bleeding symptoms, rather than by the incidental finding of an abnormal laboratory test result,25 and current diagnostic criteria for VWD also require the presence of bleeding symptoms.26 Unfortunately, there is no consensus about the number and severity of bleeding symptoms that define a significant bleeding history. This is mainly the result of the overlap of the number of reported hemorrhagic symptoms in VWD patients and in healthy controls,27-30 with at least one hemorrhagic symptom reported in up to 25% of normal subjects.5 One retrospective International Multicenter Study (IMS) evaluated the predictive value of the bleeding history using a physician-administered standard questionnaire (http://www.isth.org/LinkClick.aspx?fileticket = VIZW-fKaFnI%3d&tabid = 78)13 and proved that having at least 3 distinct hemorrhagic symptoms is a very specific diagnostic criterion for VWD (specificity, 99.5%; sensitivity, 50%). In our patient, only 2 symptoms are reported, with a specificity of only approximately 92%, according to the IMS findings. Although there is still a good chance that the patient may actually have a bleeding disorder, approximately 8% of normal subjects do report at least 2 hemorrhagic symptoms. To refine our judgment, we could try to use a quantitative bleeding score (BS), which would account not only for the number but also for the severity of the bleeding symptoms.13 The BS is the sum of the maximum severity assigned to each bleeding symptom that has been reported by a subject, graded on a scale ranging from 0 (no or trivial symptom) to 3 (Table 1). For our patient, the BS is 3, calculated by summing the epistaxis and postextraction bleeding score (scores of 1 and 2, respectively). In the IMS, a BS higher than 3 (5 for females) was considered “abnormal”; therefore, using the BS as guidance, we could likewise consider that our patient has a history of only mild bleeding.
Does this patient have significantly reduced VWF levels?
From a formal point of view, VWF is reduced when it is below the ABO blood group-adjusted 2.5th percentile,28,31 which broadly corresponds to 2 SDs below the population mean. In a European study of VWD families (the MCMDM-1VWD study), the 2.5th percentile computed in 1155 normal controls was 47 IU/dL for both the VWF antigen (VWF:Ag) and the VWF ristocetin cofactor (VWF:RCo), with O-blood group subjects reporting lower VWF levels (44 vs 51 IU/dL in non-O). However, these cut-offs are unsatisfactory for at least 2 reasons. First, 2.5% of normal subjects would be classified as possible VWD sufferers, which is a disturbing value given that the prevalence of clinically significant VWD is much lower.32,33 Second, the MCMDM-1VWD study has clearly shown that the likelihood of a VWF mutation increases only when VWF levels are less than 40 IU/dL, and particularly for values less than 20 IU/dL.34 Interestingly, the 40 IU/dL cut-off that was proposed by the MCMDM-1VWD study is actually lower than both of the ABO-adjusted reference limits, which thus negates the need for us to adopt ABO-specific reference limits. Conservatively, the threshold has been set to 30 IU/dL.17 In conclusion, our patient has reduced VWF levels that cannot be considered diagnostic for VWD. In these cases, it is our practice to screen for alternative diagnoses in patients with a significant history, including (at least in the case of prolonged bleeding time and/or PFA-100) evaluation of platelet disorders using aggregation and secretion studies because platelet function disorders may be as common as VWD.35,36 Assuming no other detectable defect, the patient probably has a disorder of unknown origin or may simply represent a very mild case of type 1 VWD, such as in those cases who lack a demonstrable mutation and/or linkage with the VWF gene.15,16,37
Could such a diagnosis be of benefit for the patient?
This patient may be classified as having type 1 VWD, but the benefit to him of such a diagnosis remains unclear. Our practice is to diagnose a patient as “having VWD” when he/she may be at risk of significant spontaneous or provoked bleeding, which thus makes antihemorrhagic prophylaxis highly desirable. For this patient, a risk of relevant bleeding can be dismissed because he never experienced a major bleeding event over the course of 32 years. We would be more concerned if the same BS had been observed in a young boy. We also know that the more severe the VWF reduction, the more severe the bleeding history, particularly with VWF counts less than 20 IU/dL.14 Thus, it seems improbable that the patient would benefit from receiving a definite diagnosis of VWD that would require significant testing of the patient and his family members. Thus, it is our practice to only warn these patients against inappropriate use of antiplatelet drugs, advise them about the use of antifibrinolytic drugs for invasive procedures or surgery on mucous tissues (eg, dental extraction), and offer consultations on an as-needed basis. Fine-needle epidural anesthesia should be preferred to the use of catheter delivery. Heparin prophylaxis should not be contraindicated for high-risk surgery (eg, knee or hip replacement) in these patients. We also think that offering more detailed clinical and laboratory investigations in these patients may lead to an inappropriate allocation of health resources.
