Human T-cell leukemia virus type I (HTLV-I)–associated malignancies are seen in a small percentage of infected persons. Although in vitro immortalization by HTLV-I virus is very efficient, we report that Tax has poor oncogenic activity in human primary T cells and that immortalization by Tax is rare. Sustained telomerase activity represents one of the oncogenic steps required for Tax-mediated immortalization. Tax expression was required for the growth of primary T cells, but was not sufficient to propel T cells into cell cycle in the absence of exogenous interleukin-2 (IL-2). Tax was sufficient to activate the phosphoinositide-3 kinase (PI3K)/Akt pathway as shown by down regulation of Src homology phosphatase-1 and increased phosphorylation of Akt. We also found disruption of putative tumor suppressors IL-16 and translocated promoter region (TPR) in Tax-immortalized and HTLV-I–transformed cell lines. Our results confirmed previous observations that Tax activates the anaphase-promoting complex. However, Tax did not affect the mitotic spindle checkpoint, which was also functional in HTLV-I–transformed cells. These data provide a better understanding of Tax functions in human T cells, and highlight the limitations of Tax, suggesting that other viral proteins are key to T-cell transformation and development of adult T-cell leukemia.
Introduction
Human T-cell leukemia virus type I (HTLV-I) transforms human CD4+ T cells in vitro and in vivo causing adult T-cell leukemia/lymphoma (ATL).1 ATL has an extremely poor prognosis for survival, characterized by an aggressive proliferation of leukemic cells.2 The molecular basis for HTLV-I–mediated transformation of T cells is unclear. Tax has been shown to inactivate several tumor suppressors, disrupt cell cycle and DNA repair checkpoints, and stimulate cell growth, while protecting against apoptosis.3,,,–7 Importantly, the oncogenic activities of Tax have been studied mainly in transgenic models and in rodent fibroblasts.8,–10 Although these models provided useful information, they have limitations, because the transformation of human cells in vitro requires more oncogenic events than the transformation of rodent cells.11
The role of Tax in transformation is based largely on forced overexpression of Tax, which results in cell-cycle abnormalities, faulty mitosis, aneuploidy, and the formation of multinucleated cells. A major caveat to these studies is that they were performed in established cell-culture lines, which are already dysfunctional in key regulatory pathways (tumor suppressor, cell cycle, and DNA repair checkpoints), and therefore are much more susceptible than normal primary cells to Tax-induced alterations. Studying Tax in human primary T cells is preferable, yet, to date, immortalized Tax-expressing human T-cell lines able to proliferate indefinitely in culture have not been established. In one study, the pX region of HTLV-I (which expresses the Tax gene among other viral genes) was found to transform human T cells in the context of a Herpesvirus saimiri vector,12 although there is no evidence to refute that the herpes genes from the vector synergized or were activated by Tax to promote transformation or that other viral genes were involved. This prompted us to investigate the ability of Tax to immortalize human primary T cells. In the following study, we demonstrate that Tax-induced immortalization of peripheral blood mononuclear cells (PBMCs) is a very rare event. Tax alone enhanced proliferation, but appears insufficient to sustain permanent proliferation. Although most attempts were unsuccessful, we finally obtained an immortalized Tax-expressing CD4 human T-cell line, WT4, which was maintained in continuous culture for more than 4 years. Our results suggest that Tax alone is a poor oncogene that increases the life span and rate of T-cell proliferation in the presence of interleukin-2 (IL-2), thereby increasing the probability for accumulation of genetic defects leading to immortalization.
Methods
Plasmids and cell lines
The HTLV-I tax gene was cloned into the HRCMV or HR′ lentiviral vectors (Figure 1A). Pseudotype virus particles were produced as previously reported.13 For cell-cycle analysis, tax was cloned into an UBC-IRES-GFP lentiviral vector. The p53 gene of WT4 was sequenced and cloned as previously described.14 HTLV-I cell lines (MT2, MT4, and C8166) have been previously described.15 ATL-derived cells TL-Om1 and ED40515(−) were kindly provided by Dr Michiyuki Maeda (Kyoto University).
Real-time PCR, TRAP assays, cell-cycle analysis, FACS, and Western blots
RNA extraction, cDNA synthesis, real-time polymerase chain reaction (PCR), and telomeric repeat amplification protocol (TRAP) assays were performed as previously described.15 Fold change is calculated as the ratio of normalized expression of the target gene divided by the normalized expression of the control sample. Primers are provided in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Transfections were performed with calcium phosphate or Lipofectamine (Invitrogen) and assayed for luciferase activity. Western blots were performed using total protein extracts and antibodies from Santa Cruz Biotechnology. Tax mouse monoclonal antibody was a gift from Dr J. N. Brady (National Institutes of Health) Cell-cycle and fluorescence-activated cell sorting (FACS) analysis was as previously described.16 For transient Tax expression assays, cells were infected with high titer Tax-IRES-GFP lentiviral pseudotype virus, stained with Vybrant DyeCycle Violet (Invitrogen), and sorted for green fluorescent protein (GFP)–positive cells before cell-cycle analysis.
Telomere length measured by FISH and karyotype analyses
Experiments were performed according to the manufacturer's instructions (Telomere PNA fluorescence in situ hybridization [FISH] Kit/fluorescein isothiocyanate; DakoCytomation). Fluorescent images were captured using a Nikon TE2000E epifluorescence microscope and Metamorph software (Molecular Devices). The images were collected at the ×100 objective. For telomere quantification, the intensities of 100 fluorescence spots of telomere FISH staining were quantified by Metamorph software. For karyotype analysis, cells were treated with Colcemid KaryoMAX (GibcoBRL), and harvested by standard cytogenetic methods of trypsin dispersal, hypotonic shock with 0.075M KCl, and fixation with 3:1 methanol–acetic acid fixative. Mitotic cell slide preparations were analyzed by the Giemsa-Trypsin-Leishman (GTL) banding method. In each case 20 cells were counted and analyzed.
