Abstract
The transforming and tumor growth–promoting properties of Axl, a member of the Tyro3, Axl, and Mer (TAM) family of receptor tyrosine kinases (TAMRs), are well recognized. In contrast, little is known about the role of the TAMR ligand growth arrest–specific gene 6 (Gas6) in tumor biology. By using Gas6-deficient (Gas6−/−) mice, we show that bone marrow–derived Gas6 promotes growth and metastasis in different experimental cancer models, including one resistant to vascular endothelial growth factor inhibitors. Mechanistic studies reveal that circulating leukocytes produce minimal Gas6. However, once infiltrated in the tumor, leukocytes up-regulate Gas6, which is mitogenic for tumor cells. Consistent herewith, impaired tumor growth in Gas6−/− mice is rescued by transplantation of wild-type bone marrow and, conversely, mimicked by transplantation of Gas6−/− bone marrow into wild-type hosts. These findings highlight a novel role for Gas6 in a positive amplification loop, whereby tumors promote their growth by educating infiltrating leukocytes to up-regulate the production of the mitogen Gas6. Hence, inhibition of Gas6 might offer novel opportunities for the treatment of cancer.
Introduction
Growth arrest–specific gene 6 (Gas6) was discovered because its expression is up-regulated in fibroblasts under growth-arrest conditions.1,2 This factor exerts pleiotropic functions in health and disease: it amplifies platelet aggregation during thrombus formation,3,4 enhances erythropoiesis,5 and increases leukocyte extravasation in inflammatory conditions,6 among other functions.7,8 Gas6 binds to the Tyro3, Axl, and Mer (TAM) family of receptor tyrosine kinases (TAMRs), which consists of Tyro3 (Sky/Rse), Axl (Ufo/Ark), and Mer (Eyk), although the binding affinity of this ligand differs for each receptor (Axl>Tyro3≫Mer).9,10
TAMRs, in particular Axl, have transforming properties. Indeed, overexpression of a truncated version of Axl in premalignant cells is sufficient to induce tumors in mice.11 Axl is also highly expressed in human tumor cells in vitro,12-15 as well as in a large variety of primary human cancers, including leukemia,16 gastric cancer,17 colon cancer,18 breast cancer,19 ovarian cancer,20 and glioblastoma,21 among others. In gastric cancer, Axl expression is associated with lymph node metastasis, an adverse prognostic factor.17
However, little is known about the role of Gas6 in cancer. Gas6 is overexpressed in human ovarian, endometrial, gastric, thyroid, and glioblastoma tumors.17,20-23 In those studies, expression of Gas6 was detected in tumor cells, endothelial cells, and astrocytes,20-24 but expression of Gas6 in tumor-infiltrated leukocytes has not been studied. Gas6 promotes proliferation and survival of different cancer cell lines, including prostate and melanoma tumor cells.13,14 Interestingly, when glioma tumors coexpress high levels of Axl and Gas6, the survival of cancer patients is shortened.21 Inhibition of Gas6 by a soluble Axl trap in vitro or a dominant-negative variant of Axl in tumor cells in vivo suppresses proliferation and invasion of cancer cells, thereby prolonging survival of tumor-bearing mice.17,25
In general, little is known about the role of Gas6 in the tumor stroma, especially about its expression in tumor-inflaming leukocytes, which play a key role in cancer progression.26,27 Intratumoral leukocyte infiltrates vary in size, composition, and distribution, and, in most but not in all instances, they are believed to promote tumor progression via several mechanisms, including secretion of growth factors, proangiogenic cytokines, and proteases.26 Tumor-associated macrophages (TAMs) are prominent components of leukocyte infiltrates and frequently are polarized to an M2 phenotype, characterized by growth-promoting, proangiogenic, and immunosuppressive properties.26-28 As a result, the presence of high numbers of macrophages in tumors often confers a negative prognosis for cancer patients.28 However, TAMs exhibit a remarkable level of complexity and also can be skewed toward an M1-polarized phenotype with a potential to kill tumor cells via phagocytosis or to activate antitumoral immune effector cells.28
Because Gas6 is present in hematopoietic cells,29-31 we explored whether stromal and, in particular, macrophage-derived Gas6 might play a role in tumor biology, and therefore implanted wild-type (WT) tumors in Gas6−/− mice. Here, we unravel a previously unknown mechanism whereby tumors educate infiltrating leukocytes to up-regulate Gas6 and as such promote their growth.
Methods
For further details, refer to the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Animals and syngeneic tumor models
The generation of Gas6-deficient mice (Gas6−/−) was described.4 All tumor models were performed as described previously.32 All cell lines were maintained as described.32 All experimental animal procedures were approved by the Institutional Animal Care and Research Advisory Committee of the K.U.Leuven.
In vitro assays
Concentrations of Gas6, sAxl, and plasminogen-activator inhibitor-1 were quantified as described.33 Interleukin-10 (IL-10) and macrophage colony-stimulating factor (M-CSF) protein levels were determined as recommended (R&D Biosystems). Quantitative reverse-transcription polymerase chain reaction (RT-PCR) was performed as described34,35 by use of the probes and primers listed in supplemental Table 1. Human recombinant Gas6 was produced as described.36 As already described, human Gas6 binds to murine Axl.8 Proliferation was measured by the use of the Cell Proliferation Reagent WST-1 (Roche). F4/80+ cells underwent fluorescent-activated cell sorting (FACS) from CT26 tumors and seeded into 24-well plates in RPMI medium (supplemented with 10% fetal bovine serum [FBS] and 1% penicillin/streptomycin; Invitrogen). After 24 hours, they were cocultured with CT26 tumor cells seeded onto cell culture inserts (pore size 0.4 μm; Becton Dickinson) in RPMI medium (supplemented with 0.1% FBS and 1% penicillin/streptomycin; Invitrogen). Forty-eight hours later, proliferation was assessed in both compartments.
Isolation of peritoneal macrophages and stimulation with cytokines
We isolated peritoneal macrophages by flushing the intraperitoneal cavity of 8-week-old mice with RPMI medium (supplemented with 10% FBS and 1% penicillin/streptomycin; Invitrogen) and allowed them to become adherent overnight. Subsequently, they were stimulated for 24 hours with recombinant vascular endothelial growth factor (VEGF), placental growth factor (PlGF), M-CSF, IL-4, or IL-10 (VEGF and PlGF were used at a concentration of 50 ng/mL, all others at 10 ng/mL; all cytokines were from R&D Biosystems). White blood cells, bone marrow cells, spleen cells, and cells from peritoneal and alveolar lavages and from tumors were harvested as described.37,38
Differential blood counts
Differential blood counts were determined by the use of the Cell-Dyn 3500 apparatus (Abbott). Prothrombin time and activated partial thromboplastin time were assessed on a KC10 machine (Amelung).
