Abstract
To understand the mechanisms underlying 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE]–induced angiogenesis, we studied the role of Egr-1. 15(S)-HETE induced Egr-1 expression in a time-dependent manner in human dermal microvascular endothelial cells (HDMVECs). Blockade of Egr-1 via forced expression of its dominant-negative mutant attenuated 15(S)-HETE–induced HDMVEC migration and tube formation as well as Matrigel plug angiogenesis. 15(S)-HETE–induced Egr-1 expression requires Src activation. In addition, adenovirus-mediated expression of dominant-negative mutant of Src blocked 15(S)-HETE's effects on migration and tube formation of HDMVECs and Matrigel plug angiogenesis. 15(S)-HETE induced fibroblast growth factor-2 (FGF-2) expression rapidly via Src-mediated production of Egr-1. Cloning and mutational analysis of FGF-2 promoter revealed that Egr-1 binding site proximal to transcription start site is required for 15(S)-HETE–induced FGF-2 expression. Neutralizing antibody-mediated suppression of FGF-2 function also attenuated the effects of 15(S)-HETE on HDMVEC migration and tube formation as well as Matrigel plug angiogenesis. Furthermore, in contrast to wild-type mice, 12/15-LOX−/− mice exhibited decreased Matrigel plug angiogenesis in response to AA, which was rescued by 15(S)-HETE. On the basis of these observations, we conclude that 15(S)-HETE–induced angiogenesis requires Src-mediated Egr-1–dependent rapid induction of FGF-2. These findings may suggest that 15(S)-HETE could be a potential endogenous regulator of pathologic angiogenesis associated with atherosclerosis and restenosis.
Introduction
Between the two 15-lipoxygenases (15-LOXs), 15-LOX1 converts arachidonic acid (AA) majorly to 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE] and minorly to 12(S)-HETE.1,2 15-LOX2, however, metabolizes AA exclusively to 15(S)-HETE.2,3 Both 15-LOX1 and 15-LOX2 are present in humans.4 Opposite actions have been observed for 15-LOX1 and 15-LOX2 in influencing prostate cancer, the former being procarcinogenic.5-8 However, in these responses 12(S)-HETE mimicked 15-LOX1, whereas 15(S)-HETE exhibited effects similar to 15-LOX2.9-11 Specifically, although 15-LOX1-12(S)-HETE appears to exert procarcinogenic effects, 15-LOX2-15(S)-HETE exhibited proapoptotic actions in prostate carcinomas. However, in the case of colorectal carcinomas, 15-LOX1 induced apoptosis.12,13 It was demonstrated that 15-LOX mediates oxidation of low-density lipoprotein, a factor that appears to be crucial in the pathogenesis of atherosclerosis.14,15 In addition, atherosclerotic arteries upon incubation ex vivo converted AA mainly to 15-HETE.16 Knockout mice for 12/15-LOX, a murine ortholog of 15-LOX1, exhibited decreased atherosclerotic lesions in response to a high-fat diet and reduced neointima formation in response to injury.17,18 Conversely, overexpression of 15-LOX1 in vascular endothelium enhanced atherosclerosis in low-density lipoprotein receptor-deficient mice.19
These results suggest a role for 15-LOX1-15(S)-HETE/12/15-LOX-12(S)-HETE axis in vascular diseases. In regard to other diseases, particularly, retinopathy, 15(S)-HETE levels have been found to be elevated in epiretinal membranes of proliferative vitreoretinal and proliferative diabetic retinopathy patients.20 Furthermore, hypoxia increased the expression of 15-LOX1 and production of 15(S)-HETE in pulmonary arteries, causing vasoconstriction.21 Hypoxia also increased the production of 15(S)-HETE in human retinal microvascular endothelial cells, causing angiogenesis.22-24 In addition, we showed that 15(S)-HETE–induced angiogenesis requires phosphoinositide 3-kinase-Akt–dependent fibroblast growth factor-2 (FGF-2) expression and signal transducer and activator of transcription-3–mediated vascular endothelial growth factor expression.25,26
Egr-1, a zinc finger transcriptional factor, has been shown to play an important role in the regulation of cell migration and proliferation.27,28 In addition, the involvement of Egr-1 in the pathogenesis of various diseases, including vascular diseases, has been demonstrated.29-31 In many reports32,33 authors have demonstrated that Egr-1 mediates the expression of angiogenic factors such as FGF-2 and antiangiogenic factors such as thrombospondin I in response to several cues. Because angiogenesis appears to be involved in atherosclerosis and restenosis34,35 and because both 15(S)-HETE and Egr-1 possess the capacity to modulate angiogenesis23-26,29 and both molecules are produced in these vascular lesions,16,30 we asked the question whether Egr-1 plays a role in 15(S)-HETE–induced angiogenesis. We found that 15(S)-HETE induces the expression of Egr-1 in human dermal microvascular endothelial cells (HDMVECs) and that this effect is dependent on activation of Src. In addition, 15(S)-HETE–induced angiogenesis requires Egr-1–mediated rapid expression of FGF-2. Furthermore, AA, although inducing robust angiogenesis in wild-type (WT) mice, failed to influence a similar effect in 12/15-LOX−/− mice, suggesting the importance of AA metabolism via the lipoxygenase pathway in the regulation of angiogenesis.
Methods
Reagents
15(S)-HETE and 15(S)-HETE enzyme immunoassay (EIA) kit were bought from Cayman Chemicals. Growth factor-reduced Matrigel and anti-CD31 antibodies were obtained from BD Biosciences. Phosphospecific anti-Src antibodies were from Cell Signaling Technology. Anti-Src antibodies were obtained from Upstate Biotechnology Inc. Anti–Egr-1 antibodies (SC-189), anti–FGF-2 antibodies (SC-79), anti–β-tubulin antibodies (SC-9104), and normal mouse serum (SC-2025) were purchased from Santa Cruz Biotechnology Inc. Neutralizing anti–FGF-2 antibodies were bought from Millipore. A human FGF-2 enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems. Anti–von Willebrand Factor (vWF) antibodies were supplied by Abcam. Hoechst 33342 (H3570) and Prolong Gold antifade mounting medium (P36930) were bought from Invitrogen. T4 polynucleotide kinase was procured from New England Biolabs. [γ32P]-ATP (3000 Ci/mmol) was bought from GE Healthcare Biosciences. All of the primers were made by IDT.
Adenoviral vectors
To construct adenoviral vector for dnEgr-1, its cDNA was released from pCB6-Egr-1 plasmid by digestion with HindIII and SmaI and cloned into the same sites of pBluescript vector. DnEgr-1 cDNA was retrieved from pBluescript vector by digestion with KpnI and NotI and subcloned into the same sites of entry vector pENTR3C. The pAd-dnEgr-1 was generated by specific recombination of pENTR3C-dnEgr-1 with pAdCMV5DEST (Invitrogen). The plasmid pAd-dnEgr-1 was linearized by digestion with PacI and transfected into HEK293A cells to amplify Ad-dnEgr-1 virus. The dominant-negative Egr-1 contains transcriptional repressor domain of Wilms tumor protein (aa 1-307) at the N-terminus of Egr-1 DNA binding domain (aa 327-427).36 Construction of Ad-GFP and Ad-dnSrc was described previously.37 Adenovirus was purified with cesium chloride centrifugation, and the titer was determined by plaque assay, as described previously.37
Cell culture
HDMVECs were bought from Cascade Biologics and grown in medium 131 containing microvascular growth supplements, 10 μg/mL gentamicin, and 0.25 μg/mL amphotericin B. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. HDMVECs were growth-arrested by incubating in medium 131 for 24 hours and used to perform the experiments unless otherwise indicated.