Results
Establishment of Tax-transduced human primary T-cell lines
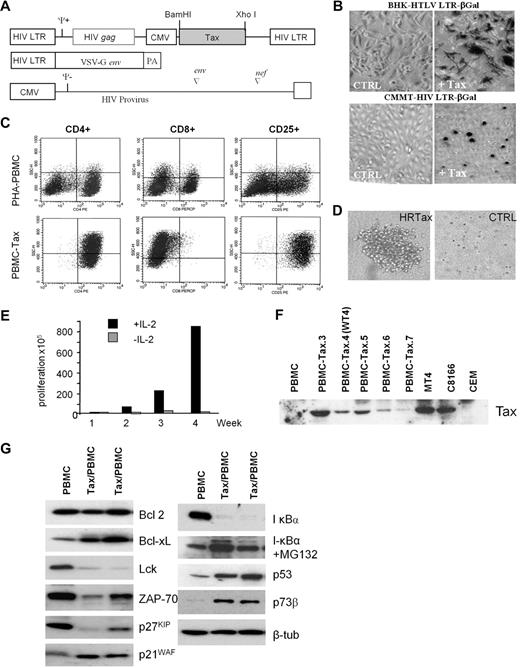
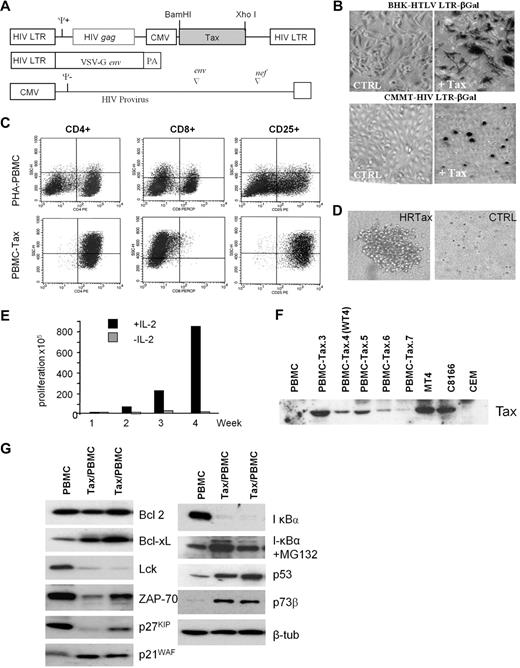
To study Tax-induced immortalization of primary human T cells, we cloned the tax gene into a lentivirus vector, which expresses Tax only when transduced into target cells (Figure 1A). The functionality of Tax-expressing pseudotype virus particles was determined by infecting 2 reporter cell lines, BHK-HTLV LTR-βGal and CMMT HIV LTR-βGal, which serve as markers for Tax-mediated activation of the cyclic adenosine monophosphate response element binding/activating transcription factor (ATF) and nuclear factor κB pathway, respectively17 (Figure 1B). PBMCs from 7 healthy donors were then infected with the Tax-expressing or control virus. After 4 weeks of culturing, control virus–transduced lymphocytes ceased to proliferate. In contrast, all Tax-transduced PBMC cultures continued to proliferate in IL-2–containing media. FACS analysis revealed that these cells had a similar phenotype as HTLV-I–infected cells in vitro and in vivo (CD4+/CD8− and CD25+; Figure 1C), and were able to form large colonies in the presence of IL-2 in soft agar assays (Figure 1D). The transformation of human T lymphocytes by HTLV-I occurs in 2 steps. In the early phase, immortalized cells are strictly dependent on exogenous interleukin 2 (IL-2). However, after several months in culture, cells acquire the ability to grow in the absence of exogenous IL-2, and they are referred to as transformed cells. The genetic events required for the transition from the immortalized to the transformed state are still largely unknown. Previous studies have shown that the transition to IL-2 independence is associated with constitutive activation of the Janus kinase/signal transducer and activator of transcription pathway and down regulation of Src homology 2 domain-containing 5′ inositol phosphatase 1 (SHP1).18,–20 The requirement of exogenous IL-2 for the continued proliferation of Tax-transduced T lymphocytes (Figure 1E) suggests that in contrast to rodent fibroblasts, Tax cannot stimulate S phase entry and cell-cycle progression in human T cells in the absence of IL-2. All Tax-transduced mixed PBMCs showed detectable Tax expression after 4 weeks of culturing (Figure 1F). Consistent with previous studies, we found high expression of Bcl-xL in Tax-transduced cells21 and decreased T-cell activation factors, Lck and ZAP-7022,23 (Figure 1G). Tax also led to constitutive activation of the nuclear factor κB pathway, as indicated by degradation of IκBα, which was stabilized after treatment with the protease inhibitor MG132. In agreement with previous findings, p53 and p73 proteins were stabilized in Tax-expressing PBMCs.24,25 Finally, deregulated expression of p27KIP and p21WAF was found, as previously described for Tax-expressing HTLV-I cell lines.26
Human primary T-cell immortalization by Tax. (A) Schematic representation of the vectors used in this study to synthesize the HIV pseudotype viral particles expressing HTLV-1 Tax protein. (B) BHK-HTLV LTR-βGal and CMMT HIV LTR-βGal established cell lines were infected with negative control virus or Tax-expressing viral particles. βGal expression was monitored to test the infectivity of Tax pseudovirus. (C) FACS analysis of CD4, CD8, and CD25 T-cell receptors in PBMCs (nontransduced control cell line) or Tax-transduced PBMCs (PBMC-Tax.3). (D) Soft agar colony formation assay on Tax-transduced PBMCs or negative control virus. (E) Proliferation of Tax-transduced PBMCs (PBMC-Tax.3) in the presence or absence of IL-2. (F) Western blot of Tax expression in a mixed population of Tax-transduced PBMCs after 4 weeks of culturing. PBMCs and CEM served as negative controls. The HTLV-I–transformed cell lines MT4 and C8166 were positive controls for Tax expression. (G) Western blot analysis of Tax-affected proteins in nontransduced PBMCs and Tax-transduced PBMCs.