Histology, immunostaining, and morphometric analyses
Cornea pocket, hind-limb ischemia, and myocardial revascularization
These assays were performed as described previously.35
Bone marrow transplantation
Balb/C and C57/Bl6 WT and Gas6−/− recipient mice were irradiated with 9 Gy and 9.5 Gy, respectively. Subsequently 5 × 105 (1 × 106 C57/Bl6) bone marrow cells from green fluorescent protein (GFP)+ WT or GFP+ Gas6−/− mice were injected intravenously via the tail vein. Tumors were implanted 6 weeks after bone marrow transplantation.
Statistics
Data represent mean plus or minus SEM of representative experiments unless otherwise stated. Statistical significance was calculated by a Student t test except for tumor growth kinetics, where analysis of variance was used (Prism Version 4.0b; GraphPad Software).
Results
Expression of Gas6 in the tumor stroma and of TAMRs in tumor cells
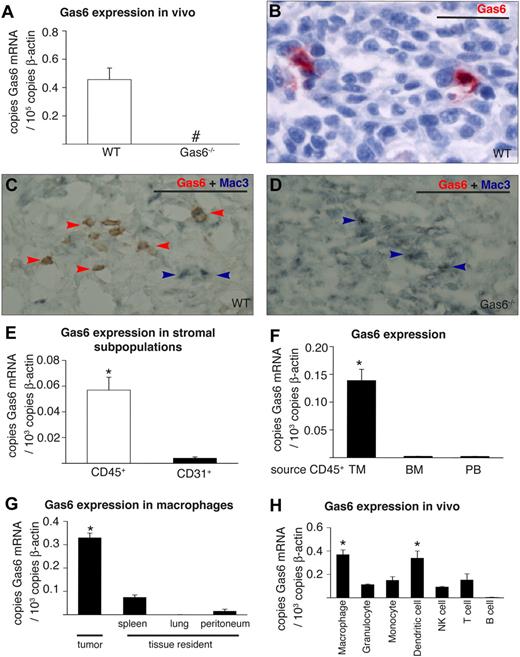
To elucidate the role of Gas6 in tumor biology, we first studied its expression in different murine tumor cell lines in vitro (CT26 colon, 4T1 breast, TSA breast, PancO2 pancreatic, B16 melanoma). By RT-PCR analysis, none of these tumor lines expressed detectable Gas6 mRNA levels in baseline conditions or in hypoxia or serum starvation, that is, conditions that resemble the tumor microenvironment (not shown). We then tested whether CT26, 4T1, or PancO2 tumors expressed Gas6 mRNA when grown in vivo. To dissect the contribution of tumor-versus-host–derived Gas6, we compared Gas6 expression in tumors grown in Gas6−/− or WT mice. Gas6 mRNA was undetectable in tumors in Gas6−/− mice but readily measurable in tumors in WT mice, indicating that Gas6 was produced by host-derived stromal cells (Figure 1A; not shown for PancO2 and 4T1). Enzyme-linked immunosorbent assay (ELISA) measurements confirmed that Gas6 was present in extracts of CT26 and PancO2 tumors in WT mice (52 ± 9.8 pg/mg, not shown for PancO2), but not of tumors in Gas6−/− mice (not shown). By immunostaining, Gas6 was detectable in tumor-infiltrating leukocytes (Figure 1B), including in macrophages in CT26 and 4T1 tumors, as shown by double immunostaining for Mac3 and Gas6 (Figure 1C-D; not shown for 4T1). Gas6 was also weakly detectable in blood vessels in CT26 and 4T1 tumors (not shown) but below the threshold of detection in other noninflammatory cell types.
Expression of Gas6 in the tumor stroma. (A) RT-PCR analysis revealing detectable Gas6 expression in CT26 tumors in WT but not in Gas6−/− mice (n = 7-8; # indicates not detectable). (B) Staining for Gas6 in CT26 tumors grown in WT mice showing Gas6 expression (red) in tumor-infiltrating leukocytes, identified on the basis of their characteristic morphology by an expert pathologist. (C) Double immunostaining of CT26 tumors for Gas6 (red) and Mac3 (blue-gray) in WT mice. Most Mac3+ macrophages express Gas6 (brownish color: merger of blue and red; red arrowheads), whereas a few macrophages do not express Gas6 (blue arrowheads). (D) Gas6 was undetectable in macrophages in Gas6−/− mice (blue-gray; blue arrows). (E) RT-PCR analysis revealing greater Gas6 expression in FACS sorted CD45+ leukocytes than CD31+ endothelial cells in CT26 tumors in WT mice (n = 4; P = .002). (F) RT-PCR analysis, showing Gas6 up-regulation in CD45+ leukocytes upon infiltration in CT26 tumors. The source of the CD45+ leukocytes is indicated: TM (tumor infiltrated), BM (bone marrow), PB (peripheral blood; n = 4; P < .001). (G) RT-PCR analysis showing increased Gas6 expression in CT26 tumor-infiltrating macrophages (n = 4) in comparison with tissue resident macrophages isolated from spleen, lung, and peritoneum (n = 3 each; P < .05). (H) RT-PCR analysis of different subfractions of WBCs infiltrating CT26 tumors (n = 3; P < .05). Scale bars represent 50 μm in panels B-D. *Denotes statistical significance (P < .05). Error bars in panels A, E, F, G, and H show SEM; all subsequent error bars are defined similarly.
Expression of Gas6 in the tumor stroma. (A) RT-PCR analysis revealing detectable Gas6 expression in CT26 tumors in WT but not in Gas6−/− mice (n = 7-8; # indicates not detectable). (B) Staining for Gas6 in CT26 tumors grown in WT mice showing Gas6 expression (red) in tumor-infiltrating leukocytes, identified on the basis of their characteristic morphology by an expert pathologist. (C) Double immunostaining of CT26 tumors for Gas6 (red) and Mac3 (blue-gray) in WT mice. Most Mac3+ macrophages express Gas6 (brownish color: merger of blue and red; red arrowheads), whereas a few macrophages do not express Gas6 (blue arrowheads). (D) Gas6 was undetectable in macrophages in Gas6−/− mice (blue-gray; blue arrows). (E) RT-PCR analysis revealing greater Gas6 expression in FACS sorted CD45+ leukocytes than CD31+ endothelial cells in CT26 tumors in WT mice (n = 4; P = .002). (F) RT-PCR analysis, showing Gas6 up-regulation in CD45+ leukocytes upon infiltration in CT26 tumors. The source of the CD45+ leukocytes is indicated: TM (tumor infiltrated), BM (bone marrow), PB (peripheral blood; n = 4; P < .001). (G) RT-PCR analysis showing increased Gas6 expression in CT26 tumor-infiltrating macrophages (n = 4) in comparison with tissue resident macrophages isolated from spleen, lung, and peritoneum (n = 3 each; P < .05). (H) RT-PCR analysis of different subfractions of WBCs infiltrating CT26 tumors (n = 3; P < .05). Scale bars represent 50 μm in panels B-D. *Denotes statistical significance (P < .05). Error bars in panels A, E, F, G, and H show SEM; all subsequent error bars are defined similarly.