Could a Bayesian approach be useful?
We acknowledge that the patient's preferences and values should also be considered and that such factors may influence these cost/benefit considerations. For instance, at least in affluent societies, there may be considerable pressure from patients and health providers to issue a definitive diagnosis. Recently, we developed a Bayesian model to better appreciate the level of uncertainty in situations similar to this case.38 Under this approach, the bleeding history in the patient (summarized in the BS) and VWF measurements in the patient and in as many first-degree relatives as possible are translated into likelihood ratios, thereby producing a “final probability” of having VWD. Because we do not have a “gold standard” for diagnosing clinically significant VWD, at time of the development of our model, we provisionally assumed that cases with proven autosomal dominant hemorrhagic disease should be taken as the VWD paradigm.38 Thus, our model quantifies the probability with which a patient fits this model, which, in this patient, is estimated as 77% (or odds of 3.5). According to this model, the identification of additional family members with reduced VWF greatly increases the final VWD probabilities in borderline patients, and it could be particularly useful when the patient is too young to have experienced significant hemostatic challenges. For instance, if our patient had 2 other siblings and one of them exhibited VWF less than 40 IU/dL, then his final VWD probability would be raised to 97% (or 35.5 expressed as odds).
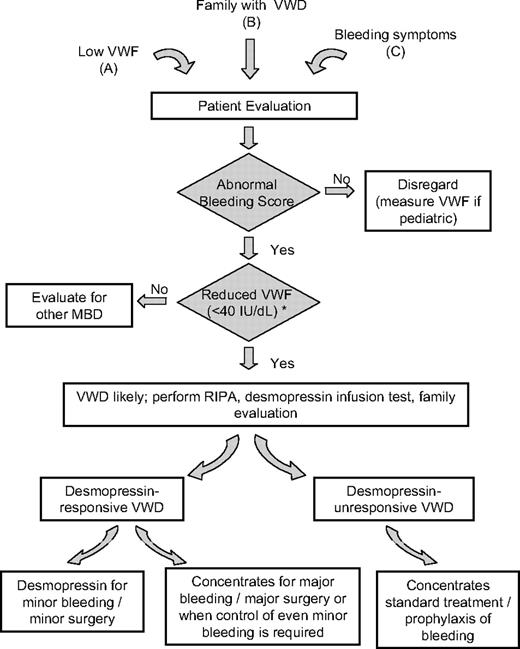
At present, we do not know the probability cut-off that best discriminates those patients who may benefit from receiving a diagnosis from those who may not, and prospective studies on VWD patients who are diagnosed with graded criteria are needed to confirm a prognostic significance in the context of the diagnosis. At our center, however, we do not label patients as “definite VWD” when the final odds are lower than 5.0 in the Bayesian model; and, in such patients, we rely on practical advice as explained previously. This practice is supported by the finding that withholding a diagnosis in less clear-cut cases does not cause significant harm to the patient.13 This approach may help avoid a large number of healthy subjects or subjects with a very mild bleeding disorder other than VWD being labeled as affected (Figure 1).
Diagnostic and therapeutic flowchart for VWD. The finding of low VWF (A), the confirmation in a family member with established VWD (B), or the evaluation of bleeding symptoms of unknown origin (C) demands a diagnostic process for VWD only when both the bleeding score is significantly elevated and the VWF:Ag or VWF:RCo levels are less than 40 IU/dL (either confirmed or consistently reported in patient clinical records). For children and young subjects with few historical hemostatic challenges, the requirement for a high bleeding score is less stringent. VWD type 2N may only be apparent with a reduced FVIII:C level. Once VWD is confirmed, if not already available in a related family member, a desmopressin trial infusion should be performed in all cases in which RIPA is not enhanced. Type-specific diagnosis is not required for clinical practice. Desmopressin should be the first treatment of choice for either treatment or prophylaxis of any minor spontaneous, traumatic, or surgical bleeding or delivery. Home treatment is feasible for selected patients. Replacement therapy should be used for major bleeding or major surgery in desmopressin-responsive patients and for all nontrivial bleeding symptoms that fail local or ancillary interventions in desmopressin-unresponsive VWD patients (or those with enhanced RIPA). *Discrepantly reduced FVIII may suggest type 2N VWD.