Human primary T-cell immortalization by Tax. (A) Schematic representation of the vectors used in this study to synthesize the HIV pseudotype viral particles expressing HTLV-1 Tax protein. (B) BHK-HTLV LTR-βGal and CMMT HIV LTR-βGal established cell lines were infected with negative control virus or Tax-expressing viral particles. βGal expression was monitored to test the infectivity of Tax pseudovirus. (C) FACS analysis of CD4, CD8, and CD25 T-cell receptors in PBMCs (nontransduced control cell line) or Tax-transduced PBMCs (PBMC-Tax.3). (D) Soft agar colony formation assay on Tax-transduced PBMCs or negative control virus. (E) Proliferation of Tax-transduced PBMCs (PBMC-Tax.3) in the presence or absence of IL-2. (F) Western blot of Tax expression in a mixed population of Tax-transduced PBMCs after 4 weeks of culturing. PBMCs and CEM served as negative controls. The HTLV-I–transformed cell lines MT4 and C8166 were positive controls for Tax expression. (G) Western blot analysis of Tax-affected proteins in nontransduced PBMCs and Tax-transduced PBMCs.
Persistent Tax expression leads to karyotypic abnormalities in human primary T cells
In contrast to cocultivation of primary PBMCs with lethally irradiated MT-2 cells, which readily leads to HTLV-I–immortalized cell lines (Sinha-Datta et al27 and data not shown), Tax-transduced primary T lymphocytes proliferated for several months, but eventually ceased to grow after 12 to 15 months in culture. These findings imply that Tax has poor oncogenic activity in primary human T cells and suggests the requirement of additional genetic events or viral protein(s) for efficient immortalization. We next sought to determine the reasons associated with the poor oncogenicity of Tax in human T cells. Although studies suggest that Tax can induce mitotic defects leading to severe aneuploidy in transfected cells,28 these effects have never been evaluated in primary human T cells. Microscopic examination on a polyclonal population of Tax-transduced primary T cells during early (less than 1 month) or late (after several months) culture revealed many genetic defects, including anaphase bridges, micronuclei formation, multinucleated cells, and asymmetrical divisions (Figure 2A). These defects were confirmed in different Tax-transduced PBMC cultures derived from different donors, excluding the possibility that outcomes were due to a particular donor's genetic makeup. Notably, these defects were not seen in early passaged Tax-transduced cells (less than 1 month) or in WT4 cells.
Genomic instability leads to premature senescence in Tax-expressing cells. (A) Tax-transduced PBMCs during either early (< 1 month) or late (> 6 months) passage were fixed, stained with DAPI (4,6 diamidino-2-phenylindole), and then analyzed for the presence of cell division abnormalities such as asymmetric division, multinucleated cell, micronuclei, and anaphase bridges. (B) Karyotype analysis of Tax-transduced PBMCs during late passage. Arrows indicate telomere associations (Tas). (C) Western blot of Tax expression in early and late Tax-transduced PBMCs (Tax/PBMC no. 3). (D) Real-time PCR analysis of hTERT expression between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). Normalized values are represented as the percentage of activation of early Tax-transduced PBMCs. (E) TRAP analysis of telomerase activity between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer was a negative control. (F) Fluorescence in situ hybridization (FISH) of telomere sequences in early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3) compared with MT2 cells. Histograms represent the intensities of fluorescence spots of telomere FISH staining with their respective numbers in each cell line. A minimum of 100 spots was quantified for each cell line. (G) Double immunofluorescence staining of late-cultured Tax-transduced PBMCs (Tax/PBMC no. 3) with anti-TRF2 and anti–phospho-H2AX antibodies. The contact of telomere-induced foci (red) and DNA double-strand breaks (green) is indicated by the arrows in the overlay image. (H) Immunofluorescence of p53 transcriptional activity in Tax-transduced PBMCs (PBMC/Tax no. 3) as indicated by phosphorylation on serine 20 of p53. (I) Real-time PCR expression of p53-responsive genes p21WAF and mdm2 in nontransduced PBMCs, and early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3). Assays were performed in duplicate. Fold changes were calculated by comparing values with that of nontransduced PBMC normalized gene expression. Error bars represent the CT SD of the gene of interest.
Genomic instability leads to premature senescence in Tax-expressing cells. (A) Tax-transduced PBMCs during either early (< 1 month) or late (> 6 months) passage were fixed, stained with DAPI (4,6 diamidino-2-phenylindole), and then analyzed for the presence of cell division abnormalities such as asymmetric division, multinucleated cell, micronuclei, and anaphase bridges. (B) Karyotype analysis of Tax-transduced PBMCs during late passage. Arrows indicate telomere associations (Tas). (C) Western blot of Tax expression in early and late Tax-transduced PBMCs (Tax/PBMC no. 3). (D) Real-time PCR analysis of hTERT expression between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). Normalized values are represented as the percentage of activation of early Tax-transduced PBMCs. (E) TRAP analysis of telomerase activity between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer was a negative control. (F) Fluorescence in situ hybridization (FISH) of telomere sequences in early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3) compared with MT2 cells. Histograms represent the intensities of fluorescence spots of telomere FISH staining with their respective numbers in each cell line. A minimum of 100 spots was quantified for each cell line. (G) Double immunofluorescence staining of late-cultured Tax-transduced PBMCs (Tax/PBMC no. 3) with anti-TRF2 and anti–phospho-H2AX antibodies. The contact of telomere-induced foci (red) and DNA double-strand breaks (green) is indicated by the arrows in the overlay image. (H) Immunofluorescence of p53 transcriptional activity in Tax-transduced PBMCs (PBMC/Tax no. 3) as indicated by phosphorylation on serine 20 of p53. (I) Real-time PCR expression of p53-responsive genes p21WAF and mdm2 in nontransduced PBMCs, and early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3). Assays were performed in duplicate. Fold changes were calculated by comparing values with that of nontransduced PBMC normalized gene expression. Error bars represent the CT SD of the gene of interest.