Cell migration
Cell migration was performed by the use of the modified Boyden Chamber method as described by us previously.38 The cell culture inserts containing membranes with 10 mm in diameter and 8.0-μm pore size (Nalgene Nunc International) were placed in a 24-well tissue culture plate (Costar; Corning Inc). The lower surface of the porous membrane was coated with 70% Matrigel at 4°C overnight and then blocked with 0.1% heat-inactivated bovine serum albumin at 37°C for 1 hour. HDMVECs were quiesced for 24 hours in medium 131, trypsinized, and neutralized with trypsin-neutralizing solution. Cells were seeded into the upper chamber at 105 cells/well. Vehicle or 15(S)-HETE was added to the lower chamber. Both the upper and lower chambers contained medium 131. When the effect of dominant-negative Src and Egr-1 mutants was tested on 15(S)-HETE–induced HDMVEC migration, cells were infected first with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 at 40 multiplicity of infection (moi) and quiesced before they were subjected to migration assay. In the case of testing the effect of neutralizing anti–FGF-2 antibodies on 15(S)-HETE–induced HDMVEC migration, cells were incubated with antibodies (3 μg/mL) for 30 minutes at 37°C followed by washing with medium 131. Cells were then seeded into each well, and wherever appropriate, the antibodies were added to both the upper and lower chambers before the addition of 15(S)-HETE. After 6 hours of incubation at 37°C, nonmigrated cells were removed from the upper side of the membrane with cotton swabs and the cells on the lower surface of the membrane were fixed in methanol for 15 minutes. The membrane was then stained with DAPI in VECTASHIELD mounting medium (Vector Laboratories Inc) and observed under Nikon diaphot fluorescence microscope with photometrics CH250 CCD camera (Nikon). Cells were counted in 5 randomly selected squares per well and presented as number of migrated cells per field.
Tube formation
Tube formation assay was performed as described by us previously.38 Twenty-four-well culture plates (Costar; Corning Inc) were coated with growth factor-reduced Matrigel (BD Biosciences) in a total volume of 280 μL/well and allowed to solidify for 30 minutes at 37°C. HDMVECs were trypsinized, neutralized with TNS, and resuspended at 5 × 105 cells/mL, and 200 μL of this cell suspension was added into each well. Vehicle or 15(S)-HETE, at the indicated concentration, was added to the appropriate well, and the cells were incubated at 37°C for 6 hours. When the effect of dominant-negative Src and Egr-1 mutants was tested on 15(S)-HETE–induced HDMVEC tube formation, cells were infected first with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 at 40 moi and quiesced before they were subjected to tube formation. In the case of testing the effect of neutralizing anti–FGF-2 antibodies on 15(S)-HETE–induced HDMVEC tube formation, cells were incubated with antibodies (3 μg/mL) for 30 minutes at 37°C followed by washing with medium 131. Cells were then seeded into each well, and wherever appropriate, the antibodies were added to the well before the addition of 15(S)-HETE. Tube formation was observed under an inverted microscope (Eclipse TS100; Nikon). Images were captured with a CCD color camera (KP-D20AU; Hitachi) attached to the microscope and tube length was measured with National Institutes of Health (NIH) Image J.
Western blotting
After appropriate treatments and rinsing with cold phosphate-buffered saline (PBS), HDMVECs were lysed in 500 μL of lysis buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 100 μg/mL phenylmethylsulphonyl fluoride, 100 μg/mL aprotinin, 1 μg/mL leupeptin, and 1mM sodium orthovanadate) and scraped into 1.5-mL Eppendorf tubes. After standing on ice for 20 minutes, the cell lysates were cleared by centrifugation at 13 000g for 20 minutes at 4°C. Cell lysates containing equal amount of protein were resolved by electrophoresis on 0.1% SDS and 10% polyacrylamide gels. The proteins were transferred electrophoretically to a nitrocellulose membrane (Hybond; Amersham Pharmacia Biotech). After blocking in 10mM Tris-HCl buffer (pH 8.0) containing 150mM sodium chloride, 0.1% Tween 20, and 5% (wt/vol) nonfat dry milk, the membrane was treated with appropriate primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected with the use of a chemiluminescence reagent kit (Amersham Pharmacia Biotech). The band intensities were quantified with NIH Image J1.31v program.
Quantitative RT-PCR
After appropriate treatments, total cellular RNA was isolated from HDMVECs by the use of Trizol reagent as per the manufacture's guidelines (Invitrogen). Reverse transcription (RT) was carried out with Superscript III First-Strand Synthesis System for RT-polymerase chain reaction (PCR) on the basis of the supplier's protocol (Invitrogen). The cDNA was then used as template for PCR with Taqman Gene Expression Assay kit for human Egr-1, human FGF-2, and human β-actin. The amplification was carried out on Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems), with the use of the following amplification conditions for the aforementioned genes as follows: 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds with extension at 60°C for 1 minute. The amplification was examined with the use of the 7300 Real-Time PCR System-SDS, Version 1.4, program.
Cloning of human FGF-2 promoter-luciferase reporter constructs
The human FGF-2 promoter was cloned from human genomic DNA by nested PCR method. The promoter was initially amplified by the outer sense primer, 5′-TAG GGC AAA TGC CAG GTC CGA GTT AAG-3′ (hFGF2p-S), and the antisense primer, 5′-AAG CTT GGC GTC ACA TCT TCT ACA TCT CCA CCC-3′ (hFGF2p-R). The resulting PCR product was then used as a template to amplify the internal region encompassing nucleotides from −494 to +125 relative to the transcription start site with an inner sense primer, 5′-CCC CTC GAG CTC CCC TTC TCT GGC CTC T-3′ (hFGF2p-F) incorporating a XhoI restriction enzyme site at the 5′-end and the outer reverse primer, hFGF2p-R, incorporating a HindIII restriction enzyme site at the 5′-end.
The PCR products were subcloned into the pGEM-T vector, sequenced, and then released from the vector by digestion with XhoI and HindIII. The released fragments were cloned into XhoI and HindIII sites of the pGL3 basic vector (Promega) to yield hFGF2p-wt-Luc. Site-directed mutagenesis of the Egr-1 binding sites was done by a PCR-based overlap extension method with the hFGF2p-wt-Luc as a template. The hFGF2p-F and hFGF2p-R were used as external primers in combination with following primers incorporating mutations, forward, 5′-TGG TGC GGG GGT TGG GTT AAG GTG ACT TTT GGG GGA-3′ (Egr1-M2-F) and reverse, 5′-TCC CCC AAA AGT CAC CTT AAC CCA ACC CCC GCA CCA-3′ (Egr1-M2-R); forward, 5′-CCC TCG CCT CTC CCC CTT AAC CGA CTG AGG CCG GGC T-3′ (Egr1-M1-F) and reverse, 5′-AGC CCG GCC TCA GTC GGT TAA GGG GGA GAG GCG AGG G-3′ (Egr1-M1-R) for generation of mutated reporter constructs targeting Egr-1 sites at −165 and −65 nt, respectively. The bold letters indicate the mutations. The constructs were confirmed by restriction enzyme analysis and DNA sequencing.
Luciferase assay
HDMVECs were transfected with FGF-2 promoter-luciferase constructs or pGL3 basic vector with Lipofectamine 2000 reagent. After growth synchronization in growth factor-free medium for 12 hours, cells were treated with and without 15(S)-HETE (0.1μM) for 30 minutes. Cells were then washed once with ice-cold PBS and lysed with 200 μL of lysis buffer. The cell extracts were collected into microcentrifuge tubes and centrifuged for 2 minutes at 12 000g at 4°C. The supernatants were collected and assayed for luciferase activity with the Luciferase Assay System (Promega) and a single-tube luminometer (TD20/20 Turner Designs), and the values are expressed as relative luciferase units.