When analyzed in culture, all aforementioned tumor lines expressed each of the Gas6 receptors, with Axl being more abundant than Tyro3 and Mer (not shown). In CT26 and 4T1 tumors, the expression of Axl also was more prominent than the 2 other TAMRs, however, with no differences in their expression between WT and Gas6−/− hosts (mRNA copies per 103 copies β-actin in CT26 tumors in WT vs Gas6−/− mice: 4.0 ± 0.35 vs 4.1 ± 0.35 for Axl; 0.04 ± 0.004 vs 0.05 ± 0.004 for Tyro3; 0.07 ± 0.008 vs 0.07 ± 0.006 for Mer; n = 7-10; P = NS; not shown for 4T1). Comparable Axl protein levels also were detected in CT26 tumors grown in either genotype (pg/mg: 493 ± 26 in WT mice vs 565 ± 47 in Gas6−/− mice). All 3 TAMRs were also detectable in CD45+ tumor-infiltrating leukocytes isolated from CT26 tumors, and, consistent with previous findings, in tumor endothelial cells, when analyzed by quantitative RT-PCR (not shown).21
Tumors educate infiltrated leukocytes to produce Gas6
To further analyze the expression of Gas6 in tumor-infiltrating leukocytes, by the use of FACS we sorted CD45+ leukocytes and CD31+ endothelial cells from CT26 tumors grown in WT mice and analyzed Gas6 expression by RT-PCR immediately after isolation without further subculturing. These experiments revealed that Gas6 transcript levels were 20-fold greater in leukocytes than in endothelial cells (n = 4, P < .005; Figure 1E). In contrast to these tumor-infiltrated leukocytes, CD45+ leukocytes that were freshly harvested from the bone marrow or peripheral blood of tumor-bearing mice expressed only minimal levels of Gas6 (n = 4, P < .001; Figure 1F). In addition, Gas6 levels were negligible in peripheral blood– and bone marrow–derived leukocytes from nontumor-bearing mice (not shown).
Further analysis revealed that Gas6 mRNA levels were abundant in F4/80+ TAMs, consistent with the aforementioned immunostaining data (Figure 1G). To determine whether the tumor microenvironment itself might “educate” TAMs to up-regulate Gas6 expression in situ, we sorted via FACS, as controls, resident F4/80+ tissue macrophage populations from the peritoneum, lung, and spleen of CT26 tumor-bearing mice and analyzed, by RT-PCR analysis, their Gas6 expression. These experiments revealed substantially greater Gas6 expression in TAMs than in resident tissue macrophages (n = 4/3, P < .001; Figure 1G). These data were confirmed in mice bearing orthotopic PancO2 tumors (not shown). Hence, the tumor microenvironment educates TAMs to express Gas6. This up-regulation of Gas6 was specific because mRNA levels of its homologue Protein S were not up-regulated in TAMs compared with tissue macrophages (supplemental Figure 1A) or in tumor-infiltrating CD45+ cells, compared with circulating leukocytes (supplemental Figure 1B).
To elucidate whether other types of tumor-infiltrating leukocytes express Gas6 within the tumor stroma, we sorted, via FACS, granulocytes (Gr1hiCD11blow-high), monocytes (Gr1−CD11bhigh), dendritic cells (CD11c+), T cells (CD3+), B cells (B220+), and natural killer cells (Dx5+CD3−) from CT26 tumors and compared their Gas6 expression to that in TAMs. RT-PCR analysis indicated comparable Gas6 levels in dendritic cells, whereas a significantly lower production of Gas6 was detectable in all other leukocyte subfractions, with even undetectable Gas6 levels in B cells (n = 3; P < .05; Figure 1H). Because macrophages are more abundant than dendritic cells in CT26 tumors (percent of all tumor and stromal cells: 4.8% ± 0.6% vs 0.8% ± 0.2%; P < .001; n = 4-5), the bulk of Gas6 in the tumor milieu is thus released by TAMs.
Gas6 deficiency inhibits tumor growth and metastasis
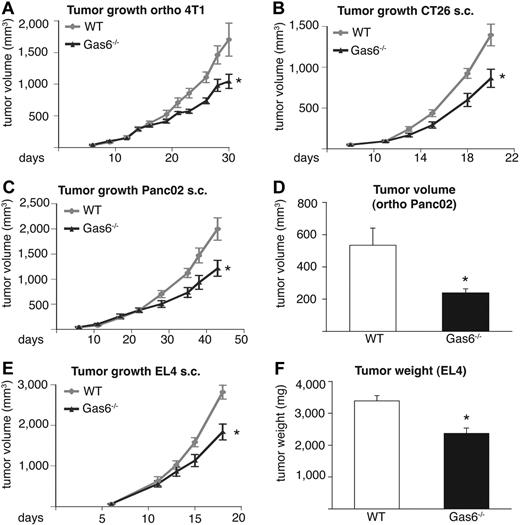
Given the up-regulation of Gas6 in tumor-infiltrating leukocytes, we analyzed the role of Gas6 in tumor growth by using 4 different syngeneic tumor models in 2 different genetic backgrounds. In Gas6−/− mice, the size of end-stage subcutaneous CT26 tumors was reduced on average by 38% (n = 6-8; P < .05), whereas the size of orthotopic 4T1 tumors was decreased by 36% (n = 7-8; P < .05). Likewise, growth of subcutaneous PancO2 tumors was diminished by 39% (n = 8-10; P < .05) and that of orthotopic PancO2 tumors by 55% (n = 7-8; P < .05; Figure 2A-D). Measurements of end-stage tumor weight confirmed these findings (supplemental Figure 1C-F).
Gas6 deficiency inhibits tumor growth in different tumor models. In all panels, the tumor size in Gas6−/− mice is smaller than in WT mice. (A) Growth of orthotopic 4T1 breast tumors (n = 6-8; P < .05). (B) Growth of subcutaneous (s.c.) CT26 colon tumors (n = 7-9; P = .01). (C) Growth of subcutaneous Panc02 pancreatic tumors (n = 8-10; P = .02). (D) End-stage tumor volume of orthotopic Panc02 pancreatic tumors (n = 7-8; P = .01). (E) Growth of subcutaneous EL4 tumors (n = 6; P < .05). (F) End-stage tumor weight of subcutaneous EL4 tumors (n = 6; P = .001).