To investigate the severity and the nature of the genetic abnormalities present in Tax-transduced lymphocytes, we performed karyotype analyses. For each analysis, at least 20 metaphase spreads were analyzed (for a total of 160 metaphases). Frequent structural and numeric karyotypic abnormalities were found, varying from balanced translocations to mild to severe aneuploidy, to several tetraploid genomes (Figure 2B and supplemental Figure 2). Our studies also revealed that Tax-transduced lymphocytes from a given donor had variable changes over time, and aneuploidy was more frequently observed in later passages (> 6 months), suggesting the involvement of additional genetic changes and a selective process for these clones.
Dysfunctional telomeres lead to a high frequency of telomere fusions and genomic instability, which may prevent Tax-induced immortalization
In vitro, dysfunctional telomeres have been associated with specific cell division abnormalities, such as anaphase bridges and multipolar mitoses,29 both of which were readily observed during long-term culturing of Tax-expressing lymphocytes (Figure 2A). In late passage cells, karyotypes presented signs of critically short telomeres characterized by telomere fusions (tas; Figure 2B). By studying numerous metaphases from the Tax-transduced lymphocytes, we found these defects were acquired in culture because different chromosomes were affected. We next sought to identify the basis for the telomere dysfunction and genomic instability found in Tax-transduced lymphocytes. The continuous proliferation of HTLV-I–transformed cells is dependent on sustained telomerase activity, because inhibition of telomerase leads to in vitro and in vivo senescence of tumor cells.14 We have previously shown that Tax stimulates the catalytic subunit of telomerase reverse-transcriptase (hTERT) promoter in primary T cells.27 Although both tax and hTERT gene expression was comparable in early and late passaged Tax-transduced cells (Figure 2C-D), we found that telomerase activity was 5-fold weaker in late passage, nonimmortalized cells compared with early passage cells. These findings suggest acquisition of posttranscriptional events regulating telomerase enzymatic activity (Figure 2E).
Loss of telomerase activity led to shorter telomeres in Tax-transduced PBMCs and individual telomeres had different lengths within a cell, with the existence of allelic length differences (Figure 2F). The relative number of telomere spots detected by FISH in MT2 cells was twice that of late passage cells, suggesting that in late passage Tax-transduced lymphocytes, many of the chromosomes have lost the majority of their telomere sequences, thus preventing detectable hybridization of the telomere–fluorescein isothiocyanate probe. In fact, the presence of dysfunctional telomeres in late passaged Tax-transduced lymphocytes was demonstrated by detection of telomere-induced foci (Figure 2G). In contrast, telomere-induced foci were not detected in early passage cells (data not shown). Dysfunctional telomeres are strongly linked to the activation of the ATM-p53 DNA damage response.30 Phosphorylation of p53 on serine 20 and specific localization were readily detected in late passage Tax-transduced PBMCs (Figure 2H). Expression of p53-responsive genes, p21waf and mdm2, was also increased (Figure 2I). These data suggest that despite the Tax-mediated transcriptional activation of hTERT, disruption of posttranscriptional checkpoints is required for persistent telomerase activity. In the absence of such alterations, progressive telomere shortening opposes Tax-mediated T-cell immortalization.
Tax-induced immortalization of T lymphocytes requires a permissible host cellular environment
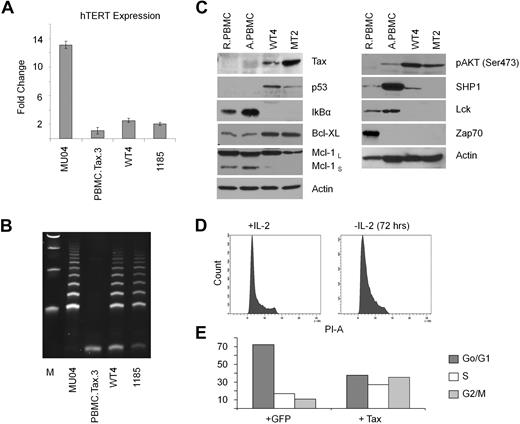
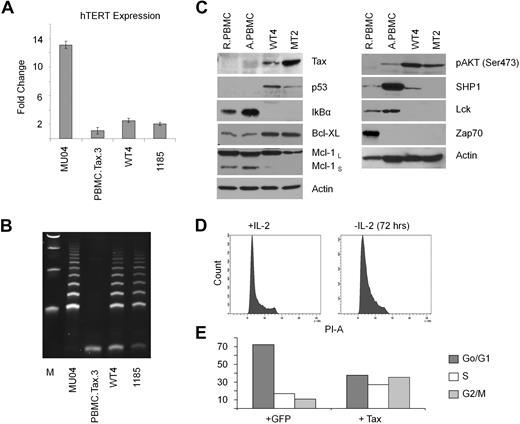
Among all cultures initiated in this study, only one Tax-immortalized cell line (named WT4) could be established. Immortalization was dependent on Tax, and not related to pre-existing genetic defects, because various Tax mutants (M47, M22, G148V, K88A, V89A, L90A) were unable to immortalize PBMCs from that same donor in experiments that were performed at the same time. It took approximately 3 years of continuous culture to obtain the WT4 line that proliferates actively with a doubling time of approximately 36 hours. We found high levels of telomerase activity in WT4 cells, compared with nonimmortalized Tax-transduced PBMCs (Figure 3A-B); and Tax expression was readily detectable in WT4 cells (Figure 3C). Expression of the tumor suppressor p53 was stabilized in WT4 compared with PBMCs. We also found that IκBα was constitutively degraded and the antiapoptotic protein, Bcl-xL, increased (Figure 3C). Constitutive activation of the prosurvival pathway AKT was detected in WT4 and MT2 cell lines. In contrast, expression of Lck and Zap70 was decreased in WT4 and MT2 cell lines. SHP1 expression was higher in WT4 cells, compared with MT2, suggesting that WT4 may not be fully transformed and may require IL-2 for sustained growth. In fact, IL-2 withdrawal from WT4 cells led to accumulation of cells arrested in the G1 phase of the cell cycle (Figure 3D). Several laboratories have reported cell-cycle arrest or senescence after HTLV-I infection. We investigated the effect of Tax in transiently transduced lymphocytes in the presence of IL-2. Our results revealed an increase of Tax-expressing cells in the S and G2/M phases of the cell cycle (Figure 3E). This supports several reports, demonstrating that transient transduction of Tax stimulates G1/S entry but blocks mitosis.31–32 Because only a fraction of Tax-expressing cells were delayed/arrested in S and G2/M, this suggest that either the levels of Tax or the presence of genetic defects affects proliferation of Tax-expressing cells. In our experimental procedures, transduction of primary T cells with high-titer vesicular stomatitis virus pseudotype particles results in more than 80% of cells transduced. Therefore we believe that differences are related to intrinsic differences between primary T cells and established Tax T-cell lines as further corroborated by the findings of genomic translocations.