Electrophoretic mobility shift assay
After appropriate treatments, nuclear extracts were prepared from HDMVECs as described previously.26 The protein content of the nuclear extracts was determined with the use of a Micro BCA Protein Assay Reagent Kit (Pierce). Protein-DNA complexes were formed by incubating 10 μg of nuclear protein in a total volume of 25 μL consisting of 1mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.9; 3mM Tris-HCl, pH 7.9; 60mM KCl; 1mM EDTA (ethylenediaminetetraacetic acid); 1mM phenylmethylsulphonyl fluoride; 1mM dithiothreitol; 2.5 μg/mL bovine serum albumin; 1 μg/mL poly (dI-dC), 15% glycerol, and 100,000 cpm of [32P]-labeled oligonucleotide probe for 30 minutes on ice. The protein-DNA complexes were resolved by electrophoresis on a 4% polyacrylamide gel with the use of 1X Tris-glycine-EDTA buffer (25mM Tris-HCl, pH 8.5; 200mM glycine; 0.1mM EDTA). Double-stranded oligonucleotides (5′-CGCCTCTCCCCCGCCCCCGACTGAG-3′, 3′-GCGGAGAGGGGGCGGGGGCTGACTC-5′) encompassing a Egr-1 binding sequence from −57 to −65 region of human FGF-2 promoter (accession no. S81809) were used as [32P]-labeled probe to measure Egr-1 DNA binding activity. Double-stranded oligonucleotides were labeled with [γ32P]-ATP with the T4 polynucleotide kinase kit following the supplier's protocol.
ChIP
Chromatin immunoprecipitation (ChIP) assay was performed on HDMVECs by use of the ChIP assay kit following supplier's protocol (Upstate Biotechnology Inc). Egr-1-DNA complexes were immunoprecipitated with an anti–Egr-1 antibody. Preimmune rabbit serum was used as a negative control. The immunoprecipitated DNA was uncross-linked, subjected to Proteinase K digestion, and purified with QIAquick columns (QIAGEN). The purified DNA was used as a template for PCR amplification by the use of primers (forward, 5′-AAGCCTGCTCTGACACAGAC-3′; reverse, 5′-GTTCGGCCACAACACGCAA-3′) flanking the putative Egr-1–binding site located at −57 to −65 nt in human FGF-2 promoter region (accession no. S81809). The PCR products were resolved on 1.2% agarose gel, stained with ethidium bromide, and the band intensities were quantified by the use of NIH Image J.
Matrigel plug angiogenesis
All the experiments that used animals were performed in accordance with the relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center. Matrigel plug assay was performed essentially as described by us previously.37 The C57BL/6 and 12/15-LOX−/− mice were obtained from The Jackson Laboratory. C57BL/6 mice or 12/15-LOX−/− mice (8 weeks old) were lightly anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) and were injected subcutaneously with 0.5 mL of Matrigel that was premixed with vehicle or 5μM of either AA or 15(S)-HETE along the abdominal midline. The injections were made rapidly with a B-D 30G1/2 needle to ensure the entire content was delivered as a single plug. Wherever the effect of Ad-GFP (5 × 109 pfu/mL), Ad-dnSrc (5 × 109 pfu/mL), Ad-dnEgr-1 (5 × 109 pfu/mL), preimmune serum (20 μg/mL), or neutralizing anti–FGF-2 antibodies (20 μg/mL) was tested on 15(S)-HETE–induced angiogenesis, they were added to the Matrigel before injecting into mice.
The mice were allowed to recover, and 7 days later, unless otherwise stated, the animals were sacrificed by inhalation of CO2 and the Matrigel plugs were harvested from underneath the skin. The plugs were homogenized in 1 mL of deionized H2O on ice and cleared by centrifugation at 9000g for 6 minutes at 4°C. The supernatant was collected and used in duplicate to measure hemoglobin content with Drabkin reagent along with hemoglobin standard essentially according to the manufacturer's protocol (Sigma-Aldrich). The absorbance was read at 540 nm in an ELISA plate reader (Spectra Max 190; Molecular Devices). These experiments were repeated at least 3 times with 6 mice for each group, and the values are expressed as grams of hemoglobin per deciliter per milligram of plug.
Double immunofluorescence staining
After retrieving the Matrigel plugs from mice, they were snap-frozen in OCT compound. Cryosections (5 μm) were made with the use of Leica Kryostat (CM3050S; Leica). After blocking in goat serum, the cryosections were incubated with rabbit anti–mouse vWF antibodies and rat anti–mouse CD31 antibodies for 1 hour. After washing in PBS, all slides were incubated with tetramethylrhodamine isothiocyanate-conjugated goat anti–rabbit and fluorescein-conjugated goat anti–rat secondary antibodies. In the case of negative control, incubation with primary antibodies was omitted. Fluorescence images of the Matrigel sections were captured by the use of an inverted Zeiss fluorescence microscope, (AxioVision AX10) via a 10×/0.6 numeric aperture objective and AxioCam MRm camera without any enhancements.
ELISA
FGF-2 released into the culture medium was measured with the use of an ELISA kit following the manufacturer's instructions (R&D Systems).
EIA
At 7 days after Matrigel injection, the plugs were retrieved from mice, minced, and incubated with AA (10μM) along with ionomycin (10μM) for 3 hours. The eicosanoids were extracted with 10% (vol/vol) methanol. After adjusting the pH to 2.5 with 10% (vol/vol) glacial acetic acid, the mixture was passed through Pre-Sep C18 column. The eicosanoids were eluted with 100% methanol followed by evaporation to dryness and then reconstituted in 50 μL of EIA buffer (Cayman Chemicals). 15(S)-HETE was assayed with the use of 15(S)-HETE EIA kit following the manufacturer's (Cayman Chemicals) instructions.
Statistics
All the experiments were repeated 3 times, and data are presented as mean plus or minus SD. The treatment effects were analyzed by the Student t test, and P values less than .05 were considered to be statistically significant. In the case of ChIP assay, double immunofluorescence staining, electrophoretic mobility shift assay (EMSA), and Western blotting analysis, 1 representative set of data are shown.
Results
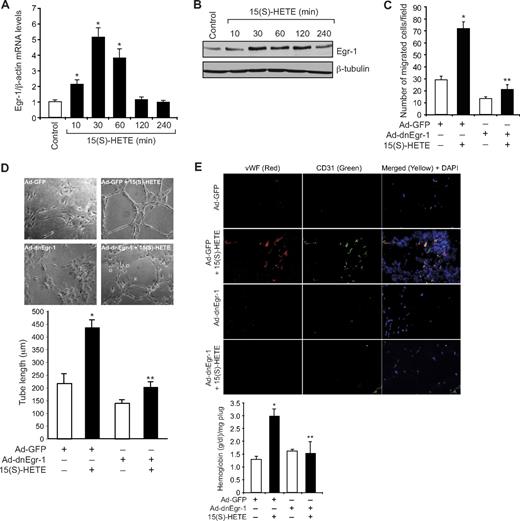
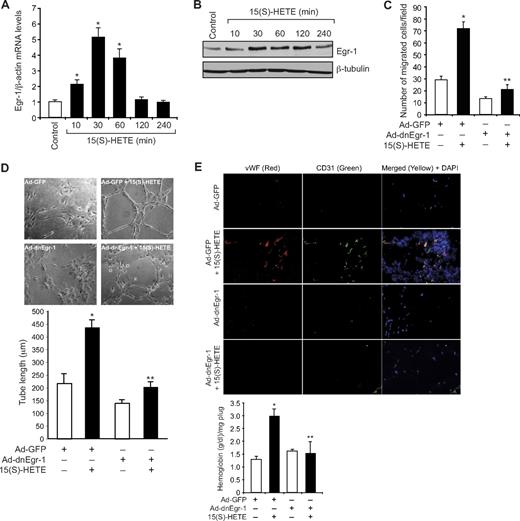
Previously we showed that 15(S)-HETE, a major AA metabolite of 15-LOX1/2, plays a role in the induction of angiogenesis.24-26,38 To understand the mechanisms underlying 15(S)-HETE–induced angiogenesis, we have studied the role of Egr-1, a zinc finger transcriptional factor.29 15(S)-HETE induced the expression of Egr-1 mRNA in a time-dependent manner, with a 5-fold increase at 30 minutes and declining thereafter (Figure 1A). The induction of Egr-1 by 15(S)-HETE also was confirmed at protein level as determined by Western blot analysis. A 3- to 4-fold increase in Egr-1 levels was observed at 30 minutes in response to 15(S)-HETE (Figure 1B). Adenovirus-mediated expression of dominant-negative Egr-1 inhibited 15(S)-HETE–induced HDMVEC migration and tube-like structure formation (Figure 1C-D). Consistent with its effects on HDMVEC migration and tube-like structure formation, 15(S)-HETE induced angiogenesis in a Matrigel plug model, and it was attenuated by dn-Egr-1, as determined by double immunofluorescence staining for CD31 and vWF and measuring hemoglobin levels (Figure 1E).