Diagnostic and therapeutic flowchart for VWD. The finding of low VWF (A), the confirmation in a family member with established VWD (B), or the evaluation of bleeding symptoms of unknown origin (C) demands a diagnostic process for VWD only when both the bleeding score is significantly elevated and the VWF:Ag or VWF:RCo levels are less than 40 IU/dL (either confirmed or consistently reported in patient clinical records). For children and young subjects with few historical hemostatic challenges, the requirement for a high bleeding score is less stringent. VWD type 2N may only be apparent with a reduced FVIII:C level. Once VWD is confirmed, if not already available in a related family member, a desmopressin trial infusion should be performed in all cases in which RIPA is not enhanced. Type-specific diagnosis is not required for clinical practice. Desmopressin should be the first treatment of choice for either treatment or prophylaxis of any minor spontaneous, traumatic, or surgical bleeding or delivery. Home treatment is feasible for selected patients. Replacement therapy should be used for major bleeding or major surgery in desmopressin-responsive patients and for all nontrivial bleeding symptoms that fail local or ancillary interventions in desmopressin-unresponsive VWD patients (or those with enhanced RIPA). *Discrepantly reduced FVIII may suggest type 2N VWD.
Case 2
A 37-year-old woman is referred with a diagnosis of VWD and a history of iron-deficient anemia resulting from menorrhagia. She and her 12-year-old daughter exhibit reduced VWF:RCo (15 IU/dL) with discrepant VWF:Ag (42 IU/dL). Browsing the Internet, she has been confused by the many subtypes of VWD, and she questions their relevance in the context of the best management of bleedings after menarche in her daughter.
What further tests are needed for this patient?
Finding a discrepant reduction between a VWF functional measurement (VWF:RCo) and its antigenic concentration (VWF:Ag) is suggestive for the presence of a qualitative deficiency (type 2). Although there is no formal consensus on the VWF:RCo/VWF:Ag ratio that best identifies patients with type 2 VWD, we conservatively consider a ratio less than 0.6 as abnormal.39 In patients with suspected type 2 VWD, we routinely measure FVIII:C levels and test for ristocetin-induced platelet aggregation (RIPA) at a final concentration of 0.5 and 1 mg/dL, but we do not routinely perform measurements of other VWF activities, such as the functionality of the collagen binding domain, the multimeric assay, or molecular testing. Assessing the VWF/collagen interaction requires a specific testing (VWF:CBA); but, to date, cases with isolated defects in this binding function have not been sufficiently demonstrated and the VWF:CBA assay is of little clinical utility. Enhanced RIPA suggests type 2B VWD. A discrepant FVIII:C to VWF:Ag ratio (< 0.7) raises the suspicion of the Normandy variant (type 2N). RIPA and FVIII:C are easily available even in a nonspecialized laboratory, whereas more specific tests, such as binding studies of FVIII to patient plasma or the multimeric assay, would require a specific commitment of the laboratory to VWD diagnosis. Furthermore, other than identification of type 2B VWD, there is no evidence that a more finely tuned VWD diagnosis could improve the clinical management of VWD. In patients with suspected type 2 VWD and without hypersensitivity to ristocetin, we regularly perform a desmopressin trial infusion (“Case 3”). Response to desmopressin cannot be accurately predicted by the multimeric pattern and/or by genotype analysis.39 Consequently, patients should always be tested with an in vivo trial rather than by other tests. Thus, in our patient and her daughter, we would perform FVIII:C, platelet aggregation to ristocetin at low concentration, and a desmopressin trial infusion, after obtaining informed consent from both persons. Additional hemostatic defects should be examined in VWD patients under certain circumstances, particularly when the patient has a bleeding history that is significantly different from other affected family members. In such cases, evaluation of platelet function by aggregometry and measurement of other clotting factors, such as FXI, may be useful. Use of global tests, such as PFA-100 or Impact-R, is not useful to detect platelet abnormalities because these tests are usually abnormal in VWD patients.40
Treatment of VWD
Case 3
A 21-year-old man with type 1 VWD requires a tooth extraction. His basal FVIII:C and VWF levels are moderately reduced (FVIII:C 35 IU/dL; VWF:Ag 24 IU/dL; VWF:RCo 22 IU/dL). During his lifetime, he had suffered only minor spontaneous hemorrhages that were controlled with oral tranexamic acid or local measures. He has not undergone any invasive procedures and had never been treated with desmopressin. A trial infusion with the compound was carried out; and, 1 hour after infusion, his FVIII:C and VWF levels were largely corrected (FVIII:C 118 IU/dL; VWF:Ag 96 IU/dL; VWF:RCo 89 IU/dL) and remained above 50 IU/dL after 6 hours.