Gas6 deficiency inhibits tumor growth in different tumor models. In all panels, the tumor size in Gas6−/− mice is smaller than in WT mice. (A) Growth of orthotopic 4T1 breast tumors (n = 6-8; P < .05). (B) Growth of subcutaneous (s.c.) CT26 colon tumors (n = 7-9; P = .01). (C) Growth of subcutaneous Panc02 pancreatic tumors (n = 8-10; P = .02). (D) End-stage tumor volume of orthotopic Panc02 pancreatic tumors (n = 7-8; P = .01). (E) Growth of subcutaneous EL4 tumors (n = 6; P < .05). (F) End-stage tumor weight of subcutaneous EL4 tumors (n = 6; P = .001).
Because orthotopic 4T1 breast tumors metastasize to the lungs and orthotopic PancO2 tumors spread to mesenteric lymph nodes, we used these models to study the effect of Gas6 deficiency on tumor metastasis. Loss of stromal Gas6 inhibited pulmonary metastasis of 4T1 tumors by 43% (pulmonary metastases/mouse: 37.1 ± 6.8 vs 21.2 ± 2.8; n = 6-8; P < .05) and mesenteric lymph node metastases of PancO2 tumors by 59% (mesenteric metastases/mouse 30.9 ± 2.6 vs 12.6 ± 1.7; n = 8-9; P < .01). Calculation of the metastatic indices (number of metastases per gram of primary tumor) revealed, however, that the reduced tumor metastasis in Gas6−/− mice was related to the size of the primary tumor in both models (supplemental Figure 1G-H), suggesting that the reduced metastasis in Gas6−/− mice was secondary to the reduced primary tumor growth.
To elucidate whether Gas6 deficiency also inhibits the growth of tumors that are resistant to VEGF inhibitors,41 we implanted EL4 lymphoma tumors subcutaneously in syngeneic Gas6−/− mice. The deficiency of stromal Gas6 impaired the growth of these tumors, which reduced end-stage tumor volume by 35% (n = 6; P < .01) and end-stage tumor weight by 30% (n = 6, P < .01; Figure 2E-F). Thus, Gas6 deficiency inhibits the growth of different murine tumor models in vivo in addition to one resistant to VEGF inhibitors. For the analysis of the underlying mechanisms of Gas6, we focused on the CT26 tumor model, in part because colorectal cancer is a leading cause of cancer-related mortality in humans.
Gas6 deficiency impairs proliferation of tumor cells in vivo
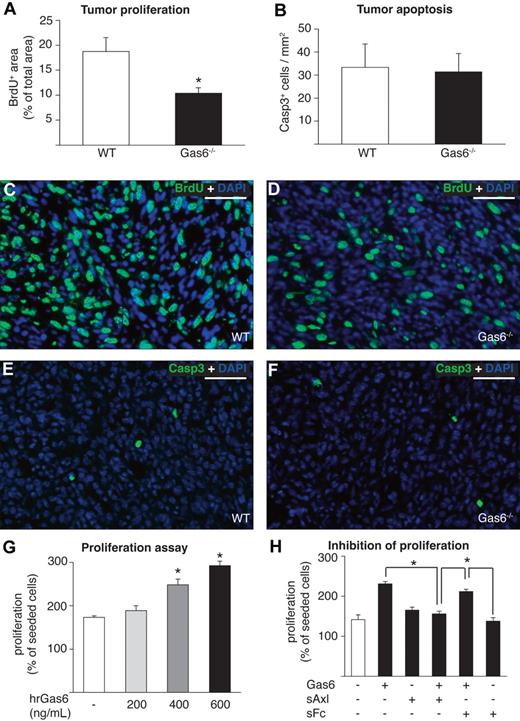
To investigate whether the reduced tumor growth in Gas6−/− mice was the result of decreased tumor cell proliferation and/or survival, we injected bromodeoxyuridine (BrdU) intraperitoneally before the dissection of end-stage CT26 tumors. Analysis of BrdU+ tumor cells revealed a decrease of the fraction of proliferating tumor cells in Gas6−/− mice by 45% (n = 8, P < .01; Figure 3A,C,D). Subsequently, we analyzed apoptosis in the same series of tumors by immunostaining of activated Caspase 3 and found no difference in the number of apoptotic nuclei present in both genotypes (Figure 3B,E,F). The findings were confirmed in the orthotopic PancO2 model (not shown). Hence, the reduced tumor growth in Gas6−/− mice is likely attributable to the decreased tumor cell proliferation.
Gas6 induces proliferation of tumor cells in vitro and in vivo. (A) Morphometric analysis of proliferation on BrdU-stained CT26 tumor sections revealing reduced proliferation of tumor cells in Gas6−/− vs WT mice (n = 8; P < .005). (B) Morphometric analysis of Caspase 3 (Casp3) staining showing similar tumor cell apoptosis in CT26 tumors in both genotypes (n = 8; P = NS). (C-D) Representative micrographs of immunofluorescent BrdU stainings (BrdU+ nuclei stained in green, all nuclei stained in blue with DAPI) of CT26 tumors grown in WT mice and Gas6−/− mice indicate reduced proliferation of tumor cells in Gas6−/− mice. (E-F) Representative micrographs of immunofluorescent stainings of activated Caspase 3 (activated Caspase 3+ nuclei stained in green, all nuclei stained in blue with DAPI) indicating similar numbers of apoptotic cells in CT26 tumors grown in WT mice and Gas6−/− mice. (G) Recombinant human Gas6 (hrGas6) dose-dependently induces proliferation of CT26 tumor cells in vitro after 48 hours (n = 3; P < .05). (H) Axl-Fc reduced proliferation of CT26 tumor cells induced by 400 ng/mL Gas6 compared with control without Fc (bar 4 vs bar 2; P = .001; n = 3), whereas the addition of control sFC led to a nonsignificant inhibition (bar 5 vs bar 2; P = NS; n = 3). Scale bars represent 100 μm in panels C-F.
Gas6 induces proliferation of tumor cells in vitro and in vivo. (A) Morphometric analysis of proliferation on BrdU-stained CT26 tumor sections revealing reduced proliferation of tumor cells in Gas6−/− vs WT mice (n = 8; P < .005). (B) Morphometric analysis of Caspase 3 (Casp3) staining showing similar tumor cell apoptosis in CT26 tumors in both genotypes (n = 8; P = NS). (C-D) Representative micrographs of immunofluorescent BrdU stainings (BrdU+ nuclei stained in green, all nuclei stained in blue with DAPI) of CT26 tumors grown in WT mice and Gas6−/− mice indicate reduced proliferation of tumor cells in Gas6−/− mice. (E-F) Representative micrographs of immunofluorescent stainings of activated Caspase 3 (activated Caspase 3+ nuclei stained in green, all nuclei stained in blue with DAPI) indicating similar numbers of apoptotic cells in CT26 tumors grown in WT mice and Gas6−/− mice. (G) Recombinant human Gas6 (hrGas6) dose-dependently induces proliferation of CT26 tumor cells in vitro after 48 hours (n = 3; P < .05). (H) Axl-Fc reduced proliferation of CT26 tumor cells induced by 400 ng/mL Gas6 compared with control without Fc (bar 4 vs bar 2; P = .001; n = 3), whereas the addition of control sFC led to a nonsignificant inhibition (bar 5 vs bar 2; P = NS; n = 3). Scale bars represent 100 μm in panels C-F.