Egr-1 mediates 15(S)-HETE–induced HDMVEC migration and tube-like structure formation in vitro and Matrigel plug angiogenesis in vivo. (A-B) Quiescent human dermal microvascular endothelial cells (HDMVECs) were treated with and without 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE; 0.1μM) for the indicated time periods, and either RNA was isolated or cell extracts were prepared. (A) RNA was analyzed by QRT-PCR for Egr-1 and β-actin mRNA levels. (B) Cell extracts were analyzed by Western blotting for Egr-1 levels by the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C-D) HDMVECs were transduced with Ad-GFP or Ad-dnEgr-1 at a moi of 40, quiesced, and subjected to 15(S)-HETE–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE along with and without Ad-GFP or Ad-dnEgr-1 (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for von Willebrand Factor (vWF) and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content with Drabkin reagent. The bar graphs in panels A, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control or Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
Egr-1 mediates 15(S)-HETE–induced HDMVEC migration and tube-like structure formation in vitro and Matrigel plug angiogenesis in vivo. (A-B) Quiescent human dermal microvascular endothelial cells (HDMVECs) were treated with and without 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE; 0.1μM) for the indicated time periods, and either RNA was isolated or cell extracts were prepared. (A) RNA was analyzed by QRT-PCR for Egr-1 and β-actin mRNA levels. (B) Cell extracts were analyzed by Western blotting for Egr-1 levels by the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C-D) HDMVECs were transduced with Ad-GFP or Ad-dnEgr-1 at a moi of 40, quiesced, and subjected to 15(S)-HETE–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE along with and without Ad-GFP or Ad-dnEgr-1 (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for von Willebrand Factor (vWF) and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content with Drabkin reagent. The bar graphs in panels A, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control or Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
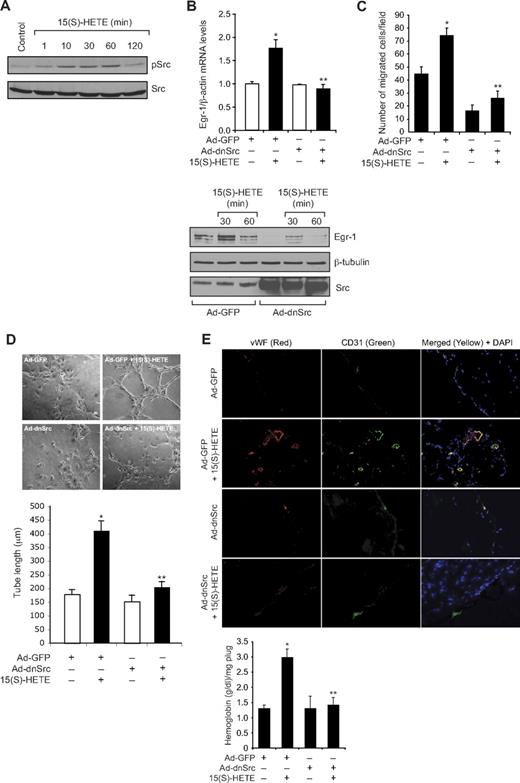
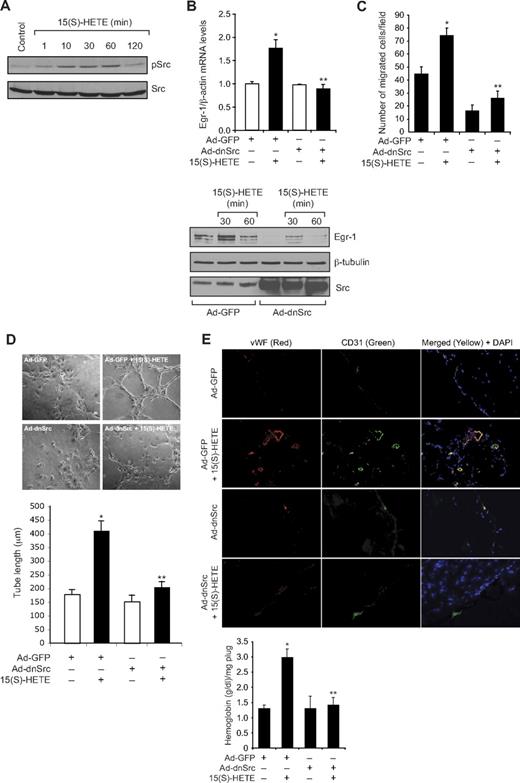
To understand the mechanism(s) by which 15(S)-HETE induces the expression of Egr-1, we tested the role of Src. Consistent with our previous observations,25 15(S)-HETE stimulated tyrosine phosphorylation of Src in a time-dependent manner (Figure 2A). Interference with Src activation via adenovirus-mediated expression of its dominant-negative mutant significantly blocked 15(S)-HETE–induced Egr-1 expression both at mRNA and protein levels (Figure 2B). Adenovirus-mediated expression of dnSrc also blocked 15(S)-HETE–induced HDMVEC migration and tube-like structure formation as well as Matrigel plug angiogenesis (Figure 2C-E).