WT4 cells maintain high telomerase activity. (A-B) Real-time PCR expression of hTERT (A) and TRAP analysis of telomerase activity (B) in Tax-transduced PBMCs (Tax/PBMC no. 3), WT4, and the HTLV-I–immortalized cell lines, MU04 and 1185. Real-time PCR was performed in duplicate; error bars represent the threshold cycle (CT) SD of the gene of interest. Fold changes were calculated by comparing values with that of MU04 normalized gene expression. (C) Western blot analysis of Tax, p53, Bcl-xL, Mcl-1, IκBα, and actin in WT4 cells compared with the HTLV-I–transformed cell line, MT4. Nontransduced resting PBMCs (RPBMCs) and activated PBMCs (ActPBMCs) served as controls. (D) Cell-cycle analysis of WT4 cells cultured 72 hours without IL-2 and/or restimulated overnight with IL-2. (E) Cell-cycle analysis of PBMCs transiently transduced with a Tax-GFP or GFP-control virus. FACS analysis was performed 48 hours after infection on GFP (infected) sorted cells.
WT4 cells maintain high telomerase activity. (A-B) Real-time PCR expression of hTERT (A) and TRAP analysis of telomerase activity (B) in Tax-transduced PBMCs (Tax/PBMC no. 3), WT4, and the HTLV-I–immortalized cell lines, MU04 and 1185. Real-time PCR was performed in duplicate; error bars represent the threshold cycle (CT) SD of the gene of interest. Fold changes were calculated by comparing values with that of MU04 normalized gene expression. (C) Western blot analysis of Tax, p53, Bcl-xL, Mcl-1, IκBα, and actin in WT4 cells compared with the HTLV-I–transformed cell line, MT4. Nontransduced resting PBMCs (RPBMCs) and activated PBMCs (ActPBMCs) served as controls. (D) Cell-cycle analysis of WT4 cells cultured 72 hours without IL-2 and/or restimulated overnight with IL-2. (E) Cell-cycle analysis of PBMCs transiently transduced with a Tax-GFP or GFP-control virus. FACS analysis was performed 48 hours after infection on GFP (infected) sorted cells.
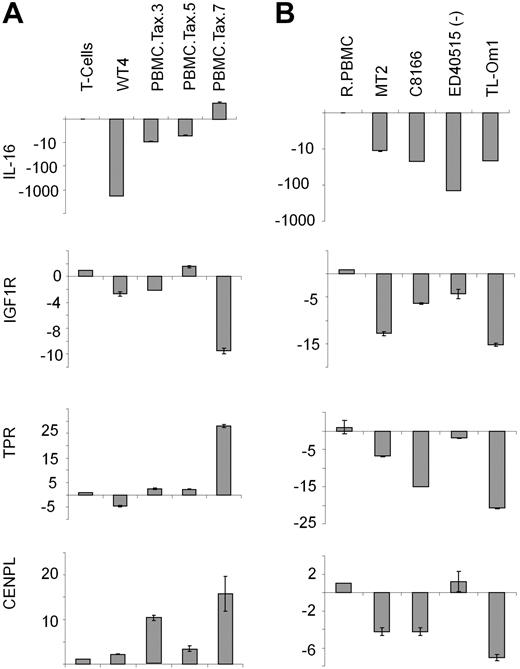
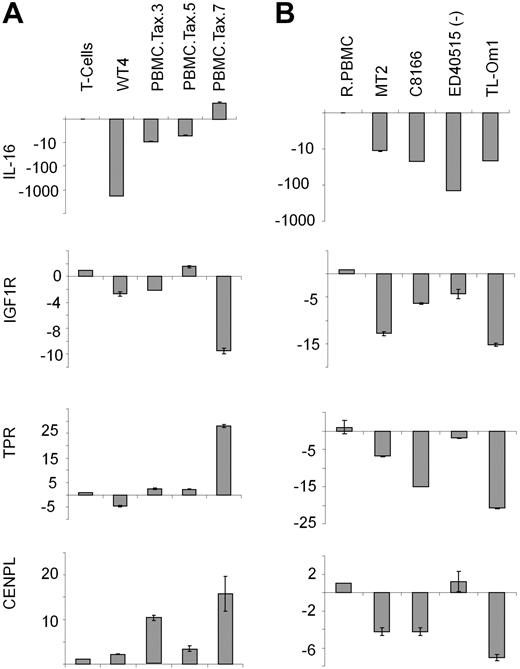
Karyotype analyses of clonal WT4 identified a balanced chromosome translocation involving 15q26.2 and 1q25. Because several putative tumor suppressor genes are located in these regions, including interleukin-16 (IL-16: 15q26.3), insulin-like growth factor-1 (IGF1R: 15q26.3), translocated promoter region (TPR: 1q25), and centromere protein L (CENPL: 1q25.1), we focused our attention on chromosomal regions 15q26.2 and 1q25. Real-time PCR analysis revealed that IL-16 and TPR expression was substantially altered between WT4 and other Tax-transduced PBMCs (Figure 4A). IL-16 expression was inhibited at a much greater level in WT4 cells compared with nonimmortalized, Tax-transduced cells. In addition, TPR expression was increased in 3 Tax-transduced cells, but decreased in WT4 cells. This suggests that IL-16 and TPR may contribute to WT4 immortalization and this is currently under investigation. With the exception of IGF1R, expression of these 4 tumor suppressor genes has not been previously reported in HTLV-I established cell lines or ATL-like cells. Real-time PCR analysis found IL-16, IGF1R, and TPR expression to be down-regulated in 2 HTLV-I cell lines (MT2 and C8166) and 2 ATL cell lines (TL-Om1 and ED40515(−); Figure 4B). Importantly, WT4 cells showed similar levels of IL-16 and TPR compared with the HTLV-I cell lines.