How should dental extraction or minor surgery be managed in this patient?
The goal of the treatment of VWD is the correction of the low FVIII:C and of the low VWF. Clinical experience indicates that FVIII level is the best predictor of soft tissue or surgical bleeding, whereas VWF:RCo normalization is required for an appropriate treatment of mucosal bleeding. Desmopressin and transfusion therapy with blood products represent the 2 treatments of choice in VWD41 (Figure 1). Other forms of treatment can be considered as adjunctive or as an alternative to these 2 modalities.
Desmopressin is a synthetic analog of vasopressin that elevates FVIII and VWF plasma concentrations in most patients with mild hemophilia A and VWD. It is relatively inexpensive, carries no risk of transmitting blood-borne viruses, and can be used for home treatment. Desmopressin is usually administered at a dose of 0.3 μg/kg body weight by a subcutaneous or intravenous route or at a fixed dose of 150 to 300 μg by a metered-dose intranasal spray. To test patient responsiveness, a therapeutic dose of desmopressin is administered, and VWF:Ag, VWF:RCo, and FVIII:C are measured at baseline and after 60, 120, and 240 minutes. We define patients as “desmopressin responders” when they exhibit a peak level for both VWF:RCo and FVIII:C of more than 50 IU/dL, which is a concentration that may be considered sufficient for effective hemostasis during minor invasive procedures.39 Assessment at 4 hours from infusion is advised to identify patients with increased clearance who are possible candidates for alternative treatments.39,41-43 However, patients with VWD Vicenza, which is associated with the R1205H mutation, and who show the shortest half-life of FVIII and VWF after desmopressin, can be effectively treated with desmopressin for most instances, including parturition.43,44 Patients with type 3 VWD are usually unresponsive to desmopressin, although some patients do experience an increase of FVIII:C to effective hemostatic levels, even without a change in the bleeding time (BT).45 The compound is also acceptable to Jehovah's Witnesses. Because the responses in a given patient and within his/her family are consistent on different occasions, a test dose of desmopressin administered at the time of diagnosis will allow for future treatment plans to be drawn up.
Therapeutic infusions can be repeated every 8 to 24 hours, depending on the type and severity of the bleeding episode.46 Tachyphylaxis may ensue after 3 or 4 infusions, so FVIII and VWF measurements should be monitored to confirm a continuing response. Furthermore, significant fluid retention and hyponatremia may occur, which requires body weight and serum electrolyte control, particularly in children younger than 2 years. Other side effects are mild and may include tachycardia, headache, and flushing. The drug should be used with caution in elderly patients with atherosclerotic disease or in the presence of cardiovascular disorders because a few cases of myocardial infarction and stroke have occurred in hemophiliacs and uremic patients given desmopressin.47
The present case proved to be responsive to the desmopressin trial infusion. For dental procedures, we usually administer a single desmopressin subcutaneous (or intravenous) injection of the concentrated formulation. Oral tranexamic acid (15-25 mg/kg body weight 3 times a day) is usually also given for 5 to 7 days. Tranexamic mouthwash (as 5% wt/vol water solution every 6 hours for 5-7 days) is an effective and useful alternative. In the case of minor surgery, a few additional desmopressin infusions at 8 to 12 intervals should be considered.
Case 4
A 49-year-old woman with type 3 VWD requires a hysterectomy. Her basal FVIII:C and VWF levels were both less than 3 IU/dL. She had been treated in the past on several occasions for epistaxis, menorrhagia, and posttraumatic knee hemarthrosis with plasma-derived FVIII and VWF concentrates.