Src mediates 15(S)-HETE–induced Egr-1 expression in HDMVECs, leading to their migration and tube-like structure formation. (A) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for pSrc with the use of its phosphospecific antibodies. The blot was reprobed with anti-Src antibodies for normalization. (B) HDMVECs were transduced with Ad-GFP or Ad-dnSrc at a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and 60 minutes for cell extract preparation. RNA was analyzed for Egr-1 and β- actin mRNA levels by QRT-PCR, and cell extracts were analyzed by Western blotting for Egr-1 with the use of its specific antibodies. The blot was reprobed sequentially with anti–β-tubulin antibodies and anti-Src antibodies for normalization and to show the overexpression of dnSrc, respectively. (C-D) HDMVECs that were transduced with Ad-GFP or Ad-dnSrc at a moi of 40 and quiesced were subjected to 15(S)-HETE (0.1μM)–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with and without Ad-GFP or Ad-dnSrc (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels B, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
Src mediates 15(S)-HETE–induced Egr-1 expression in HDMVECs, leading to their migration and tube-like structure formation. (A) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for pSrc with the use of its phosphospecific antibodies. The blot was reprobed with anti-Src antibodies for normalization. (B) HDMVECs were transduced with Ad-GFP or Ad-dnSrc at a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and 60 minutes for cell extract preparation. RNA was analyzed for Egr-1 and β- actin mRNA levels by QRT-PCR, and cell extracts were analyzed by Western blotting for Egr-1 with the use of its specific antibodies. The blot was reprobed sequentially with anti–β-tubulin antibodies and anti-Src antibodies for normalization and to show the overexpression of dnSrc, respectively. (C-D) HDMVECs that were transduced with Ad-GFP or Ad-dnSrc at a moi of 40 and quiesced were subjected to 15(S)-HETE (0.1μM)–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with and without Ad-GFP or Ad-dnSrc (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels B, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
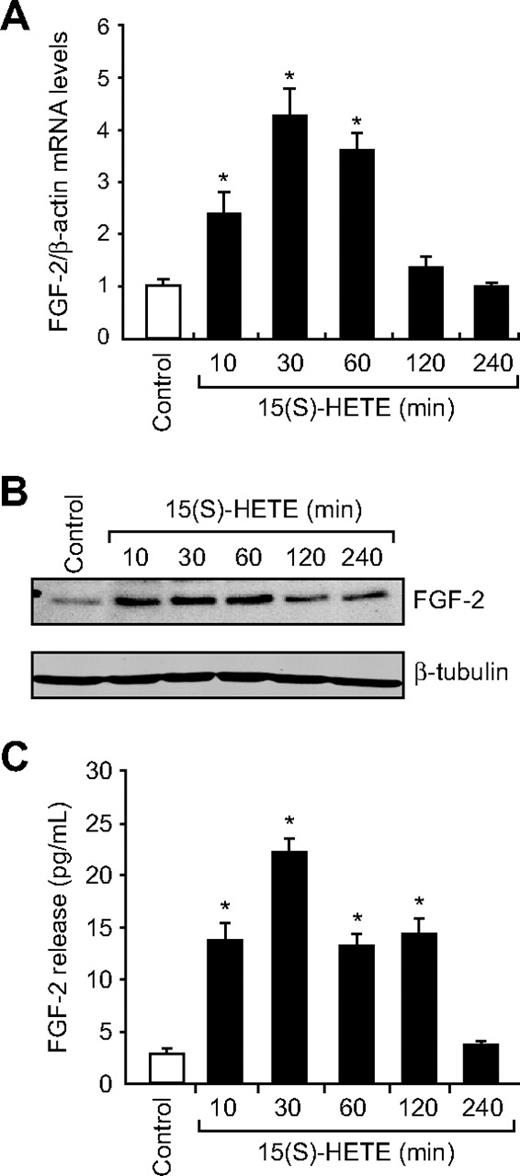
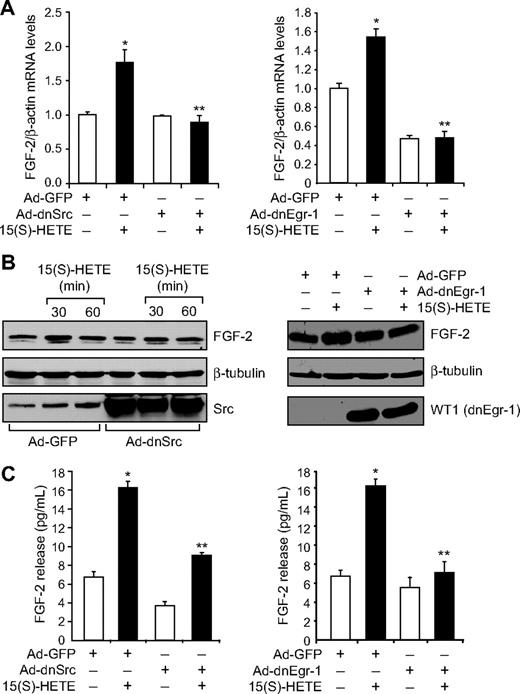
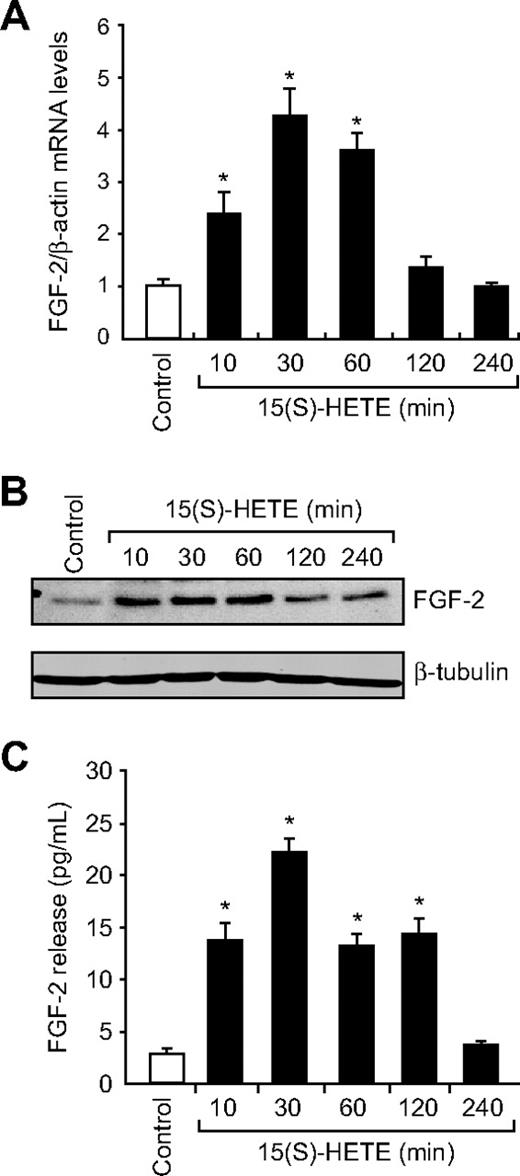
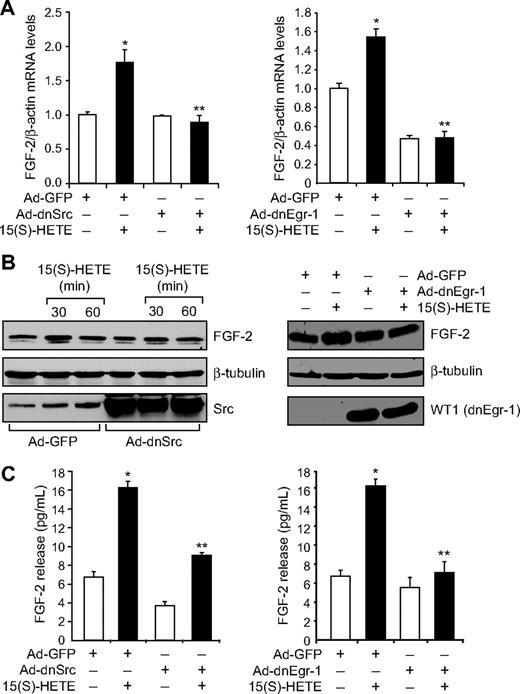
Jin et al32 have indicated a direct role for Egr-1 in the regulation of FGF-2. Hence, to find the downstream effector molecules of Egr-1 in mediating 15(S)-HETE–induced angiogenesis, we examined the role of FGF-2. 15(S)-HETE induced FGF-2 mRNA expression rapidly, as determined by quantitative reverse-transcription (QRT)-PCR. Maximum increase in FGF-2 mRNA expression occurred at 30 minutes after treatment with 15(S)-HETE (Figure 3A). The rapid increase in FGF-2 mRNA levels was confirmed at the protein level as well. Western blot analysis and ELISA data showed a 3- to 5-fold increase in the expression and release of FGF-2 by 15(S)-HETE acutely with maximum effect at 30 minutes (Figure 3B-C). In addition, blockade of either Src or Egr-1 via adenovirus-mediated expression of their dominant-negative mutants suppressed 15(S)-HETE–induced FGF-2 expression both at mRNA and protein levels (Figure 4A-C). To understand the molecular mechanisms of FGF-2 regulation by 15(S)-HETE, 0.5 kb of human FGF-2 promoter was cloned and analyzed for transcription factor binding sites by TRANSFAC search. TRANSFAC analysis revealed the presence of 2 Egr-1 binding sites in the promoter (Figure 5A).
15(S)-HETE induces FGF-2 expression rapidly in HDMVECs. (A-B) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for various time periods, and either RNA was isolated or cell extracts were prepared. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C) All the conditions were the same as in panel B except that medium was collected and analyzed for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs control.
15(S)-HETE induces FGF-2 expression rapidly in HDMVECs. (A-B) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for various time periods, and either RNA was isolated or cell extracts were prepared. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C) All the conditions were the same as in panel B except that medium was collected and analyzed for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs control.
