In this issue of Blood, Gratacap and colleagues analyze the effects of the multikinase inhibitor dasatinib on platelets, helping to explain the occurrence of bleeding related to its use for CML patients resistant to or intolerant of imatinib.1 In addition, by virtue of careful experimentation using multiple functional platelet assays, these authors are paving the way for the development of kinase inhibitors as antithrombotic agents.
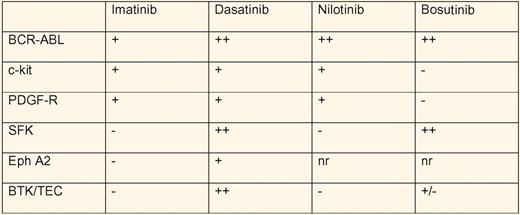
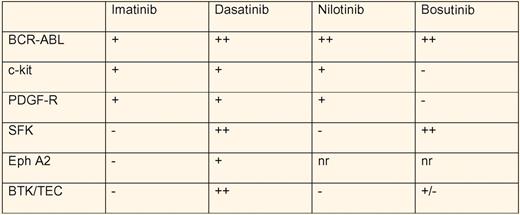
Dasatinib is one of the second generation BCR-ABL inhibitors, along with bosutinib and nilotinib,2,3 used to treat chronic myeloid leukemia (CML) patients resistant to or intolerant of imatinib. These ATP-competitive inhibitors share potent inhibition of BCR-ABL, but differ significantly in their spectrum of multikinase inhibition (see table).4,5 The fact that imatinib also inhibits the kinases c-kit and PDGF-R has been appreciated for some time and taken advantage of clinically. Both dasatinib and nilotinib inhibit c-kit and PDGF-R, while bosutinib does not. However, both dasatinib and bosutinib also inhibit src-family kinases (SFK). Although bleeding during dasatinib use had been reported in association with thrombocytopenia, Quintas-Cardama, Cortes, and colleagues noted dose-related internal and mucosal bleeding in the absence of thrombocytopenia.2,6 The bleeding could not be explained by concomitant use of antiplatelet or anticoagulant medications. They went on to show defective platelet function in the PFA-100 assay to epinephrine/collagen, but not ADP/collagen, when dasatinib was present at a concentration of 400 nM. Aggregation of platelets from CML patients receiving dasatinib was abnormal in response to arachidonic acid and epinephrine, but normal with low- and high-dose ADP, ristocetin, and high-dose collagen. Of note, imatinib use resulted in decreased aggregation in response to arachidonic acid only, while neither nilotinib-treated nor bosutinib-treated patients showed abnormal aggregation to any agonist tested.
Multikinase profile of the BCR-ABL inhibitors in CML treatment. − indicates no inhibition; +, moderate inhibition; ++, strong inhibition at clinically relevant concentrations; and nr, not reported.
Multikinase profile of the BCR-ABL inhibitors in CML treatment. − indicates no inhibition; +, moderate inhibition; ++, strong inhibition at clinically relevant concentrations; and nr, not reported.
It is in this context that the contribution by Gratacap, Payrastre, and colleagues can be examined further. These authors studied human platelet activation by thrombin, ADP, collagen (low- and intermediate-dose), and agonists of the FcγRIIa receptor in the presence of dasatinib. The potency of dasatinib, active at concentrations of less than 20 nM in inhibiting platelet activation via both collagen receptor GPVI/FcRγ and FcγRIIa, is striking. These receptors share the immunoreceptor tyrosine activation motif (ITAM) mode of activating platelets via SFKs and syk. Dasatinib affected thrombin-mediated activation only at low doses of thrombin, whereas imatinib did not affect any of the platelet functions tested. Indeed, thrombus volume was decreased by dasatinib but not imatinib in flow of either human or mouse blood over a collagen surface at arterial shear rates. Mice treated with dasatinib doses resulting in 100 to 150 nM plasma concentration of dasatinib, equivalent to human levels achieved with standard dosing, experienced significantly increased tail bleeding times. Finally, sera from patients with heparin-induced thrombocytopenia, which activates platelets in the presence of heparin via FcγRIIa, were unable to activate platelets pretreated with dasatinib.
Gratacap et al attribute the effects of dasatinib to its inhibition of SFKs, critical nonreceptor kinases directly involved in GPVI/FcRγ, and FcγRIIa activation signals, as well as in αIIb/β3 outside-in signaling. It will be interesting in future studies to compare bosutinib, which also inhibits SFKs but likely not platelet BTK or Eph kinases, with dasatinib to see how much of the platelet inhibition is specifically related to SFK inhibition. Their observations provide further proof of concept that platelet nonreceptor protein tyrosine kinases, such as the SFKs or syk7,8 may be excellent targets for therapy of atherothrombosis or heparin-induced thrombocytopenia and thrombosis.
Conflict-of-interest disclosure: S.E.M. and M.P.R. report receiving research support from Portola Pharmaceuticals. S.E.M. also reports receiving research support from NovoNordisk. ■