How should this patient be scheduled for surgery?
Type 3 VWD is unresponsive to desmopressin. Replacement therapy with blood products containing FVIII and VWF, which were originally developed for hemophilia A, is the treatment of choice for type 3, type 2B, and all desmopressin-unresponsive cases.41 Replacement therapy is also required for patients responsive to desmopressin who are undergoing major surgery or procedures with a high bleeding risk or in which even minor bleeding should be avoided, such as spinal surgery. Commercially available concentrates feature a variable content of FVIII and VWF; thus, different levels are obtained after infusion. One should prefer those concentrates that are labeled for both VWF (VWF:RCo) and FVIII (FVIII:C) content and that have a VWF/FVIII ratio equal or higher than 1. Accepting that not all concentrates are labeled for both VWF and FVIII content and that FVIII:C more reliably predicts delayed bleeding, we advise to base substitution schedules on FVIII content and to daily monitor FVIII:C during the first 5 to 7 days after major surgery to maintain its level above 50 IU/dL. VWF:RCo level monitoring is not strictly required, and it is not our common practice. In our case, a loading dose of 50 U/kg FVIII was given 1 hour before surgery, followed by a similar daily dose for the next 2 days. On the third day, the pre-infusion FVIII:C level was 180 IU/dL and 4 infusions were administered on alternate days. The daily FVIII:C measurement did not exceed 150 IU/dL. Prophylaxis with low-molecular-weight heparin commenced 2 hours after surgery and continued for 10 days.
High postinfusion levels of VWF are consistently obtained with plasma-derived FVIII concentrates.48 Furthermore, an FVIII increment lasting for up to 24 hours and at a level much higher than predicted is regularly observed because of the stabilizing effect of exogenous VWF on endogenous FVIII, which is synthesized at a normal rate in these patients. High levels of postinfusion FVIII:C have recently been associated with episodes of deep vein thrombosis reported in VWD patients who have received repeated infusions of FVIII/VWF concentrates after surgery.49 Recombinant FVIII or concentrates obtained by immunoaffinity chromatography (FVIII > 2000 IU/mg) contain no or very small amounts of VWF and are therefore unsuitable. However, in those rare patients with type 3 VWD resulting from homozygous gene deletions or nonsense mutations who develop alloantibodies with the risk of life-threatening anaphylaxis, these concentrates remain the first therapeutic option.9 Exceptionally, recombinant activated factor VII could be used.50
Recently, a very high purity VWF concentrate, which is not yet available in North America, proved clinically effective in a small cohort of type 3 VWD cases. Its very low FVIII content requires its infusion 6 to 8 hours before surgery to allow the infused VWF to stabilize the endogenously synthesized FVIII. Therefore, this concentrate is unsuitable for the treatment of acute bleeding episodes and for emergency surgeries, but it does offer the advantage of predictable FVIII increments while avoiding exceedingly high FVIII levels. Recombinant VWF concentrates are currently under development. The dosages of concentrates that are recommended for the control of bleeding episodes are summarized in Table 2.
These products are not always effective in correcting the BT48 because they do not contain a completely functional VWF, as tested in vitro by evaluating the multimeric pattern because VWF proteolysis occurs during purification. Nevertheless, several retrospective studies have shown excellent or good hemostasis in almost all cases of surgery and in the context of acute spontaneous bleeding.41 A recent prospective study evaluated the choice of doses through a careful pharmacokinetic analysis of 29 cases with VWD who were undergoing surgery.51 This study demonstrated a constant incremental recovery over a wide range of doses of VWF/FVIII concentrate (dose linearity of the infused dose with postinfusion increment), which suggested that the pretreatment pharmacokinetic results may be used to determine the treatment plan.
For the rare cases in which bleeding persists or occurs despite adequate replacement therapy, other therapeutic options are available. Desmopressin, given after cryoprecipitate, further shortens or normalizes the BT in patients with type 3 VWD for whom cryoprecipitate failed to correct the BT.52 Platelet concentrates (administered before or after cryoprecipitate, at doses of 4-5 × 1011 platelets) have achieved similar effects in patients who are unresponsive to cryoprecipitate alone, both in terms of BT correction and bleeding control.53
Case 5
A 16-year-old girl is referred for evaluation of menorrhagia. Since menarche at age 11, she has suffered from heavy menses, which were empirically treated with oral tranexamic acid. No gynecologic abnormalities were identified. She also reported easy bruising and a few episodes of epistaxis. Laboratory evaluation performed elsewhere showed slightly reduced FVIII:C and VWF levels (FVIII:C 38 IU/dL, VWF:Ag 33 IU/dL, VWF:RCo 35 IU/dL) and microcytic anemia, which was being treated with iron supplements.