15(S)-HETE–induced FGF-2 expression is mediated by Src-Egr-1 signaling in HDMVECs. (A-B) HDMVECs were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and/or 60 minutes for cell extract preparation. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with its specific antibodies. The blots were reprobed with anti–β-tubulin antibodies and/or anti-Src antibodies or anti-WT1 antibodies for normalization and to show overexpression of dnSrc or dnEgr-1, respectively. (C) All the conditions were the same as in panel A except that medium was collected and tested for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
15(S)-HETE–induced FGF-2 expression is mediated by Src-Egr-1 signaling in HDMVECs. (A-B) HDMVECs were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and/or 60 minutes for cell extract preparation. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with its specific antibodies. The blots were reprobed with anti–β-tubulin antibodies and/or anti-Src antibodies or anti-WT1 antibodies for normalization and to show overexpression of dnSrc or dnEgr-1, respectively. (C) All the conditions were the same as in panel A except that medium was collected and tested for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
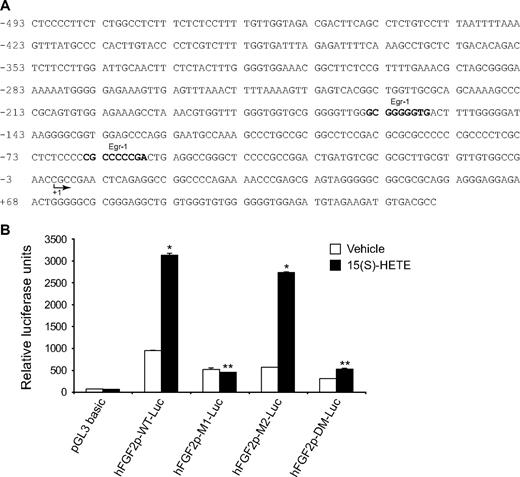
15(S)-HETE–induced FGF-2 promoter-luciferase expression requires Egr-1-binding site. (A) Sequence of a 0.5-kb cloned human FGF-2 promoter region showing the Egr-1 binding sites. Sequence in bold represents Egr-1-binding site; +1 indicates transcription start site. (B) HDMVECs were transfected with either empty vector or FGF-2 promoter-luciferase constructs, quiesced, and treated with and without 0.1μM 15(S)-HETE for 30 minutes, and cell extracts were prepared and analyzed for luciferase activity. The bar graph in panel B represents the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs hFGF2p-WT-Luc vehicle control; **P < .01 vs hFGF2p-WT-Luc + 15(S)-HETE. hFGF2p-WT-Luc indicates human FGF-2 promoter-luciferase construct; hFGF2p-M1-Luc, human FGF-2 promoter-luciferase construct with mutation in the first Egr-1 binding site from the transcriptional start site; hFGF2p-M2-Luc, human FGF-2 promoter-luciferase construct with mutation in the second Egr-1 binding site from the transcriptional start site; hFGF2p-DM-Luc, human FGF-2 promoter-luciferase construct with mutations in both the Egr-1 binding sites.
15(S)-HETE–induced FGF-2 promoter-luciferase expression requires Egr-1-binding site. (A) Sequence of a 0.5-kb cloned human FGF-2 promoter region showing the Egr-1 binding sites. Sequence in bold represents Egr-1-binding site; +1 indicates transcription start site. (B) HDMVECs were transfected with either empty vector or FGF-2 promoter-luciferase constructs, quiesced, and treated with and without 0.1μM 15(S)-HETE for 30 minutes, and cell extracts were prepared and analyzed for luciferase activity. The bar graph in panel B represents the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs hFGF2p-WT-Luc vehicle control; **P < .01 vs hFGF2p-WT-Luc + 15(S)-HETE. hFGF2p-WT-Luc indicates human FGF-2 promoter-luciferase construct; hFGF2p-M1-Luc, human FGF-2 promoter-luciferase construct with mutation in the first Egr-1 binding site from the transcriptional start site; hFGF2p-M2-Luc, human FGF-2 promoter-luciferase construct with mutation in the second Egr-1 binding site from the transcriptional start site; hFGF2p-DM-Luc, human FGF-2 promoter-luciferase construct with mutations in both the Egr-1 binding sites.
To further characterize the promoter, the Egr-1 binding sites were mutated and tested for their involvement in 15(S)-HETE–induced FGF-2 promoter-luciferase reporter gene activity. Site-directed mutation of Egr-1 binding site proximal to transcriptional start site abolished 15(S)-HETE–induced FGF-2 promoter-luciferase reporter gene activity (Figure 5B). EMSA analysis with Egr-1 elements proximal to transcriptional start site as a [32P]-labeled probe demonstrated that 15(S)-HETE induced Egr-1 binding to this site in a time-dependent manner with maximum effect at 30 minutes (Figure 6A). In addition, dominant-negative Src or Egr-1 blocked 15(S)-HETE–induced Egr-1 DNA binding activity (Figure 6B). To confirm this result further, we performed the ChIP assay. As shown in Figure 6C, 15(S)-HETE induced Egr-1 binding to FGF-2 promoter in a time-dependent manner with approximately a 2-fold increase at 30 minutes. Adenovirus-mediated expression of either dnSrc or dnEgr-1 completely prevented Egr-1 binding to the FGF-2 promoter in response to 15(S)-HETE (Figure 6D).
Egr-1 binds to FGF-2 promoter in response to 15(S)-HETE both in vitro and in vivo in Src-dependent manner. (A,C) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity in vitro with the use of [32P]-labeled double stranded oligonucleotide probe that corresponds to the Egr-1 binding site proximal to the transcriptional start site in the FGF-2 promoter (A) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (C). (B,D) HDMVECs that were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40 and quiesced were treated with and without 15(S)-HETE (0.1μM) for 30 minutes and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity as described in panel A (B) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (D).
Egr-1 binds to FGF-2 promoter in response to 15(S)-HETE both in vitro and in vivo in Src-dependent manner. (A,C) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity in vitro with the use of [32P]-labeled double stranded oligonucleotide probe that corresponds to the Egr-1 binding site proximal to the transcriptional start site in the FGF-2 promoter (A) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (C). (B,D) HDMVECs that were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40 and quiesced were treated with and without 15(S)-HETE (0.1μM) for 30 minutes and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity as described in panel A (B) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (D).
