Abstract
The C-type lectin-like receptor 2 (CLEC-2) activates platelets through Src and Syk tyrosine kinases via a single cytoplasmic YxxL motif known as a hem immunoreceptor tyrosine-based activation motif (hemITAM). Here, we demonstrate using sucrose gradient ultracentrifugation and methyl-β-cyclodextrin treatment that CLEC-2 translocates to lipid rafts upon ligand engagement and that translocation is essential for hemITAM phosphorylation and signal initiation. HemITAM phosphorylation, but not translocation, is also critically dependent on actin polymerization, Rac1 activation, and release of ADP and thromboxane A2 (TxA2). The role of ADP and TxA2 in mediating phosphorylation is dependent on ligand engagement and rac activation but is independent of platelet aggregation. In contrast, tyrosine phosphorylation of the GPVI-FcRγ-chain ITAM, which has 2 YxxL motifs, is independent of actin polymerization and secondary mediators. These results reveal a unique series of proximal events in CLEC-2 phosphorylation involving actin polymerization, secondary mediators, and Rac activation.
Introduction
Dectin-1, C-type lectin-like receptor 2 (CLEC-2), and CLEC-9A are C-type lectin receptors which have been shown to signal through an immunoreceptor tyrosine-based activation motif (ITAM)–like pathway via a single YxxL in their short cytoplasmic tails known as a hemITAM.1-4 Tyrosine phosphorylation of the conserved tyrosine and both SH2 domains of the tyrosine kinase Syk have been shown to be essential for activation of Syk by CLEC-2 and dectin-1, favoring a model in which the tyrosine kinase is activated by the cross-linking of 2 receptors.2 This is consistent with the observations that CLEC-9A is a disulphide-linked dimer and that CLEC-2 is expressed as a noncovalent dimer.1,5
CLEC-2 is highly expressed in platelets and at lower levels in other hematopoietic cells, including neutrophils and dendritic cells.6,7 It is a type II transmembrane protein and a nonclassical C-type lectin that lacks the conserved amino acids that mediate binding to glycans.7 CLEC-2 was identified by affinity chromatography as a ligand for the snake venom protein rhodocytin, purified from the Malayan pit viper, Calloselasma rhodostoma.3 Independent crystal structures of rhodocytin show that it assembles as a tetramer, which leads to the suggestion that it mediates activation of CLEC-2 through clustering.8,9 Consistent with this clustering model of activation, whole antibodies and F(ab)2 fragments have been reported to activate CLEC-2, whereas antibody Fab fragments were inhibitory.3,10,11
Physiologic roles of CLEC-2 are now beginning to emerge. Recently, CLEC-2 has been implicated in tumor metastasis via its interaction with the type I sialoglycoprotein podoplanin, which is also expressed on the surface of kidney podocytes, lung type I alveolar cells, and lymphatic endothelium.12-14 CLEC-2 has also been shown to play a role in neutrophil phagocytosis.15 Evidence obtained using antibody depletion of CLEC-2 from mouse platelets has shown that it plays an important role in hemostasis and thrombosis at arteriolar rates of flow.11
Studies on platelets and mutant cell lines have demonstrated that CLEC-2 and the other hemITAM receptors share many of the signaling features of receptors that signal via ITAMs. The ITAM motif is defined by the sequence YxxLx6-12YxxL, and is found in receptors such as the T-cell receptor and the platelet collagen receptor, GPVI-FcRγ-chain.16 The characteristic ITAM signaling features include sequential activation of Src and Syk family kinases, adapter proteins such as LAT, SLP-76, and BLNK, and activation of PLCγ2.2,3,17 Nevertheless, we have demonstrated that the proximal events in the CLEC-2 signaling cascade are distinct from that for ITAM receptors in that phosphorylation of the hemITAM is mediated by both Src and Syk tyrosine kinases, whereas phosphorylation of the ITAM is mediated solely by Src kinases.10
ITAM receptors signal through cholesterol-rich lipid membrane domains, known as lipid rafts or GEMS.18-20 These specialized regions of the membrane are enriched in many signaling proteins, including Src family kinases and the membrane adapter protein LAT.21 In addition, the cytoskeleton plays a contributory, but poorly understood role in signaling by ITAM receptors.22 Recently, dectin-1 has also been shown to signal in lipid rafts,23 but it is not known if this is also the case for CLEC-2.
