Abstract
Deep venous valves are frequent sites of deep venous thrombosis initiation. However, the possible contribution of the valvular sinus endothelium has received little attention in studies of thrombosis risk. We hypothesized that the endothelium of valve sinus differs from that of vein lumen with up-regulation of anticoagulant and down-regulation of procoagulant activities in response to the local environment. In pursuit of this hypothesis, we quantified endothelial protein C receptor (EPCR), thrombomodulin (TM), and von Willebrand factor (VWF) by immunofluorescence in great saphenous veins harvested at cardiac bypass surgery. We found significantly increased expression of EPCR and TM in the valvular sinus endothelium as opposed to the vein lumenal endothelium, and the opposite pattern with VWF (paired t test for TM and EPCR, each P < .001; for VWF, P = .01). These data support our hypothesis and suggest that variation in valvular sinus thromboresistance may be an important factor in venous thrombogenesis.
Introduction
Direct evidence from autopsy studies and phlebography, as well as circumstantial evidence such as the correlation between frequency of deep venous thrombosis and the number of valves in individuals, have established the venous valvular sinus as a frequent location of thrombosis initiation.1-4 This phenomenon has been attributed to stasis, one of the components of Virchow triad. In the 1960s, contrast media was shown to linger in valve sinuses an average of 27 minutes postvenography.5 Valvular sinus stasis has also been associated with hypoxia and increased hematocrit,6 as well as preventing the efflux of activated clotting factors and influx of clotting factor inhibitors, constituting a potentially hypercoagulable microenvironment, another component of Virchow triad. Stasis alone, however, is not a sufficient explanation for the propensity of thrombi to form in deep venous valve sinuses because, for example, prolonged periods of stasis associated with sleep are not associated with thrombus formation. Accordingly, there must be other factors interacting with stasis in the generation of venous valvular thrombi
In recent years, attention has been focused on the importance of endothelial heterogeneity in different vascular beds.7,8 Venous endothelium manifests multiple distinct phenotypes in different organs such as the kidney and liver. Gene expression microarray studies have shown that endothelial cells from macro- versus microvascular beds, from arteries versus veins, and from different organs have distinctly different and characteristic gene expression profiles.9 In response to local changes in flow, shear stress, or oxygenation, endothelial cells often adapt by increasing or decreasing expression of critical cell-surface and cytoplasmic proteins.10 Thus, we hypothesized that valvular sinus endothelium would maintain a thromboresistant phenotype with the expression of the anticoagulant proteins thrombomodulin (TM) and endothelial protein C receptor (EPCR) up-regulated and the procoagulant protein von Willebrand factor (VWF) down-regulated, compared with vein lumen endothelium.
Methods
Eleven great saphenous veins were collected from eleven patients undergoing coronary artery bypass grafting at Fletcher Allen Health Care (Burlington, VT). The mean age of the patients was 71 years (SD = 9; range =51-81), and 55% were male. This study was reviewed and approved by the Human Experimentation Committee of the University of Vermont, and informed consent was obtained in accordance with the Declaration of Helsinki. Immediately following surgical removal, the specimens were gently perfused in a retrograde fashion with the donor's heparinized blood and then immersed in heparinized donor blood in a bowl on the surgical tray. All harvested veins were received for processing within 3 to 4 hours of harvest. Each specimen was opened longitudinally, pinned to a cork board, and fixed in 10% neutral buffered formalin for 24 hours. The fixed specimens were trisected longitudinally and embedded on edge in paraffin. Hematoxylin and eosin stained slides were prepared in the Fletcher Allen Health Care routine histology laboratory.
Tissue sections 5 μm thick were cut for immunofluorescence from each paraffin block, including both valvular and vein lumen wall. The tissue sections were subjected to routine processing for immunofluorescence, including deparaffinization, rehydration, and antigen retrieval with 10 mM sodium citrate. Slides were then blocked in preimmune donkey serum for 1 hour at room temperature in preparation for immunofluorescence staining. The following primary IgG antibodies were used for an overnight incubation at 4°C: anti-TM (mouse clone no. 1009; Lab Vision); anti-EPCR (goat, AF2245; R&D Systems); and anti-VWF (rabbit, A0082; DAKO). Secondary antibodies were incubated 1 hour at room temperature: donkey anti–rabbit Alexa 488, donkey anti–goat Alexa 555, and donkey anti–mouse Alexa 647 (Invitrogen; see supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Nuclei were visualized with 4′, 6-diamidino-2-phenylindole hydrochloride (DAPI). A secondary negative control was used to check for the nonspecific binding of the secondary antibodies. For this control, slides previously treated with preimmune donkey serum for a routine blocking step were left in serum during primary antibody incubation. A cocktail of species-appropriate fluorescence conjugated secondary antibodies was then added to the slides, including donkey anti–mouse Alexa 647, donkey anti–rabbit Alexa 488, and donkey anti–goat Alexa 555 (Invitrogen). No immunoflourescence labeling of these slides was observed.