That Gas6 is mitogenic for tumor cells was further underscored by in vitro studies demonstrating that recombinant Gas6 dose-dependently increased proliferation of cultured CT26 and 4T1 tumor cell lines (Figure 3G; not shown for 4T1), consistent with previous reports.13,14 Recombinant sAxl, supplemented in 10-fold molar excess of Gas6, significantly inhibited the mitogenic activity of Gas6 (Figure 3H). This effect was specific because an unrelated sFc-fragment, capturing VEGF (sVEGFR-1/2), failed to significantly inhibit Gas6-induced proliferation of CT26 tumor cells (Figure 3H).
Gas6 deficiency does not regulate tumor angiogenesis or inflammation
To assess whether the reduced tumor growth in the absence of stromal Gas6 was attributable to any effects on the tumor stroma, we characterized tumor angiogenesis, a plausible hypothesis, given that Gas6 and its receptors are expressed at low levels in tumor endothelial cells (not shown) and that previous studies documented a role for TAMRs in vascular biology.42-44 When analyzing CT26 and 4T1 tumor angiogenesis in Gas6−/− mice in comparison with WT mice, we could, however, not detect any differences in microvessel density or vessel lumen area after staining for CD31 (supplemental Figure 2A; 4T1 not shown), whereas tumor vessel perfusion (analyzed after injecting FITC-labeled lectin; supplemental Figure 2B; not shown for 4T1) or tumor hypoxia (analyzed by staining for the hypoxia-marker pimonidazole; supplemental Figure 2C; not shown for 4T1) was also comparable. Furthermore, analysis of tumor vessel maturation, by determining pericyte coverage of vessels via double staining for CD31 and α-smooth muscle actin, did also not reveal significant differences between Gas6−/− and WT mice (supplemental Figure 2D; not shown for 4T1). Finally, assessment of necrotic area by morphometric quantification of autofluorescent area revealed no differences (4T1 and CT26 not shown). Altogether, these results suggest that absence of Gas6 has a negligible impact on tumor angiogenesis. Consistent herewith, we could not detect any differences in pathologic angiogenesis in Gas6−/− mice, compared with WT control mice, when using additional angiogenesis models, including the cornea pocket assay or the hind-limb ischemia or coronary artery ligation assays (supplemental Figure 3A-E). In 4T1 tumors grown in Gas6−/− mice, no differences in lymphatic vessel area or density could be detected (supplemental Figure 3F; density not shown).
Given that TAMRs are expressed by leukocytes,10 we also analyzed leukocyte infiltration in CT26, 4T1, and PancO2 tumors but could not detect any genotypic differences in the number of CD45+ cells inside tumors (supplemental Figure 3G; not shown for 4T1 and PancO2). In addition, there were no significant genotypic differences in TAMR expression in CD45+ tumor-infiltrating leukocytes or F4/80+ macrophages (not shown). Furthermore, morphometric quantification of the number of vimentin+ cells revealed no genotypic differences in the accumulation of cancer-associated fibroblasts in CT26 and 4T1 tumors (supplemental Figure 3H; not shown for 4T1). Because Gas6 has been implicated in thrombus stabilization,4 we also investigated different parameters of coagulation in tumor-bearing WT and Gas6−/− mice. Analysis of the prothrombin time and the activated partial thromboplastin time, as well as measurement of plasma levels of the plasminogen-activator inhibitor-1 or intratumoral levels of fibrin(ogen)+ or von Willebrand factor+ thrombi showed, however, no genotypic differences, rendering it unlikely that Gas6 deficiency influenced intratumoral coagulation substantially (supplemental Figure 4A-E).
Transplantation of WT bone marrow rescues tumor growth in Gas6−/− mice
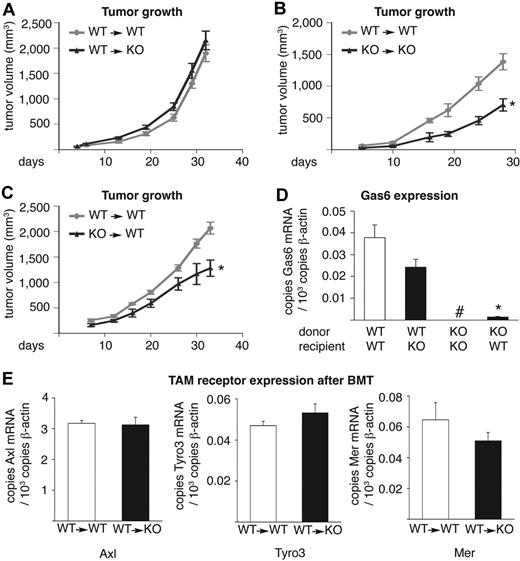
To further explore whether the reduced tumor growth in Gas6−/− mice was attributable to the lack of Gas6 expression by tumor-infiltrating leukocytes, we transplanted WT bone marrow of syngeneic mice, ubiquitously expressing GFP, into lethally irradiated Gas6−/− recipients (referred to as WT→KO mice) or performed the reverse experiment (KO→WT mice). The transplantation of WT bone marrow in WT hosts (WT→WT mice) or Gas6−/− bone marrow in Gas6−/− hosts (KO→KO mice) was used as positive and negative control, respectively. At 6 weeks after bone marrow transplantation, when CT26 colon tumors were injected subcutaneously, hematopoietic reconstitution and differential blood counts were comparable in all genotypes (not shown).
The growth of CT26 tumors in WT→WT mice was slower than tumor growth in WT hosts without bone marrow transplantation; similarly, CT26 tumor growth was slower in KO→KO mice than in Gas6−/− hosts without bone marrow transplantation (compare Figure 4A with Figure 2B and Figure 4B with Figure 2B). A delay in tumor growth in bone marrow–transplanted mice has been previously ascribed to radiation-induced damage of the host vasculature and connective tissue.45-51 Notably however, within the group of bone marrow–transplanted mice, tumor growth was reduced in KO→KO mice compared with WT→WT mice and could be rescued by transplantation of WT bone marrow in Gas6−/− mice (WT→KO mice) to similar levels as observed in WT→WT mice (Figure 4A,B), indicating that WT bone marrow is sufficient to restore tumor growth in Gas6−/− mice. Similar results were obtained when analyzing end-stage tumor weights (supplemental note). Conversely, tumor growth was reduced in KO→WT mice, compared with WT→WT mice, to a similar extent as in KO→KO mice (Figure 4B-C; supplemental note). These data were confirmed in the PancO2 and 4T1 tumor models (supplemental Figure 5A-B). Thus, Gas6 expression by bone marrow–derived leukocytes determines overall tumor growth in these crossover tumor experiments.