The aim of the present study was to investigate the role of lipid rafts and the actin cytoskeleton in platelet activation by CLEC-2 alongside that of GPVI to investigate whether this could explain the differential reliance of the 2 receptors on Src and Syk kinases in mediating hemITAM and ITAM phosphorylation.
Methods
Reagents
Rhodocytin was purified from C rhodostoma venom as previously described.24 Antibodies used included goat anti–human CLEC-2 antibody (R&D Systems), mouse antiphosphotyrosine antibody (4G10; Upstate Biotechnology, TCS Biologicals Ltd), mouse anti-Rac antibody, 23A8 (Upstate Biotechnology), rabbit anti-PLCγ2, and rabbit anti-Syk polyclonal antibodies were from previously described sources.25 Mouse anti-integrin αIIb-specific antibody SZ22 was from Immunotech, rabbit anti-LAT antibody was from Upstate Biotechnology, and rat anti-podoplanin antibody NZ-1 was from AngioBio. Horseradish peroxidase (HRP)–conjugated anti–mouse and anti–rabbit IgGs were obtained from GE Healthcare, and HRP-conjugated anti–rat IgG was obtained from Santa Cruz Biotechnology. EHT 1864 was obtained from ExonHit Therapeutics, and Cangrelor, a P2Y12 inhibitor, was obtained from The Medicines Company. Eptifibatide was obtained from the University Hospital Birmingham's pharmacy. All other reagents were purchased from Sigma-Aldrich.
Preparation of human platelets
Venous blood from healthy drug-free volunteers was taken into 10% sodium citrate, and washed platelets were prepared as previously described.2 They were resuspended in a modified HEPES Tyrode buffer (composition: 134mM NaCl, 2.9mM KCl, 0.34mM Na2PO4.12H2O, 12mM NaHCO3, 20mM HEPES, 1mM MgCl2, and 5mM glucose [pH 7.3]).
Platelet aggregation
Aggregation was monitored by light transmission using a Born lumi-aggregometer (Chronolog).
MβCD treatment
For cholesterol depletion, washed platelets were incubated with 5mM, 2.5mM, or 1mM methyl-β-cyclodextrin (MβCD) for 1 hour at room temperature before stimulation.
Lipid raft isolation
For membrane preparation, cells (0.5 mL at 1.5 × 109 platelets/mL) were disrupted in ice-cold lysis buffer (25mM Tris-HCL [pH 7.6], 150mM NaCl, 5mM EDTA, 1% Brij 58, 1mM phenylmethylsulfonyl fluoride [PMSF], 2mM sodium orthovanadate [Na3VO4], 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A). The same buffer was used for the sucrose gradient solutions. The lysate was mixed with an equal volume of 80% (wt/vol) sucrose, producing a final concentration of 40%. The lysate was loaded on the bottom of an ultracentrifuge tube and overlaid with 2 mL of 30% (wt/vol) sucrose followed by 1 mL of 5% (wt/vol) sucrose. Lipid rafts were separated from nonraft and cytoskeleton components by ultracentrifugation (48 000g for 16 hours). After fraction isolation, 1% (wt/vol) N-dodecyl-β-D-gluco-maltoside was added to solubilize the lipid rafts. Fractions were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Immunoprecipitation studies
Washed resting or stimulated platelets (1.5 × 108), pretreated with 9μM eptifibatide to inhibit platelet activation and aggregation through integrin αIIbβ3, were lysed with an equal volume of 2 × lysis buffer (20mM Tris/HCL [pH 7.5], 300mM NaCl, 2mM EGTA, 2mM EDTA, 2% NP-40, 2mM PMSF, 5mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin). Cell debris was removed by centrifugation (15 000g for 10 minutes). After preclearing, proteins were captured by antibodies bound to either protein G–sepharose or protein A–sepharose. Samples were separated on a SDS-PAGE along side a molecular weight marker and transferred onto a polyvinylidene difluoride (PVDF) membrane. After blocking, the membranes were incubated with primary antibody overnight, washed, and then incubated with HRP-conjugated secondary antibody for 1 hour. Immunoprecipitated proteins were visualized by chemiluminescence (ECL; Pierce).