What is the best management for this patient?
Menorrhagia may occur in approximately 10% of women.54 It is subjectively reported as excessive menstrual blood loss, but the current definition of menorrhagia requires a menstrual blood loss of more than 80 mL. To avoid troublesome measurements, a pictorial chart for the semiquantitative assessment of blood loss has recently been adopted, with reasonable sensitivity and specificity.55 In this patient, the coexistence of iron-deficient anemia was thought to be sufficient evidence for a clinically relevant menorrhagia and would have been assigned a score of 2. Menorrhagia may be the most prominent manifestation of a congenital bleeding disorder.56,57 For example, a primary coagulation defect was found in 20% of adolescents who presented with menorrhagia.58 In some studies, up to 20% of women with menorrhagia turned out to have mild VWD.59 In a recent study that included 150 women with objectively confirmed menorrhagia but without anatomic or hormonal causes, 14% had mild VWD and 3% had mild FXI deficiency.56 Using a standardized questionnaire, 2 recent studies reported menorrhagia in up to 70% of women with VWD who had more severe symptoms in VWD compared with age-matched controls. To avoid an exceedingly high number of false-positive VWD diagnoses despite these data, we do not routinely investigate women with isolated menorrhagia for VWD unless accompanying bleeding symptoms, as in this case, yield a significantly elevated bleeding score (> 5 in women).25
Treatment options are similar to those adopted in women without a bleeding disorder, with the exception of using desmopressin or a replacement therapy. In general terms, it seems preferable to avoid combined oral contraceptives at menarche or in young girls if they respond to antifibrinolytics, such as oral tranexamic acid (15-25 mg/kg body weight) given 3 times a day for 4 to 5 days. Oral contraception may be preferred for older persons. Recently, a levonorgestrel-releasing intrauterine device (trade name: Mirena) proved efficacious and safe in primary menorrhagia, and it also seems promising in women with bleeding disorders,60 although it would be preferred for older women. Desmopressin has been successfully used as a concentrated formulation for subcutaneous home-treatment administration, with an effective response in 86% of 43 self-treated cycles.61 A similar value (88%) was obtained using the intranasal spray formulation,62 even though a crossover trial failed to demonstrate a substantial benefit with the use of desmopressin.63
In the present patient, tranexamic acid (15 mg/kg, 3 times a day during the entire menstrual period) was scarcely effective and produced abdominal discomfort. Combined oral contraceptive was suggested as a second choice, but the patient refused. Home treatment with subcutaneous desmopressin injection every 12 hours on the first day of menses and repeated once 24 hours later proved sufficient to significantly reduce the blood loss. After 6 months of therapy with ferrous sulfate tablets, her hemoglobin level rose to 13.5 g/dL and her mean corpuscular value to 81 fL. Reassessment a year later showed normal hemoglobin and ferritin.
Case 6
A 25-year-old woman with unclassified von Willebrand disease asks for advice because she is 12 weeks into her first pregnancy. Her basal FVIII:C and VWF measurements are significantly reduced (FVIII:C 30 IU/dL, VWF:Ag 33 IU/dL, VWF:RCo 28 IU/dL).
How should her pregnancy and parturition be managed?