Tumor growth–promoting Gas6 is delivered by bone marrow–derived cells. For technical reasons, the data shown in panels A through C were generated in different experiments; therefore, WT→WT mice were included as reference controls in each group. Tumor growth of subcutaneous CT26 tumors in (A) WT→WT– and WT→KO bone marrow–transplanted mice (n = 6-8; P = NS) and in (B) WT→WT and KO→KO mice (n = 5; P < .05). The reduced growth of CT26 tumors in KO→KO mice (B) was completely rescued by transplantation of WT bone marrow in Gas6−/− mice (WT→KO mice; A), to similar levels as observed in WT→WT mice (A). (C) Tumor growth of subcutaneous CT26 tumors in WT→WT and KO→WT mice (n = 5; P < .01). Tumor growth was reduced in KO→WT mice compared with WT→WT mice to a similar extent as in KO→KO mice. Hence, Gas6 expression by bone marrow–derived cells promotes tumor growth. (D) RT-PCR analysis, showing Gas6 expression of CT26 tumors grown in chimeric bone marrow–transplanted mice (n = 5-8). Gas6 mRNA levels were undetectable in KO→KO mice but were rescued in WT→KO mice to nearly similar levels as present in WT→WT mice (#undetectable; n = 7-8; P = NS). Conversely, in KO→WT mice, Gas6 mRNA levels were very low. Thus, bone marrow–derived cells deliver Gas6 into tumors (data were generated by the use of tumors from different experiments shown in panels A-C). (E) RT-PCR analysis showing similar TAM receptor expression in CT26 tumors grown in bone marrow–transplanted mice (n = 6-8; P = NS). BMT indicates bone marrow transplantation.
Tumor growth–promoting Gas6 is delivered by bone marrow–derived cells. For technical reasons, the data shown in panels A through C were generated in different experiments; therefore, WT→WT mice were included as reference controls in each group. Tumor growth of subcutaneous CT26 tumors in (A) WT→WT– and WT→KO bone marrow–transplanted mice (n = 6-8; P = NS) and in (B) WT→WT and KO→KO mice (n = 5; P < .05). The reduced growth of CT26 tumors in KO→KO mice (B) was completely rescued by transplantation of WT bone marrow in Gas6−/− mice (WT→KO mice; A), to similar levels as observed in WT→WT mice (A). (C) Tumor growth of subcutaneous CT26 tumors in WT→WT and KO→WT mice (n = 5; P < .01). Tumor growth was reduced in KO→WT mice compared with WT→WT mice to a similar extent as in KO→KO mice. Hence, Gas6 expression by bone marrow–derived cells promotes tumor growth. (D) RT-PCR analysis, showing Gas6 expression of CT26 tumors grown in chimeric bone marrow–transplanted mice (n = 5-8). Gas6 mRNA levels were undetectable in KO→KO mice but were rescued in WT→KO mice to nearly similar levels as present in WT→WT mice (#undetectable; n = 7-8; P = NS). Conversely, in KO→WT mice, Gas6 mRNA levels were very low. Thus, bone marrow–derived cells deliver Gas6 into tumors (data were generated by the use of tumors from different experiments shown in panels A-C). (E) RT-PCR analysis showing similar TAM receptor expression in CT26 tumors grown in bone marrow–transplanted mice (n = 6-8; P = NS). BMT indicates bone marrow transplantation.
To correlate Gas6 expression by infiltrating leukocytes with tumor growth, we also measured, by RT-PCR analysis, Gas6 expression in CT26 tumors of the aforementioned chimeric mouse models. This analysis revealed that tumor Gas6 mRNA levels, which were undetectable in KO→KO mice, were rescued in WT→KO mice to levels nearly approaching those found in WT→WT mice (Figure 4D). Conversely, in KO→WT mice, Gas6 mRNA levels were very low (Figure 4D). This low Gas6 expression in CT26 tumors in KO→WT mice was, however, not caused by the infiltration of fewer infiltrating leukocytes, and neither was the expression of Gas6 in tumors of WT→KO mice caused by the infiltration of more leukocytes because the number of tumor-infiltrated CD45+ cells was comparable in tumors grown in all different bone marrow chimeras and comparable with tumors grown in nontransplanted WT and Gas6−/− mice (compare supplemental Figure 3G with supplemental Figure 5C). In addition, expression of Axl, Tyro3, and Mer was comparable in tumors grown in WT→WT and in WT→KO mice (Figure 4E). These findings underline that bone marrow-derived (inflammatory) cells are indeed responsible for production of Gas6 and stimulation of tumor growth.
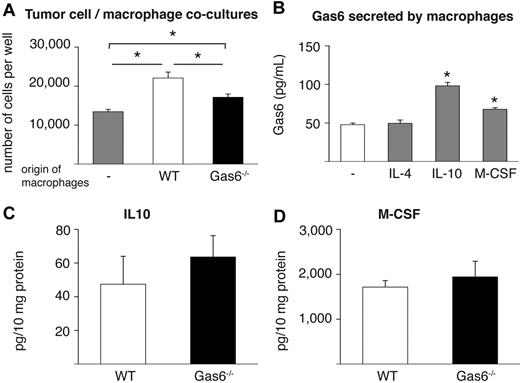
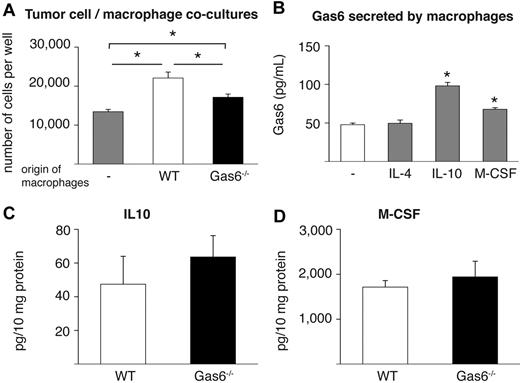
In attempt to further prove the role of leukocyte-derived Gas6 in tumor growth, we focused on tumor-infiltrating macrophages because they are well known to enhance tumor growth26,27 and express abundant levels of Gas6 (see “Tumors educate infiltrated leukocytes to produce Gas6”). We thus isolated F4/80+ macrophages from end-stage CT26 tumors grown in Gas6−/− and WT mice and subsequently cocultured them in vitro with CT26 tumor cells. These experiments revealed that tumor cell proliferation was enhanced when they were cocultured with WT macrophages compared with when tumor cells were cultured alone (Figure 5A). In contrast, when Gas6−/− macrophages were cocultured with CT26 tumor cells, tumor proliferation was only minimally enhanced (Figure 5A). Importantly, numbers of Gas6−/− and WT macrophages were comparable after the coculture period (not shown). Hence, the presence of Gas6 in tumor-infiltrating macrophages promotes proliferation of tumor cells in vitro, corroborating the aforementioned role of TAM-derived Gas6 for tumor growth.