Rac pull-down assay
Eptifibatide-treated platelets were lysed with 2× lysis buffer (100mM Tris-HCL [pH 7.5], 1M NaCl, 20mM MgCl2, 1% deoxycholate, 0.2% SDS, 2% Triton X-100, 2mM PMSF, 10 μg/mL leupeptin, 20 μg/mL aprotinin, and 5mM EGTA). Lysates were centrifuged at 18 000g at 4°C for 10 minutes. Equal volumes of lysates were incubated with glutathione-S-transferase–PAK bound to glutathione-sepharose beads for 30 minutes at 4°C. The beads were washed 3 times with wash buffer (50mM Tris-HCL [pH 7.5], 150mM NaCl, 10mM MgCl2, 1% Triton X-100, 1mM PMSF, 10 μg/mL leupeptin, 5 μg/mL aprotinin, and 5mM EGTA). The Rac1 proteins bound to the beads were detected by Western blotting using anti-Rac1 antibodies.
Generation of podoplanin-expressing CHO cells
Chinese Hamster Ovary (CHO) cells were transfected with pcDNA3 containing full-length human podoplanin using a calcium phosphate transfection method. Stable transfectants were obtained using media containing 1 mg/mL geneticin (G418) and clonal cell populations obtained after serial dilutions into 96-well plates. Surface expression of human podoplanin was assessed by flow cytometry.
Results
CLEC-2 localizes to lipid rafts upon stimulation with rhodocytin
Lipid rafts are plasma microdomains which have been shown to play an important role in signaling by ITAM receptors. To assess the role of these specialized membrane regions in signaling by CLEC-2, we measured the presence of CLEC-2 in lipid rafts under resting and stimulated conditions. Brij 58 has previously been shown to preserve lipid rafts over a wide range of concentrations and was selected as the detergent of choice in this study.26 Brij 58-solubilized platelet lysates were separated into lipid raft fractions and nonraft fractions by sucrose gradient ultracentrifugation and analyzed by SDS-PAGE. The presence of CLEC-2, which runs as a doublet due to differential glycosylation,3 was investigated by Western blotting using a specific antibody. In control, nonstimulated platelets, approximately 5% of CLEC-2 was found in the detergent-insoluble fraction (Figure 1A-B). The detergent-insoluble fraction was enriched in the lipid raft marker protein LAT but excluded the nonraft marker, the integrin αIIb subunit.26 Stimulation of platelets by rhodocytin caused an approximate 35% redistribution of CLEC-2 to the detergent-insoluble fraction (Figure 1A-B) raising the possibility that it signals through these lipid-rich membrane domains. To investigate this further, we monitored tyrosine phosphorylation of CLEC-2 after immunoprecipitation from lipid raft and nonraft fractions of rhodocytin-stimulated platelets (Figure 1C). Approximately 90% of tyrosine-phosphorylated CLEC-2 was found within the insoluble, lipid raft membrane domain consistent with a model in which signaling by the lectin receptor takes place in the cholesterol-rich membrane domains.