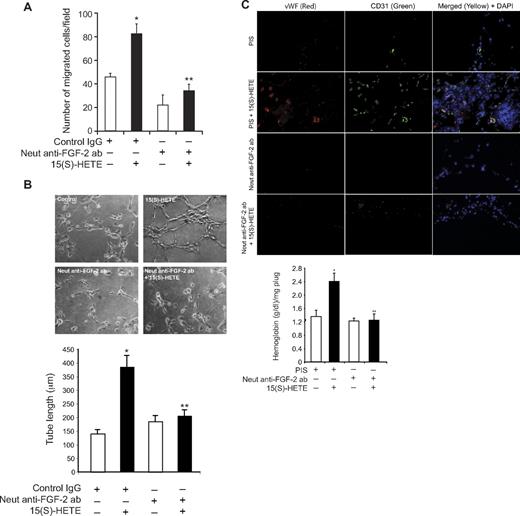
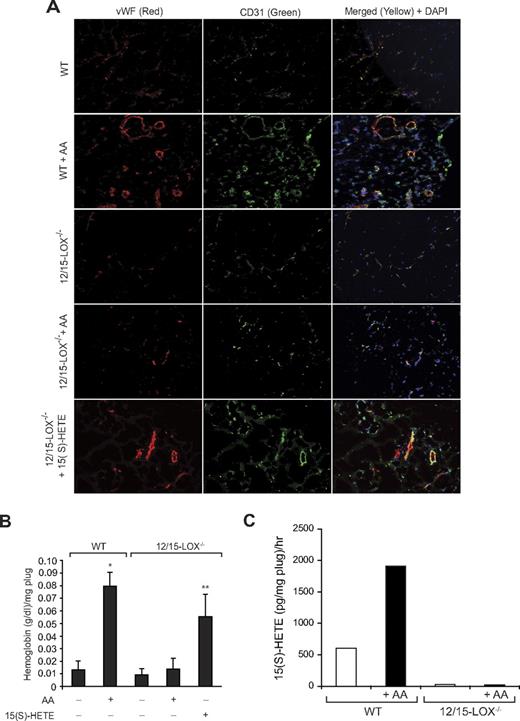
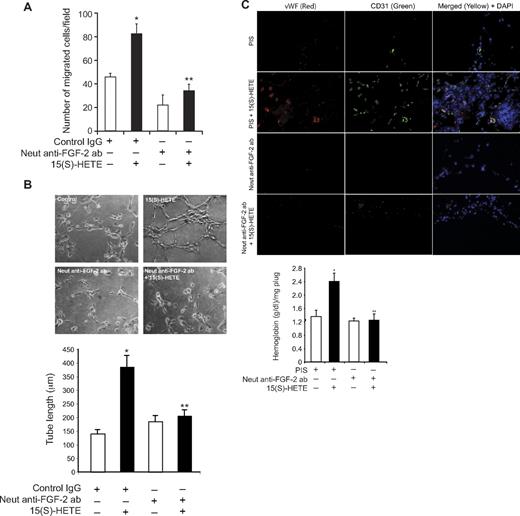
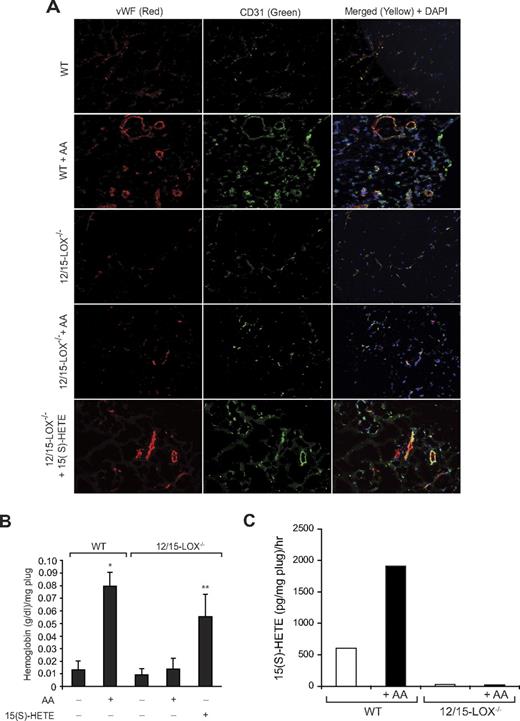
To explore the functional role of FGF-2 in 15(S)-HETE–induced angiogenesis, we used its neutralizing antibodies. Neutralizing anti–FGF-2 antibodies blocked 15(S)-HETE–induced HDMVEC migration and tube-like structure formation as well as Matrigel plug angiogenesis (Figure 7A-C). To obtain additional evidence for the role of 15(S)-HETE in the regulation of angiogenesis, we used 12/15-LOX−/− mice. AA, the substrate for cyclooxygenases, lipoxygenases, and epoxygenases, although inducing Matrigel plug angiogenesis in WT mice, failed to influence a similar effect in 12/15-LOX−/− mice (Figure 8A-B). However, the addition of 15(S)-HETE (5μM) to the Matrigel restored the angiogenic stimulus in 12/15-LOX−/− mice (Figure 8A-B). This finding suggests that AA-induced angiogenesis requires its conversion via the 12/15-LOX pathway. To gain additional evidence in support of this notion, we measured the capacity of these plugs to convert AA to 15(S)-HETE. Matrigel plugs were retrieved 7 days after injection from both WT and 12/15-LOX−/− mice that received either vehicle or AA supplementation at the time of injection, minced, incubated with 10μM AA and 10μM ionomycin for 3 hours, and assayed for 15(S)-HETE by EIA. As shown in Figure 8C, Matrigel plugs from WT mice that received AA supplementation produced several fold greater levels of 15(S)-HETE compared with Matrigel plugs from either control WT or 12-LOX−/− mice or 12-LOX−/− mice that received AA supplementation.
FGF-2 mediates 15(S)-HETE–induced angiogenesis. (A-B) Quiescent HDMVECs were treated with normal serum or neutralizing anti–FGF-2 antibodies (3 μg/mL) for 30 minutes at 37°C followed by washing with medium 131. The cells were then subjected to 15(S)-HETE (0.1μM)–induced migration (A) or tube-like structure formation (B) in the presence and absence of 3 μg/mL of preimmune serum or neutralizing anti–FGF-2 antibodies. (C) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with 20 μg/mL preimmune serum or neutralizing anti–FGF-2 antibodies. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining using their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control (normal serum); **P < .01 vs 15(S)-HETE (normal serum + 15(S)-HETE).
FGF-2 mediates 15(S)-HETE–induced angiogenesis. (A-B) Quiescent HDMVECs were treated with normal serum or neutralizing anti–FGF-2 antibodies (3 μg/mL) for 30 minutes at 37°C followed by washing with medium 131. The cells were then subjected to 15(S)-HETE (0.1μM)–induced migration (A) or tube-like structure formation (B) in the presence and absence of 3 μg/mL of preimmune serum or neutralizing anti–FGF-2 antibodies. (C) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with 20 μg/mL preimmune serum or neutralizing anti–FGF-2 antibodies. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining using their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control (normal serum); **P < .01 vs 15(S)-HETE (normal serum + 15(S)-HETE).
12/15-LOX−/− mice show lack of angiogenic response to AA. (A-B) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle, 5μM AA, or 5μM 15(S)-HETE as indicated. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 by double immunofluorescence staining by the use of their specific antibodies (A) or analyzed for hemoglobin content with Drabkin reagent (B). (C) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM AA. One week later, the Matrigel plugs were retrieved from animals, minced, incubated with 10μM AA and 10μM ionomycin, and 15(S)-HETE production was measured by EIA. The bar graphs represent the quantitative analysis of 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs WT control. **P < .01 vs WT control or 12/15-LOX−/− control or 12/15-LOX−/− + AA.
12/15-LOX−/− mice show lack of angiogenic response to AA. (A-B) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle, 5μM AA, or 5μM 15(S)-HETE as indicated. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 by double immunofluorescence staining by the use of their specific antibodies (A) or analyzed for hemoglobin content with Drabkin reagent (B). (C) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM AA. One week later, the Matrigel plugs were retrieved from animals, minced, incubated with 10μM AA and 10μM ionomycin, and 15(S)-HETE production was measured by EIA. The bar graphs represent the quantitative analysis of 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs WT control. **P < .01 vs WT control or 12/15-LOX−/− control or 12/15-LOX−/− + AA.
Discussion
The important findings of the present study are as follows. 15(S)-HETE induced Egr-1 expression in HDMVECs. Dominant-negative mutant-mediated blockade of Egr-1 attenuated the effects of 15(S)-HETE on HDMVEC migration and tube-like structure formation as well as Matrigel plug angiogenesis. 15(S)-HETE–induced expression of Egr-1 requires Src activation, and suppression of Src via forced expression of its dominant-negative mutant inhibited the migration and tube-like structure formation of HDMVECs as well as Matrigel plug angiogenesis. 15(S)-HETE induced FGF-2 expression rapidly via Src-mediated production of Egr-1. Cloning and TRANSFAC analysis of human FGF-2 promoter revealed the presence of 2 Egr-1 binding sites, and 15(S)-HETE induced Egr-1 binding to FGF-2 promoter both in vitro and in vivo in a manner that is dependent on Src activation. Site-directed mutagenesis demonstrated that between the 2 Egr-1 binding sites, the one that is proximal to transcriptional start site is crucial in mediating the effects of 15(S)-HETE on FGF-2 promoter-luciferase reporter gene activity. Blockade of FGF-2 via its neutralizing antibodies suppressed the migration and tube-like structure formation of HDMVECs and Matrigel plug angiogenesis. Although AA, the substrate for cyclooxygenases, lipoxygenases, and epoxygenases, induced angiogenesis in WT mice, it failed to stimulate a similar angiogenic response in 12/15-LOX−/− mice, which was rescued by 15(S)-HETE. These findings clearly show that 15(S)-HETE–induced angiogenesis requires Src-mediated Egr-1–dependent rapid induction of expression of FGF-2.