WT4 cells display decreased IL-16 and TPR. (A-B) Real-time PCR analysis of IL-16, IGF1R, TPR, and CENPL in Tax-expressing PBMCs (A) or HTLV-1–transformed (MT2 and C8166) or ATL-like (ED40515(−) and TL-Om1) cell lines (B). Nontransduced T cells or resting PBMCs (RPBMCs) served as controls. Real-time PCR was performed in duplicate; error bars represent the CT SD of the gene of interest.
WT4 cells display decreased IL-16 and TPR. (A-B) Real-time PCR analysis of IL-16, IGF1R, TPR, and CENPL in Tax-expressing PBMCs (A) or HTLV-1–transformed (MT2 and C8166) or ATL-like (ED40515(−) and TL-Om1) cell lines (B). Nontransduced T cells or resting PBMCs (RPBMCs) served as controls. Real-time PCR was performed in duplicate; error bars represent the CT SD of the gene of interest.
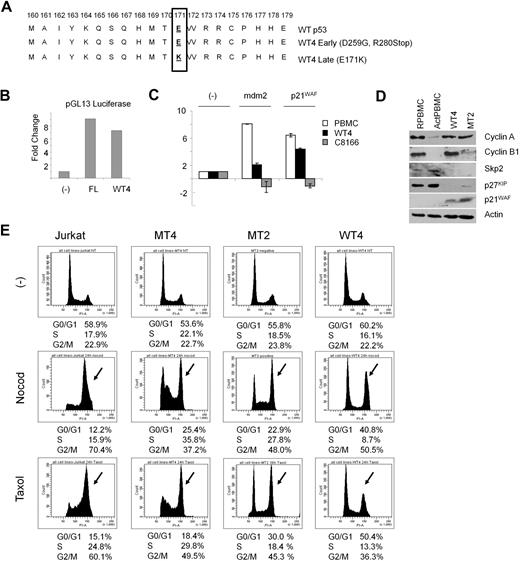
HTLV-I cell lines have a transcriptionally inactive p53. Cloning and sequencing of the p53 hot-spot region in WT4 cells revealed an E to K mutation at position 171 (Figure 5A). Transfection of WT4 p53 and a p53 luciferase-reporter plasmid confirmed previous reports that the E171K p53 mutation was still transcriptionally active (Figure 5B). Surprisingly, the E171K mutation was acquired and selected during long-term culturing, and for unknown reasons, earlier time points from preimmortalized cultures of WT4 cells, which had clones with deletions of p53 or mutations in p53 at D259G and R280Stop, were not selected (Figure 5A). Lack of p53-inactivating mutations in the hot-spot region is consistent with previous reports that Tax can stabilize and inhibit p53 function in HTLV-I–transformed cells.33 To further define the transcriptional activity of p53 in WT4 cells, we treated WT4 cells with gamma irradiation and performed real-time reverse-transcription–PCR on p53-responsive genes mdm2 and p21WAF. Our results show that p53 transcriptional activities are only partially inactivated in WT4 compared with the Tax-expressing HTLV-I–transformed C8166 cell line. Although both cell lines express different levels of Tax, C8166 also expresses small regulatory HTLV-I genes p12, p13, p30, and HBZ (M.B., unpublished data, 2009), which are not present in WT4. Recent studies have shown that Tax can activate the anaphase-promoting complex (APC) ahead of schedule and induce mitotic defects.26 We examined the levels of APC-related proteins in WT4 cells and compared their expression to MT2 cells. As previously reported in Tax-expressing cells, we found inhibition of Skp2, indicating activation of APC (Figure 5D). This was associated with lower levels of p27KIP and stabilization of p21WAF. An alteration in the mitotic checkpoint is needed for mitotic defects to accumulate and be tolerated. We analyzed the mitotic spindle checkpoint, controlled in part by the Mad1/2 proteins, in WT4 and 2 HTLV-I transformed cell lines MT2 and MT4. As demonstrated in Figure 5E, treatment with microtubule-disrupting agents nocodazole or paclitaxel activated the mitotic spindle checkpoint and efficiently arrested all cell lines in G2/M, suggesting that this checkpoint is not altered and remains functional in WT4- and HTLV-I–transformed MT2 and MT4 cells.
WT4 cells activate the anaphase-promoting complex. (A) Sequencing of the p53 hot spot in early passage and immortalized WT4 cells, compared with wild-type p53. (B) p53 Luciferase (pGL13) activity between wild-type p53 (FL) and WT4-p53 in Jurkat cells. (C) Real-time PCR expression of p21WAF and mdm2 in PBMCs, C8166, and WT4 cells after gamma irradiation (10 Gy). RNA was extracted 2.5 hours after irradiation. Real-time PCR was performed in duplicate; error bars represent the CT SD of the gene of interest. (D) Western blot analysis of cyclin A, cyclin B, Skp2, p27KIP, and p21WAF in WT4 and MT2 cells compared with nontransduced resting (RPBMCs) or activated (ActPBMCs) PBMCs. (E) Mitotic spindle checkpoint studies. Cells were treated for 24 hours with either nocodazole (400 ng/mL) or paclitaxel (10μM), followed by cell-cycle analysis by flow cytometry.