CLEC-2 translocates to lipid rafts upon stimulation. (A) Resting and rhodocytin-stimulated platelets were lysed in the presence of 1% Brij 58 and separated into lipid raft and nonraft fractions by sucrose gradient ultracentrifugation. The localization of CLEC-2 was determined by immunoblotting with specific antibodies. The lipid raft fractions were identified using an anti-LAT blot, and nonraft fractions were identified using an anti–integrin αIIb blot. (B) Characterization of fractions from basal and rhodocytin stimulated platelets pretreated with DMSO or 10μM cytochalasin D. Data represent the mean and standard error of 3 independent experiments. (C) CLEC-2 was immunoprecipitated from lipid raft and nonraft fractions of platelets stimulated with 100nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of at least 3 independent experiments.
CLEC-2 translocates to lipid rafts upon stimulation. (A) Resting and rhodocytin-stimulated platelets were lysed in the presence of 1% Brij 58 and separated into lipid raft and nonraft fractions by sucrose gradient ultracentrifugation. The localization of CLEC-2 was determined by immunoblotting with specific antibodies. The lipid raft fractions were identified using an anti-LAT blot, and nonraft fractions were identified using an anti–integrin αIIb blot. (B) Characterization of fractions from basal and rhodocytin stimulated platelets pretreated with DMSO or 10μM cytochalasin D. Data represent the mean and standard error of 3 independent experiments. (C) CLEC-2 was immunoprecipitated from lipid raft and nonraft fractions of platelets stimulated with 100nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of at least 3 independent experiments.
As CLEC-2 phosphorylation by Src and Syk family kinases is critical for platelet activation, we investigated whether movement of CLEC-2 to the lipid raft fraction was blocked by pretreatment of platelets with the Src kinase inhibitor PP2. Incubation of platelets with PP2 completely inhibits tyrosine phosphorylation of CLEC-2 (data not shown), in confirmation of previous publications,3,10 but had no effect on its movement to the lipid raft fraction (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), demonstrating that translocation occurs before tyrosine phosphorylation.
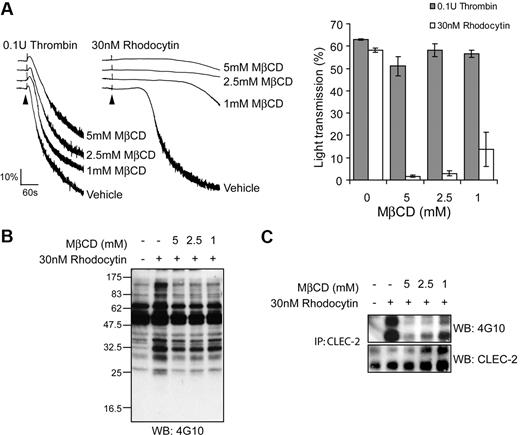
The cholesterol-lowering agent MβCD was used to further investigate the role of lipid rafts in rhodocytin signaling. In agreement with previous reports,27,28 we found that pretreatment of platelets with a relatively high concentration (5mM) of MβCD caused mild (< 20%) inhibition of thrombin induced aggregation, whereas there was no effect at lower MβCD concentrations (Figure 2A). In contrast, MβCD caused a marked, concentration-dependent inhibition of rhodocytin-induced aggregation that reduced the response by greater than 95% at 5mM, with a partial effect observed at lower concentrations (1-2mM). Consistent with this, both whole-cell tyrosine phosphorylation and CLEC-2 tyrosine phosphorylation by rhodocytin were inhibited by MβCD in a concentration-dependent manner (data not shown; Figure 2B). Together, these data demonstrate a critical role for lipid rafts in signaling by the snake venom toxin and support a model in which translocation to the raft fractions occurs before phosphorylation of the hemITAM receptor.
Lipid raft disruption by MβCD impairs CLEC-2 signaling. (A) Platelets were pretreated with indicated concentrations of MβCD and stimulated with 30nM rhodocytin or 0.1 U/mL thrombin. (Left) Representative aggregation traces are shown for control and MβCD-treated platelets. Addition of agonist is indicated by an arrowhead. The aggregation traces have been staggered. (Right) The percentage of transmittance of platelets pretreated with decreasing concentrations of MβCD-treated platelets stimulated with rhodocytin or thrombin was measured after 5 minutes. Data represent the mean and standard error of 3 independent experiments. (B) CLEC-2 was immunoprecipitated from platelets pretreated with indicated concentrations of MβCD and stimulated with vehicle or 30nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of 3 independent experiments.