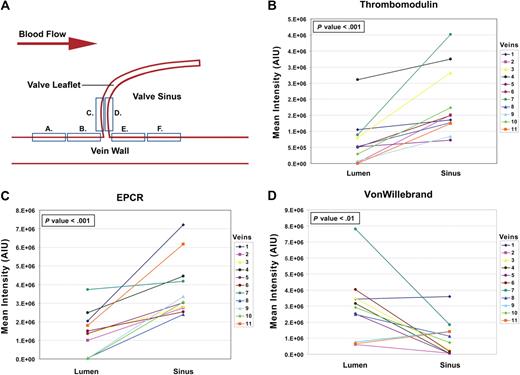
Immunostained slides were imaged with a Zeiss LSM 510 META confocal laser scanning microscope (Zeiss MicroImaging). A Plan-Neofluar 25×/0.8 NA multi-immersion objective lens was used for quantitative analysis. All images were acquired as 12-bit in multitrack mode, with appropriate laser excitation and emission filters as previously described.11 To capture confocal images for quantitative analysis, the detector gain was adjusted for each specimen so that individual fluorophore channels had only one pixel of saturation for each of the 3 antibodies. Conversely, amplifier gain was held constant for all antibodies. The detector gain adjustment was necessary to avoid unquantifiable pixel saturation resulting from the variable staining intensities observed between the patient samples. The detector gain adjustment was held constant for each specimen allowing for intraspecimen comparisons of valvular sinus versus vein luminal endothelium. However, between specimen differences in gain settings prevented the direct comparison of pixel intensities for the same antibody among the different patient samples. Detector gain settings were determined using the valve images from areas C and D in Figure 1A, then held constant throughout all other representative areas. Images were analyzed using MetaMorph Imaging Series 7.1 image analysis software (Molecular Devices) as described in supplemental data. For each factor the difference in arbitrary intensity units between the mean of areas A through C on the vein lumen and the mean of areas D-F in the valvular sinus was tested using a paired t test.
Diagrammatic representation of the differences of the means measured on the valvular versus the nonvalvular vein endothelium. (A) Diagram showing the locations of the endothelial confocal measurements made on the vein lumenal surface and in the valve sinus. (B-D) Graphic representations of the differences in mean intensity between the vein lumenal endothelium (combined areas A-C) and the valvular sinus endothelium (combined areas D-F) for EPCR, TM, and VWF, respectively. Paired t tests were used to test the differences in the means for each vein (see supplemental data and supplemental Table 1 with supporting data for this figure).
Diagrammatic representation of the differences of the means measured on the valvular versus the nonvalvular vein endothelium. (A) Diagram showing the locations of the endothelial confocal measurements made on the vein lumenal surface and in the valve sinus. (B-D) Graphic representations of the differences in mean intensity between the vein lumenal endothelium (combined areas A-C) and the valvular sinus endothelium (combined areas D-F) for EPCR, TM, and VWF, respectively. Paired t tests were used to test the differences in the means for each vein (see supplemental data and supplemental Table 1 with supporting data for this figure).
Results and discussion
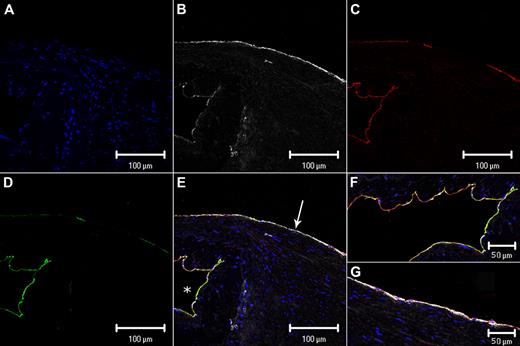
Each image was split into separate fluorophore channels to visualize each antibody separately. The split images in Figure 2 show representative immunofluorescence staining of a saphenous vein valve and luminal endothelium. The differences between the lumenal and valvular sinus endothelium are visually evident. EPCR and TM expression is higher in the sinus endothelium than the vein lumenal endothelium, and conversely VWF expression was lower in the sinus endothelium compared with the vein lumenal endothelium. Changes in the quantitative mean intensity scores for each of the 11 specimens, comparing vein lumen to valvular sinus endothelium, are shown graphically in Figure 1B-D. EPCR and TM mean intensity values are higher (P < .001) in the valvular sinus compared with vein lumenal endothelium, and VWF mean intensity is higher (P = .01) in the lumenal compared with the valvular sinus endothelium (see supplemental data and supplemental Table 1 with supporting data for Figure 1). These data support the hypothesis that the valvular sinus endothelium has a more thromboresistant phenotype compared with the vein lumen endothelium.