WT4 cells activate the anaphase-promoting complex. (A) Sequencing of the p53 hot spot in early passage and immortalized WT4 cells, compared with wild-type p53. (B) p53 Luciferase (pGL13) activity between wild-type p53 (FL) and WT4-p53 in Jurkat cells. (C) Real-time PCR expression of p21WAF and mdm2 in PBMCs, C8166, and WT4 cells after gamma irradiation (10 Gy). RNA was extracted 2.5 hours after irradiation. Real-time PCR was performed in duplicate; error bars represent the CT SD of the gene of interest. (D) Western blot analysis of cyclin A, cyclin B, Skp2, p27KIP, and p21WAF in WT4 and MT2 cells compared with nontransduced resting (RPBMCs) or activated (ActPBMCs) PBMCs. (E) Mitotic spindle checkpoint studies. Cells were treated for 24 hours with either nocodazole (400 ng/mL) or paclitaxel (10μM), followed by cell-cycle analysis by flow cytometry.
Discussion
Our data demonstrate that Tax is a poor oncogene and that immortalization of PBMCs is a very rare event in vitro. Our results also support the idea that dysfunctional telomere-driven genomic instability is associated with a tumor suppressor phenotype and opposes immortalization by Tax. These findings are consistent with previous studies, whereby inhibition of endogenous hTERT leads to decreased life span, cytogenetic abnormalities, and chromosome fusions in human T cells.34 This study confirms our previous findings that sustained telomerase activity is imperative to the survival of HTLV-I–infected cells, because inhibition of telomerase leads to critically short telomeres and induction of senescence in HTLV-I–infected cells and in nonimmortalized Tax-expressing PBMCs. Our findings also suggest the existence of posttranscriptional Tax-independent events that are required for sustained telomerase activity. These results are consistent with published literature in which transcriptional regulation of hTERT alone does not determine telomerase activity in human CD4+ T lymphocytes.35 The poor oncogenicity of Tax in human primary T cells is in stark contrast to Tax transformation of rodent fibroblasts and transgenic models. However, it is well known that introduction of 2 collaborating oncogenes is sufficient to transform rodent primary cells. Human cells are far more complex and attempts to transform human cells with 2 cooperating oncogenes have been unsuccessful to date. The combination of genetic alterations needed to transform human cells is currently unknown, but it has been proposed that at least 6 to 8 genetic events are required.
This study provides new insights about Tax's pleiotropic effects. We found that Tax is a poor oncogene in human T cells and immortalization by Tax is a rare event. Tax-expressing cells remain IL-2 dependent and Tax expression does not directly transform human T cells. Tax expression is not sufficient to stimulate cell-cycle progression from G1 to S phase in the absence of exogenous IL-2. Furthermore, Tax expression in itself is not directly associated with aneuploidy and does not affect the mitotic spindle checkpoint in human T cells. Our results are in agreement with the fact that Tax expression is sufficient to stabilize p53 and partially inactivate p53-dependent transcription. Tax expression was associated with decreased expression of IκBα, Lck, and ZAP-70, whereas Bcl-xL and p-Akt were up-regulated. These findings suggest either Tax-dependent activation of the AKT pathway or selection of specific cellular mutation(s) during the immortalization processes, which result in the activation of AKT, independent of Tax. The down-regulation of p27KIP (Figure 5D) in Tax-transduced cells, supports the latter. WT4- and HTLV-I–transformed cells demonstrated low levels of the E3 ligase, Skp2, which should result in p27KIP stabilization. In contrast, we found low levels of p27KIP in Tax-expressing cells, despite the loss of Skp2 expression. It is suggested that somatic mutations in phosphoinositide-3 kinase (PI3K)/AKT may lead to inactivation of p21WAF or p27KIP, which could occur in absence of a Skp2-mediated degradation of p27KIP.36 Previous studies have found that inhibition of the PI3K pathway in HTLV-I–infected cells causes an increase in p27KIP levels and bypasses G1 arrest.37 Therefore, maintaining low levels of p27KIP is considered a key event in HTLV-I–mediated transformation. Although some of our observations appear reminiscent of those published by Kuo et al,26 there are some notable differences. In the study by Kuo et al, HeLa cells were used and the genetic instability and senescence appeared rapidly within a few mitotic divisions after Tax expression, whereas the defects detected in our Tax-transduced PBMCs appeared only after several months in culture when telomeres reached a critically short size. This difference may be explained by the fact that most established cancer cell lines, including HeLa, have short telomeres compared with primary cells and have inactivated tumor suppressor and cell-cycle checkpoints. It is possible that some of the aberrations observed in Figure 2A have the same origin as those reported by Kuo et al, but it takes longer in primary cells, because these cells need to inactivate tumor suppressor and apoptosis pathways to be tolerated and be visible.
The establishment of the immortalized cell line WT4 was at least partly achieved by the random genetics events (loss of IL-16 and TPR). IL-16 is overexpressed in resting PBMCs and overexpression of IL-16 in activated PBMCs induces p27KIP accumulation, inhibits proliferation, and induces cell-cycle arrest. TPR is part of the nuclear pore complex and binds Mad1 and Mad2.38 A role for these genes in HTLV-I pathogenesis and ATL disease progression has not been established and warrants future studies. These data do not take into account the possibility of gene disruption that results from single nucleotide polymorphisms, epigenetic modifications, and/or posttranslational modifications to proteins, which cannot be detected by karyotype analysis and that require a more detailed analysis. It is highly likely that additional tumor suppressors and/or oncogenes are deregulated at the nucleotide and/or epigenetic level and contribute to the immortalization process. Detailed analyses of WT4 may reveal some of these genetic and epigenetic changes needed for Tax-mediated human T-cell immortalization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Jenkins for editorial assistance.
This work was supported by the National Cancer Institute (grants CA106258 and CA115398) to C.N. M.B. was partially supported by a fellowship (P20 RR016475) from the National Center for Research Resources.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.B. performed experiments shown in Figures 2 through 5 and wrote the paper; H.H.B. performed experiments shown in Figures 2 and 5; Y.Y. performed experiments shown in Figures 2, 3, and 5; and C.N. established the Tax-immortalized cell line WT4, designed the project, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Nicot, University of Kansas Medical Center, Department of Pathology and Laboratory Medicine, Center for Viral Oncology, KU Cancer Center, 3901 Rainbow Blvd, Kansas City, KS 66160; e-mail: cnicot@kumc.edu.