Lipid raft disruption by MβCD impairs CLEC-2 signaling. (A) Platelets were pretreated with indicated concentrations of MβCD and stimulated with 30nM rhodocytin or 0.1 U/mL thrombin. (Left) Representative aggregation traces are shown for control and MβCD-treated platelets. Addition of agonist is indicated by an arrowhead. The aggregation traces have been staggered. (Right) The percentage of transmittance of platelets pretreated with decreasing concentrations of MβCD-treated platelets stimulated with rhodocytin or thrombin was measured after 5 minutes. Data represent the mean and standard error of 3 independent experiments. (B) CLEC-2 was immunoprecipitated from platelets pretreated with indicated concentrations of MβCD and stimulated with vehicle or 30nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of 3 independent experiments.
Cytochalasin D inhibits rhodocytin signaling
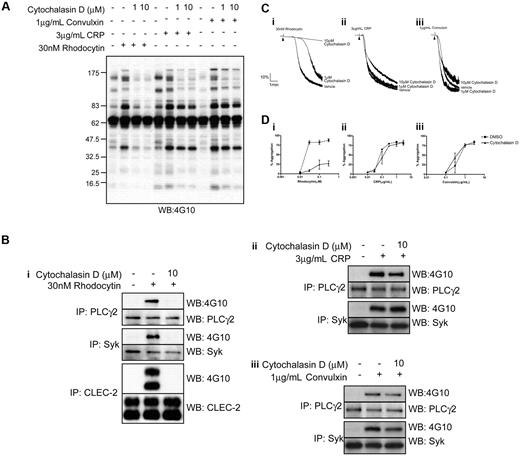
The actin cytoskeleton plays a contributory but poorly defined role in signaling by ITAM receptors. We therefore used cytochalasin D, a powerful inhibitor of actin polymerization, to investigate the role of actin polymerization in the movement of CLEC-2 to lipid rafts and platelet activation by rhodocytin. Cytochalasin D (10μM) had no effect on the translocation of CLEC-2 to lipid rafts (Figure 1B; supplemental Figure 1), but completely abrogated tyrosine phosphorylation of the lectin receptor, demonstrating a pivotal role for actin polymerization in the early events underlying receptor signaling. Consistent with this, whole-cell tyrosine phosphorylation, including tyrosine phosphorylation of Syk and PLCγ2, were completely inhibited in the presence of a maximally effective (10μM) concentration of cytochalasin D (Figure 3A-B). Furthermore, cytochalasin D (10μM) blocked aggregation to low concentrations of rhodocytin and inhibited the response to higher concentrations of the snake venom by more than 70%, while a submaximal concentration of cytochalasin D (1μM) caused a marked increase in the lag phase that precedes aggregation (Figure 3C-D). In contrast, a maximal concentration of cytochalasin D (10μM) had no significant effect on tyrosine phosphorylation of Syk and PLCγ2 induced by the GPVI-specific agonists collagen-related peptide (CRP) and convulxin (Figure 3A-B), and caused only a marginal decrease in the rate of late-stage aggregation with no effect on the maximal response (Figure 3C-D). Comparable observations for both rhodocytin- and GPVI-mediated responses were seen with a second inhibitor of actin polymerization, latrunculin A (data not shown). These results therefore demonstrate a critical role for actin polymerization in platelet activation by rhodocytin.