Without antihemorrhagic prophylaxis, approximately 30% of pregnant women with VWD exhibit postpartum hemorrhage compared with approximately 5% in the general population. In patients with VWD type 1, the levels of VWF and FVIII rise 2- to 3-fold during the second and third trimester but fall to baseline levels after delivery. Patients with the frequent VWD Vicenza and C1130F mutations show only a slight increase of these moieties during pregnancy, with the result that treatment with desmopressin is required at delivery.44,64 Patients with type 2N, which is associated with the common R854Q mutation, show a complete normalization of FVIII:C, and no treatment is usually required.65 In VWD type 2B, elevated abnormal VWF can cause or worsen thrombocytopenia. In general, VWD patients should be monitored for VWF:RCo and FVIII:C levels at least during the third trimester of pregnancy and within 10 days of the expected delivery date. The risk of bleeding is very small when FVIII:C and VWF:RCo levels are more than or equal to 50 IU/dL, which also is the minimum level that should be achieved for a safe spinal or epidural anesthesia.66,67 In type 1 VWD pregnant women with FVIII:C levels lower than 50 IU/dL, desmopressin on the day of parturition and for a couple of days thereafter is advisable. To prevent late bleeding, VWF:RCo and FVIII:C levels should be tested, and the women should be monitored clinically for at least 2 weeks postpartum. Generally, no treatment with FVIII/VWF concentrates is required for parturition, and there is no indication for a mandatory cesarean section. In type 3 VWD women, VWF and FVIII do not increase during pregnancy; thus, VWF/FVIII concentrates are required in the context of delivery, epidural anesthesia, or cesarean section. The latter should be reserved only for the usual obstetric indications. There is no apparent increased bleeding risk for neonates with VWD, but for fetuses at risk of type 2 and 3 VWD invasive monitoring techniques or vacuum extraction and forceps use at delivery should be preferably avoided.68 Prenatal diagnosis is required only in selected families with type 3 VWD.
In the present case, FVIII:C and VWF measurements were retested at the beginning of the ninth month and were found to be greater than 100 IU/dL and vaginal delivery was uneventful.
Case 7
A previously asymptomatic 15-year-old boy developed a large hematoma after a football game. A slightly prolonged activated partial thromboplastin time was observed. At the time of referral, his basal FVIII:C level was 17 IU/dL, and his VWF measurements were normal. A tentative diagnosis of mild hemophilia A was made, and the boy was successfully treated with 2 desmopressin infusions over a 24-hour interval. A FVIII:C level of 85 IU/dL was observed 1 hour after the first administration. Family investigation disclosed borderline FVIII:C levels in the parents. The younger sister, reporting some minor episodes of epistaxis, had a remarkably similar FVIII:C deficiency (FVIII:C 21 IU/dL).
Should VWD be suspected?
Type 2N VWD should be considered in a case of autosomal recessive inheritance with disproportionately low FVIII:C levels compared with VWF levels (ratio < 0.5). To definitely prove type 2N, a FVIII-VWF binding assay is required. A marked abnormality was observed in the boy and his sister (VWF:FVIIIB 10 IU/dL), and borderline values were present in the parents. VWF gene mutation screening of the D′ and D3 domains revealed that the patient and his sister were homozygotes for the R854Q mutation.
The correct identification of this type of VWD is important for genetic counseling, as advice differs from that offered to hemophilia A patients. The R854Q change at the amino-terminus of the VWF subunit is by far the most frequent mutation associated with type 2N. Although it can be present in up 2% of the general population, simple heterozygotes hardly bleed, whereas a bleeding tendency is definitely present in homozygotes or compound heterozygotes. Desmopressin induces a good FVIII:C increment in all patients with R854Q, even though the half-life may be reduced.43,69 FVIII/VWF concentrates should be reserved only for those rare mutations that fail to exhibit a significant FVIII:C increase after desmopressin treatment.69,70 A desmopressin test infusion is thus also advised in type 2N patients.
Conclusions
VWD continues to be the subject of intense investigation to fully elucidate its complex pathogenesis and its relationships with the different phenotypes. Therefore, an accurate classification of the different types is of paramount importance and should be developed in parallel with the increased knowledge of VWF structure and function in health and disease. However, the clinician requires a simplified approach to the patient. With this goal in mind, we have tried to delineate a practical approach that is sufficient to translate our current understanding of the disease into broadly applicable operational steps (Figure 1). These steps should provide reliable indications to individualize the optimal management of the patient with the most effective treatment plan. The chosen approach can vary from no treatment at all, conservative measures such as antifibrinolytics, desmopressin infusion, or substitutive treatment.
Authorship
Contribution: F.R., G.C., and A.T. wrote the paper and gave their consent to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Rodeghiero, Department of Cell Therapy and Hematology San Bortolo Hospital, Viale Rodolfi 37, 36100 Vicenza, Italy; e-mail: rodeghiero@hemato.ven.it.