![Figure 2. Genomic instability leads to premature senescence in Tax-expressing cells. (A) Tax-transduced PBMCs during either early (< 1 month) or late (> 6 months) passage were fixed, stained with DAPI (4,6 diamidino-2-phenylindole), and then analyzed for the presence of cell division abnormalities such as asymmetric division, multinucleated cell, micronuclei, and anaphase bridges. (B) Karyotype analysis of Tax-transduced PBMCs during late passage. Arrows indicate telomere associations (Tas). (C) Western blot of Tax expression in early and late Tax-transduced PBMCs (Tax/PBMC no. 3). (D) Real-time PCR analysis of hTERT expression between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). Normalized values are represented as the percentage of activation of early Tax-transduced PBMCs. (E) TRAP analysis of telomerase activity between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer was a negative control. (F) Fluorescence in situ hybridization (FISH) of telomere sequences in early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3) compared with MT2 cells. Histograms represent the intensities of fluorescence spots of telomere FISH staining with their respective numbers in each cell line. A minimum of 100 spots was quantified for each cell line. (G) Double immunofluorescence staining of late-cultured Tax-transduced PBMCs (Tax/PBMC no. 3) with anti-TRF2 and anti–phospho-H2AX antibodies. The contact of telomere-induced foci (red) and DNA double-strand breaks (green) is indicated by the arrows in the overlay image. (H) Immunofluorescence of p53 transcriptional activity in Tax-transduced PBMCs (PBMC/Tax no. 3) as indicated by phosphorylation on serine 20 of p53. (I) Real-time PCR expression of p53-responsive genes p21WAF and mdm2 in nontransduced PBMCs, and early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3). Assays were performed in duplicate. Fold changes were calculated by comparing values with that of nontransduced PBMC normalized gene expression. Error bars represent the CT SD of the gene of interest.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/12/10.1182_blood-2009-08-241117/5/m_zh89991050090002.jpeg?Expires=1766076715&Signature=t9-13M8zJQt0lADGZzoiJjkbZ5jNLZjBvyDEB3OREGg8hbxs~izOK34J5rCeYGlKwpRQ~w3AMx24ti6NW7GkrhgGKHabajKxe9l21xODcL2wIbbagEFHSzc~RDu2I~-x3~GGexwbcXe8KUKNAhQBLmd1956UDVSu5qggo5AT44qt6ENp1Mu-hNWHa4FVJf2U0noTRDlN0U-X1rXp7wiWgwtqqoFevXsXMdPyHWpJ1prtvC0E5HM5IdUunGq7sAeIDXpzzdTVgv1HnOGEhs7iA3MRK2qGqkpdQaB2Jey3fA8GgC8MKj1m5wZ0oMpwdg0GCXjuu7twghUpZbFaJpWf3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Genomic instability leads to premature senescence in Tax-expressing cells. (A) Tax-transduced PBMCs during either early (< 1 month) or late (> 6 months) passage were fixed, stained with DAPI (4,6 diamidino-2-phenylindole), and then analyzed for the presence of cell division abnormalities such as asymmetric division, multinucleated cell, micronuclei, and anaphase bridges. (B) Karyotype analysis of Tax-transduced PBMCs during late passage. Arrows indicate telomere associations (Tas). (C) Western blot of Tax expression in early and late Tax-transduced PBMCs (Tax/PBMC no. 3). (D) Real-time PCR analysis of hTERT expression between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). Normalized values are represented as the percentage of activation of early Tax-transduced PBMCs. (E) TRAP analysis of telomerase activity between early and late Tax-transduced PBMCs (Tax/PBMC no. 3). 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer was a negative control. (F) Fluorescence in situ hybridization (FISH) of telomere sequences in early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3) compared with MT2 cells. Histograms represent the intensities of fluorescence spots of telomere FISH staining with their respective numbers in each cell line. A minimum of 100 spots was quantified for each cell line. (G) Double immunofluorescence staining of late-cultured Tax-transduced PBMCs (Tax/PBMC no. 3) with anti-TRF2 and anti–phospho-H2AX antibodies. The contact of telomere-induced foci (red) and DNA double-strand breaks (green) is indicated by the arrows in the overlay image. (H) Immunofluorescence of p53 transcriptional activity in Tax-transduced PBMCs (PBMC/Tax no. 3) as indicated by phosphorylation on serine 20 of p53. (I) Real-time PCR expression of p53-responsive genes p21WAF and mdm2 in nontransduced PBMCs, and early and late passage Tax-transduced PBMCs (Tax/PBMC no. 3). Assays were performed in duplicate. Fold changes were calculated by comparing values with that of nontransduced PBMC normalized gene expression. Error bars represent the CT SD of the gene of interest.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/12/10.1182_blood-2009-08-241117/5/m_zh89991050090002.jpeg?Expires=1766984418&Signature=2zCQtzrym6LunBbGjVxijfJjZqGRQpWVICa8YH-~vlHtK77pgqSlaOU8c5-v9lmSiqTAWdtn9ZHWMl6vPwTw7Q8zTQRqFtDMSKrYXdqVe6O83D2sCxtHIRP3Slqg4vXgfAw3-~v97VCmdq754AACnMBxP3Qv~SrWMFJ7qe6ELFwVdHnuXJ2TOdjS33wtOnYCaYqCs09LuRn~0M5JqMR-J9sDGI5qng-eBtkaUNrvv6M4uH3LNfIOiOZbW-L3gNLNP5RQ6~-gDI-xpt8BKaZMK8DM~XsCbrLexk3zuUkBSSlpr2dY-Kpy0~wsXJW4Wi~JJ8dC2G15lAF-zgTTTt~qvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)