IL-10 and M-CSF up-regulate Gas6 expression. (A) Cocultures of CT26 tumor cells with tumor-infiltrating macrophages isolated from CT26 tumors grown in WT and Gas6−/− hosts reveal that absence of Gas6 in macrophages significantly reduces their ability to stimulate proliferation of tumor cells in vitro (n = 3, P < .05). (B) ELISA revealing that IL-10 and M-CSF, but not IL-4, up-regulate Gas6 levels in the medium of cultured peritoneal macrophages after 24 hours (n = 3; P < .005). (C-D) ELISA measurement of CT26 tumor lysates showing similar IL-10 and M-CSF protein levels in WT and Gas6−/− mice (n = 8-9; P = NS).
IL-10 and M-CSF up-regulate Gas6 expression. (A) Cocultures of CT26 tumor cells with tumor-infiltrating macrophages isolated from CT26 tumors grown in WT and Gas6−/− hosts reveal that absence of Gas6 in macrophages significantly reduces their ability to stimulate proliferation of tumor cells in vitro (n = 3, P < .05). (B) ELISA revealing that IL-10 and M-CSF, but not IL-4, up-regulate Gas6 levels in the medium of cultured peritoneal macrophages after 24 hours (n = 3; P < .005). (C-D) ELISA measurement of CT26 tumor lysates showing similar IL-10 and M-CSF protein levels in WT and Gas6−/− mice (n = 8-9; P = NS).
IL-10 and M-CSF up-regulate Gas6 in macrophages
Finally, in an initial effort to start elucidating how tumors educate infiltrating leukocytes to up-regulate Gas6, we analyzed a panel of possible molecular candidates that might regulate Gas6 expression in differentiated macrophages. We therefore isolated peritoneal macrophages and incubated them with different cytokines known to be prominent in the tumor microenvironment (both malignant and stromal cells), including VEGF, PlGF, IL-4, IL-10, and M-CSF.26,27,52 Analysis of mRNA and protein expression revealed that Gas6 expression was up-regulated by 2-fold in response to IL-10 and by 1.4-fold in response to M-CSF, whereas it was not affected by IL-4, VEGF, or PlGF (Figure 5B; not shown). These findings were confirmed in macrophages that were differentiated from mononuclear bone marrow cells in vitro (not shown). Notably, in lysates of CT26 and 4T1 tumors in Gas6−/− and WT mice, both cytokines were present at comparable levels (Figure 5C-D; 4T1 not shown).
Discussion
This study uncovers a novel tumor-promoting mechanism of Gas6, whereby tumor-infiltrating leukocytes are educated by the tumor microenvironment to produce elevated levels of Gas6. The key findings of this study are: (1) growth and metastasis of 5 different ectopic and orthotopic syngeneic murine tumor models are slowed down in Gas6−/− hosts; (2) Gas6 induces proliferation of tumor cells in vitro and in vivo; (3) Gas6 is up-regulated by tumor-infiltrating leukocytes, in particular by macrophages, but is minimally expressed by resident tissue macrophages or circulating leukocytes of tumor-bearing mice; and (4) the transplantation of WT bone marrow in Gas6−/− hosts rescues impaired tumor growth via restoring intratumoral Gas6 production by infiltrating leukocytes; conversely, transplantation of Gas6−/− bone marrow in WT mice phenocopies decreased tumor growth in Gas6−/− mice.
Although Gas6 was discovered more than 2 decades ago2 and identified as a ligand of the TAM family of receptor tyrosine kinases shortly thereafter,53-55 the role of Gas6 in tumor biology has remained elusive. By using previously generated Gas6−/− mice,4 we studied the role of Gas6 deficiency on tumor biology. These experiments revealed that stromal Gas6 deficiency inhibits growth and metastasis of the studied tumor models by 35% to 55%. Tumor inhibition in Gas6−/− hosts did not seem to result from changes in angiogenesis, leukocyte infiltration, or cancer-associated fibroblast accumulation. Instead, it was attributable to the absence of the mitogenic activity of Gas6 for tumor cells because tumor cell proliferation was reduced in Gas6−/− hosts in vivo and Gas6 stimulated tumor cell proliferation in vivo. The mitogenic activity of Gas6 on tumor cell lines in vitro is in concordance with the published literature.13,14 Indeed, Gas6 induces the proliferation of mammary carcinoma cells,56 melanoma cells,14 and prostate cancer cells.13
The authors of previous studies13,21 documented that Gas6 often acts in a paracrine manner on tumor cells, although autocrine production and effects have also been reported. Expression analyses in this study revealed that Gas6 was below the detection threshold in tumor cell lines in vitro and in intact tumors in Gas6−/− mice in vivo, whereas it was readily detectable in tumors grown in WT mice, indicating that Gas6 is largely, if not completely, host-derived, at least in these experimental tumor models. This offered us the opportunity to study the role of Gas6 in the tumor stroma “in isolation” and to analyze how Gas6 acts in a paracrine manner on nearby tumor cells. In the tumor stroma, Gas6 is prominently expressed by leukocytes, in particular by macrophages. This result is in line with previous in situ hybridization studies that Gas6 is expressed by macrophage-rich regions of lymph nodes and spleen, although its functional relevance (for tumor progression) was not analyzed yet.31
Interestingly, comparing Gas6 expression in tumor-infiltrating macrophages and resident tissue macrophages in the peritoneum, lung, or spleen, we found a strong up-regulation of Gas6 in tumor infiltrating macrophages; these findings suggest that tumors educate macrophages to up-regulate the release of Gas6. Similar findings were obtained when comparing the whole population of CD45+ tumor-infiltrating leukocytes with bone marrow or peripheral blood leukocytes. Additional comparison of Gas6 expression in the main subpopulations of tumor-infiltrating leukocytes further shows that macrophages are likely the most abundant producers of Gas6 in the tumor microenvironment but also reveals that granulocytes, monocytes, dendritic cells, T cells, and natural killer cells can produce Gas6 in tumors and thus amplify the effect of macrophage-derived Gas6. Our findings thus extend previous studies that Gas6 can be expressed by cells of the myeloid lineage29,30 and illustrate another level of regulation of its expression that was previously not recognized.