Split and merged confocal images of a representative venous valve and vein wall. (A) 4′,6-diamidino-2-phenylindole hydrochloride (blue) binds to DNA; (B) VWF (white); (C) EPCR (red); (D) TM (green); (E) merged image (the overlap of red and green fluorophores is perceived as yellow); (F) higher magnification of the merged image of the valve sinus and (G) higher magnification of the merged image of the vein lumenal wall just distal to the valve images in A-D. (F-G) Images were inserted to include an extended area of both the valvular sinus endothelium and the nonvalvular endothelium distal to the valve (see supplemental data). White arrow in panel E indicates vein luminal endothelium; and white asterisk, valve sinus. Scale bars indicate 100 μm (A-E) and 50 μm (F-G).
Split and merged confocal images of a representative venous valve and vein wall. (A) 4′,6-diamidino-2-phenylindole hydrochloride (blue) binds to DNA; (B) VWF (white); (C) EPCR (red); (D) TM (green); (E) merged image (the overlap of red and green fluorophores is perceived as yellow); (F) higher magnification of the merged image of the valve sinus and (G) higher magnification of the merged image of the vein lumenal wall just distal to the valve images in A-D. (F-G) Images were inserted to include an extended area of both the valvular sinus endothelium and the nonvalvular endothelium distal to the valve (see supplemental data). White arrow in panel E indicates vein luminal endothelium; and white asterisk, valve sinus. Scale bars indicate 100 μm (A-E) and 50 μm (F-G).
EPCR and TM facilitate the activation of protein C to activated protein C (APC) by thrombin, which proteolytically inactivates factors Va and VIIIa. In addition to this well-known anticoagulant effect, APC bound to EPCR has increasingly appreciated anti-inflammatory and cytoprotective effects.12-16 Inflammation has been shown to play a role in the initiation, propagation, and resolution of venous thrombosis.17-20 Thus, the anti-inflammatory effects of APC are likely important components of endothelial thromboresistance. The cytoprotective effect of APC is mediated by both an antiapoptotic mechanism15 and by enhancement of endothelial barrier function. For example, thrombin-induced increase in endothelial permeability can be reversed by APC.21-23 These anti-inflammatory and cytoprotective effects of APC on the endothelium likely play an important role in the resistance of the valvular sinus to the combination of chronic stasis and cyclic hypoxia. Thus, interindividual or age-related differences in the thromboresistance profile of valvular sinus endothelium could modulate thrombosis risk.
Our observation of decreased expression of VWF in valvular sinus endothelium is intriguing. There are few studies of VWF expression in venous endothelium across different vascular beds. A recent immunohistochemical study of VWF in human tissues showed that VWF expression varies in the microvasculature but is expressed nearly ubiquitously in the larger vessels of specific organs.24 The deep venous system and other large arteries and veins are diffusely positive for VWF. Thus, it seems reasonable to view the venous valvular sinus as a unique microenvironment in some respects similar to the microenvironments present in other specific vascular beds. In the case of the venous valvular sinus, the microenvironment is conditioned by cyclic hypoxia and stasis. The observation of decreased VWF in the valvular sinus endothelium may be part of the explanation for the relative ineffectiveness of antiplatelet therapy in the prevention of deep venous thrombosis.
In conclusion, these preliminary data suggest for the first time that the procoagulant/anticoagulant balance differs significantly between the valvular sinus endothelium and vein lumen endothelium, with the valvular sinus endothelium shifted to a more thromboresistant phenotype (see supplemental data for limitations of the study). Variation in valvular sinus endothelial thromboresistance may be an important factor in venous thrombogenesis. It is intriguing to speculate that aging, a strong risk factor for venous thrombosis, could well play a role in modifying the thromboresistance phenotype of valvular sinus endothelium.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This project was supported by National Heart, Lung, and Blood Institute grant PO1-HL046703 and a Transatlantic Network for Excellence in Cardiovascular Research grant from the Fondation Leducq, Paris, France.
National Institutes of Health
Authorship
Contribution: E.G.B. and W.T. contributed equally to the design, execution, and writing of this work; M.P.W., D.J.T., and M.F.E. contributed to the immunohistochemistry and confocal microscopy; F.P.I. contributed the saphenous veins and was involved in the study design; P.W.C. performed the statistical analysis; C.T.E. was involved in the conceptual design, discussions during the study, and writing; and E.G.B. was responsible for and involved in all aspects of the study. All authors discussed the results and contributed to the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin Bovill, MD, Chairman of Pathology, Director of Clinical Laboratories FAHC, Department of Pathology, E203 Given Bldg, University of Vermont College of Medicine, Burlington, VT 05482; e-mail: Edwin.bovill@uvm.edu.
References
Author notes
*E.G.B. and W.T. are joint first authors.