The role of 15-LOX1 and its murine ortholog, 12/15-LOX, in the pathogenesis of both atherosclerosis and restenosis has been demonstrated.17-19 Besides its involvement in the oxidation of low-density lipoprotein, the other mechanisms by which 15-LOX1 influences the pathogenesis of these vessel wall diseases are largely unknown. In this aspect, work from our laboratory showed that 15(S)-HETE, a preferential product of 15-LOX1/2, possesses the capacity to influence angiogenesis.24-26,38 12(S)-HETE, the AA metabolite of 12/15-LOX, also has been reported to stimulate angiogenesis.39 In addition, upon incubation with AA atherosclerotic arteries produced 15(S)-HETE preferentially.16,40 Recently, we have shown that forced expression of 15-LOX2 in rat vascular smooth muscle cells or 15-LOX1 in balloon-injured arteries led to production of only 15(S)-HETE.41,42 Many reports also revealed that angiogenesis plays a critical role in the progression of atherosclerosis and restenosis.34,35
Our findings showed that 12/15-LOX−/− mice fail to exhibit an angiogenic response to AA compared with WT mice. However, the addition of 15(S)-HETE to the Matrigel restored the angiogenic response in these mice. These observations imply that conversion of AA to 15(S)-HETE is essential for induction of angiogenesis. In fact, these conclusions were further reinforced by the findings that Matrigel plugs from only WT mice that were supplemented with AA but not either control WT mice or 12/15-LOX−/− mice with or without AA supplementation possess the capacity to produce 15(S)-HETE. These results also infer that the angiogenic endothelial cells convert AA to 15(S)-HETE. Previous studies by others17,18 have shown that 12/15-LOX−/− mice develop diminished atherosclerosis in response to a high-fat diet and reduced restenosis in response to vascular injury compared with WT mice. Hence, on the basis of these observations, one can assume that 15(S)-HETE by its capacity to induce angiogenesis may be an important mediator in the pathogenesis of these vascular lesions.
The authors of several studies29,43 have shown that FGF-2 plays a major role in angiogenesis. In addition, a role for Egr-1 in the regulation of FGF-2 expression has been reported in many cell types, including endothelial cells and cardiac myocytes.29,32 The present findings also show a role for Egr-1 in 15(S)-HETE–induced FGF-2 expression, and Src appears to be involved in this transcriptional up-regulation of FGF-2 upstream to Egr-1. In addition, the EMSA and ChIP analysis indicated that Egr-1 binds to FGF-2 promoter directly both in vitro and in vivo. Cloning and characterization of human FGF-2 promoter revealed the presence of 2 Egr-1 binding sites and the Egr-1 binding site that is proximal to transcriptional start site is essential for 15(S)-HETE–induced FGF-2 promoter-luciferase reporter gene activity.
The authors of other studies32,44 have shown that depending on agonist used, either both the Egr-1 binding sites at −57 nt and −157 nt or the latter site is involved in FGF-2 promoter activity.32,44 Thus, our results provide a mechanism for 15(S)-HETE–induced expression of FGF-2 in HDMVECs. A large body of evidence45,46 indicates that FGF-2 is produced in atherosclerotic and restenotic arteries and that neutralizing antibodies inhibit injury-induced vascular wall remodeling. Because we and others16,40-42 previously demonstrated that atherosclerotic and restenotic arteries produce 15(S)-HETE abundantly, it is possible that 15(S)-HETE may be a contributing factor in the expression of FGF-2 in these vascular diseases. In fact, it was shown that inhibition of 15-LOX1 via a pharmacologic approach attenuates atherosclerosis.47,48 In view of these observations, one can assume that 15-LOX1/2, via their role in the production of 15(S)-HETE, may be a crucial mediator in the progression of peripheral vascular diseases such as atherosclerosis and restenosis. In conclusion, in this report we provide mechanistic evidence for the ability of 15-LOX1/2 products of AA in the stimulation of angiogenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant (HL074860) from the National Heart, Lung, and Blood Institute/National Institutes of Health.
National Institutes of Health
Authorship
Contribution: V.K.-S. performed ChIP assays, EIA, EMSA, tube formation assay, and assisted in data analysis and the writing of the paper; J.N. performed QRT-PCR, Western blotting, ELISA, and the migration assay; D.W. performed immunohistochemistry and the Matrigel plug angiogenesis assay; D.V.Q. performed cloning; Q.Z. performed QRT-PCR; N.K.S. performed Matrigel plug angiogenesis; J.S. performed Western blot analysis; S.K. amplified and purified adenovirus; and G.N.R. designed the experiments, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gadiparthi N. Rao, PhD, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave, Memphis, TN 38163; e-mail: grao@physio1.utmem.edu.


![Figure 6. Egr-1 binds to FGF-2 promoter in response to 15(S)-HETE both in vitro and in vivo in Src-dependent manner. (A,C) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity in vitro with the use of [32P]-labeled double stranded oligonucleotide probe that corresponds to the Egr-1 binding site proximal to the transcriptional start site in the FGF-2 promoter (A) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (C). (B,D) HDMVECs that were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40 and quiesced were treated with and without 15(S)-HETE (0.1μM) for 30 minutes and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity as described in panel A (B) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/10/10.1182_blood-2009-09-241802/4/m_zh89991049530006.jpeg?Expires=1768265431&Signature=nuC41plH8xM~IxVjSS5dw15fJT7k~jXwjLTLF87CfJyPBIwu-peSuRhAwSrHqKvsizq194Pq7J5yRtiZ78jCo16iT8XqbcZfNF2FobHvainEDO5NZe9NmShEduR4-Az52Q5129Jy9u7otSTbwNOUNt1DDTFf0eBNeGaQAZJrVtym9b52iqohV5N6dWJTtTQoAC5CHfX67isEYBUya5kEst3mDm3rnycAVJCSpQZPUlaILwviJoplngv6XrkPOsdVTt~7anYvJip3wz0r7L0SEyDZ7LX7wzYRC9K9lnJ493pg6-HqzSy~30oRv5nGUvPWm6HhNesX8IijfV7806G69A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Egr-1 binds to FGF-2 promoter in response to 15(S)-HETE both in vitro and in vivo in Src-dependent manner. (A,C) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity in vitro with the use of [32P]-labeled double stranded oligonucleotide probe that corresponds to the Egr-1 binding site proximal to the transcriptional start site in the FGF-2 promoter (A) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (C). (B,D) HDMVECs that were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40 and quiesced were treated with and without 15(S)-HETE (0.1μM) for 30 minutes and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity as described in panel A (B) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/10/10.1182_blood-2009-09-241802/4/m_zh89991049530006.jpeg?Expires=1768894113&Signature=QQEBjGcM6d3nN5wAD9ckIa5Wa-yPSQ~BI~tYldYOsMCg16VBpJC8KZjt0CxLkAQDHCG~3~Ufm6fj2BjwmuYUpcCIK7H~2kwRA8OmkygnhrU~V-5mIbDlta08eMeTl5ldgmQjOahIkak7ULaCgpx5pKMkYKJlf3A3eVfmioM9Gi8sTR6F7tm0GFrmpLN8scIIbjq-n8gatPlWBGgtwIETZ6BLtngL3-drAyMI7VINfzEIDjK6N0A3IbTaGLbOYC51jpzWqCEY322BPDl9Fn4aKRmdFlthON-MnPPpSfiErLdrdwP90Hq1PH9p1L~Ogqn7r49PK01w6bUe5sZx9OS9Ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)