The enhanced production of Gas6 by bone marrow–derived leukocytes, upon infiltration in the tumor microenvironment, contributes to the growth of the primary tumor and metastasis. Indeed, replacement of Gas6−/− bone marrow by WT bone marrow not only rescued the impaired tumor growth in Gas6−/− mice but also largely restored intratumoral Gas6 levels to WT levels, without, however, inducing any change in leukocyte recruitment. This close association strongly suggests that Gas6, up-regulated by intratumoral-infiltrated leukocytes, rescues tumor proliferation and growth. Vice versa, transplantation of Gas6−/− bone marrow into WT mice sufficed to phenocopy the reduced tumor growth in Gas6−/− mice. Hence, Gas6 delivered by bone marrow–derived cells functionally enhances tumor growth. Other tumor-promoting growth factors, such as epidermal growth factor, are also predominantly produced by infiltrating TAMs.26,28,57
Initial analysis further demonstrated that IL-10 up-regulates Gas6 in peritoneal macrophages. This finding is in line with previous results that Gas6 is up-regulated in human monocytes after treatment with IL-10 in vitro58 and in monocytes of psoriatic patients after systemic treatment with IL-10.58 IL-10 is secreted by tumor cells in the cancer microenvironment.26,27 Likely, additional molecules, perhaps even a cocktail of cytokines, are involved in the up-regulation of Gas6 by tumor macrophages, although the nature of these signals remains to be further defined. Given the prominent up-regulation of Gas6 expression, specifically in tumor-infiltrated macrophages compared with resident tissue macrophages, we speculate that part of the tumor “education” of these leukocytes to increase their production of Gas6 might be attributable to effects of the tumor on macrophage differentiation, and, on top, to an additional induction of Gas6 expression by tumor-released cytokines. Interestingly, IL-10 and M-CSF are capable of promoting both processes, that is, macrophage differentiation per se26 as well as stimulation of Gas6 expression, once hematopoietic precursors differentiated to macrophages.
Leukocyte infiltration in tumors was not altered in Gas6−/− mice. Nonetheless, given the reported pleiotropic role of TAMRs on leukocyte function,10 we cannot formally exclude the possibility that Gas6, through autocrine effects on immune cells, might have effects on the immune modulation of tumor growth, which were not recognized in our experimental conditions. For instance, TAMR signaling prevents autoimmunity by silencing inflammatory responses in dendritic cells and macrophages; such an activity of Gas6 would dampen antitumoral immunity and thus promote tumor growth,10,30,31 and hence amplify the mitogenic activity of Gas6 on tumor cells. However, Gas6 and TAMRs also promote natural killer development59,60 and phagocytosis of apoptotic cells,10 activities that would be expected to silence rather than to fuel tumor growth. If this mechanism is operational in tumors, its overall effect must have been overcome by the aforementioned tumor-promoting activities of Gas6 in our conditions. In addition, it might be noteworthy that the aforementioned immunomodulatory activity of TAMRs in preventing autoimmunity has been observed in nontumor-bearing mice. Given that tumors are able to alter their microenvironment to promote their growth,26,27 it remains to be evaluated whether the immunomodulatory activities of TAMR signaling also are not changed in the tumor stroma in comparison with normal tissue.
This study also illustrates, for the first time, that absence of Gas6 does not affect angiogenesis in health or disease. This was not anticipated, given previous reports that Gas6 and its receptors are expressed by endothelial cells,6 vascular smooth muscle cells,61 fibroblasts,1 and pericytes62 in conditions of vascular injury, inflammation, and repair.42-44 In addition, Gas6 has been reported to promote survival and proliferation of these vascular cells.63-66 Some studies67-69 suggested that Gas6 would promote angiogenesis through homophilic interactions between Axl-expressing endothelial cells, whereas another70 reported inhibition of VEGF-A–induced tyrosine phosphorylation of VEGFR-2 by activated Axl. Yet another study25 showed that the expression of a dominant-negative Axl in a glioblastoma model reduced tumor growth but had no impact on tumor angiogenesis. However, none of these previous studies analyzed the endogenous role of Gas6 in health and disease in vivo. One, perhaps the most straightforward explanation for these discrepant observations is that endogenous Gas6 is redundant or has a negligible effect in vivo. An alternative explanation might be that another TAMR ligand (protein S?) might compensate for the absence of Gas6, an answer that might be provided by conditional knockout studies of protein S. Yet an alternative explanation may be that Gas6 was not accessible for endothelial cells in these tumor models. Regardless of these possible hypotheses, our current findings do not refute the possibility that Gas6 might regulate angiogenesis in other conditions.
Do these genetic findings have any medical implications? Possibly, because bone marrow–derived Gas6 promotes growth of several different tumor models, including those resistant to VEGF inhibitors. Different groups have already shown the expression of Gas6 and its Axl receptor in different human malignancies, including gastric cancer,17 glioma,21 leukemia,71 and ovarian cancer.20 Even more, in gastric cancer and glioblastoma, Gas6 expression correlates with negative prognostic parameters.17,21 However, additional preclinical and clinical studies are required to establish more conclusively the therapeutic potential of inhibiting Gas6, most likely in conjunction with established treatments, in human cancer. As for now, these genetic studies illustrate how experimental tumors may amplify their own growth, by educating infiltrating leukocytes to up-regulate the expression of Gas6.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Vesalius Research Center technicians for assistance.
S.L. is supported by a Mildred Scheel postdoctoral fellowship from Deutsche Krebshilfe; T.S. by a postdoctoral fellowship from Deutsche Forschungsgemeinschaft; M.T. by IWT and FWO; E.L. by the Humboldt Foundation (Sofja Kovalevskaja grant) and the Netherlands Organization for Scientific Research (NWO, VIDI grant to E.L.); D.V. by FWO; and A.L. by FWO, a K.U.Leuven Center of Excellence grant (EF/05/13), and an FP-7 European Research Council Starting Grant Imagined-203291. This work was supported by grants (to P.C.) from “Long-term structural funding: Methusalem funding by the Flemish Government,” the FWO (Flemish Foundation for Scientific Research), the European Union 7th Framework Programme, the Concerted Research Activities–K.U.Leuven, the Belgian Science Policy, and the Stichting Tegen Kanker (to M.D. and M.T.).
Authorship
Contribution: P.C. conceived and initiated the study and scientific direction; S.L., T.S., M.T., M.D., and P.C. designed the experiments; S.L., T.S., K.v.G., D.L., D.V., and A.L. performed the experiments; D.B. and S.P. generated research tools; S.L., T.S., M.T., K.v.G., D.L., E.L., D.V., B.J., and P.C. analyzed data; S.L., T.S., M.T., E.L., S.P., M.H., A.L., M.D., B.J., and P.C. participated in the discussion; and S.L., T.S., and P.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Carmeliet, MD, PhD, Vesalius Research Center, VIB, K.U.Leuven, Campus Gasthuisberg, Herestraat 49, B-3000, Leuven, Belgium; e-mail: peter.carmeliet@vib-kuleuven.be.
References
Author notes
*S.L. and T.S. contributed equally to this work.