Platelet responses to rhodocytin are impaired upon cytochalasin D treatment. (A) Whole-cell lysates from basal or agonist-stimulated platelets preincubated with various doses of cytochalasin D were separated by SDS-PAGE, and tyrosine phosphorylation was assessed using an antiphosphotyrosine antibody. (B) Whole-cell lysates were isolated from resting or platelets stimulated with (i) rhodocytin, (ii) CRP, or (iii) convulxin, treated with or without cytochalasin D. CLEC-2, PLCγ2, and Syk were immunoprecipitated and the samples separated by SDS-PAGE and immunoblotted for CLEC-2, PLCγ2, and Syk. Their tyrosine-phosphorylated states were assessed using an antiphosphotyrosine antibody. (C) Platelets pretreated with various concentrations of cytochalasin D were stimulated with (i) 30nM rhodocytin, (ii) 3 μg/mL CRP, and (iii) 1 μg/mL convulxin. Addition of agonist is indicated by an arrowhead. Representative aggregation traces are shown for control and cytochalasin D–treated platelets. (D) Dose responses to (i) rhodocytin, (ii) CRP, and (iii) convulxin. Platelets pretreated with DMSO or 10μM cytochalasin D are shown as mean and standard error of 3 independent experiments.
Platelet responses to rhodocytin are impaired upon cytochalasin D treatment. (A) Whole-cell lysates from basal or agonist-stimulated platelets preincubated with various doses of cytochalasin D were separated by SDS-PAGE, and tyrosine phosphorylation was assessed using an antiphosphotyrosine antibody. (B) Whole-cell lysates were isolated from resting or platelets stimulated with (i) rhodocytin, (ii) CRP, or (iii) convulxin, treated with or without cytochalasin D. CLEC-2, PLCγ2, and Syk were immunoprecipitated and the samples separated by SDS-PAGE and immunoblotted for CLEC-2, PLCγ2, and Syk. Their tyrosine-phosphorylated states were assessed using an antiphosphotyrosine antibody. (C) Platelets pretreated with various concentrations of cytochalasin D were stimulated with (i) 30nM rhodocytin, (ii) 3 μg/mL CRP, and (iii) 1 μg/mL convulxin. Addition of agonist is indicated by an arrowhead. Representative aggregation traces are shown for control and cytochalasin D–treated platelets. (D) Dose responses to (i) rhodocytin, (ii) CRP, and (iii) convulxin. Platelets pretreated with DMSO or 10μM cytochalasin D are shown as mean and standard error of 3 independent experiments.
Role of secondary mediators in rhodocytin signaling
The secondary mediators ADP and TxA2 play a critical positive feedback role in mediating platelet activation by all agonists, although for many their action is obviated at high agonist concentrations.29 To investigate the dependency on secondary mediators for platelet activation by rhodocytin, we used the ADP scavenger, apyrase, and the cyclooxygenase inhibitor, indomethacin. Strikingly, the combination of apyrase and indomethacin abolished platelet aggregation to a 30-nM concentration of rhodocytin (Figure 4Ai). The inhibition seen in the presence of secondary mediators could not be overcome with higher concentrations of rhodocytin (Figure 4Aii). Strikingly, however, the movement of CLEC-2 into lipid rafts induced by rhodocytin was not altered in the presence of the 2 inhibitors (supplemental Figure 1). The increase in whole-cell tyrosine phosphorylation induced by rhodocytin (100nM) and phosphorylation of CLEC-2 was also dramatically reduced in the presence of apyrase and indomethacin (Figure 4B; data not shown). The residual increase in tyrosine phosphorylation induced by rhodocytin in the presence of apyrase and indomethacin was inhibited in the presence of PP2 or cytochalasin D (Figure 4B).
Role of secondary mediators in rhodocytin induced aggregation and protein phosphorylation. (Ai,iii) Platelets pretreated with or without inhibitors were stimulated with 30nM rhodocytin. Addition of agonist is indicated by an arrowhead. (ii) Dose responses to rhodocytin. Platelets pretreated with vehicle or 2 U/mL apyrase/10μM indomethacin are shown as mean and standard error of 3 independent experiments. Protein phosphorylation measurements (B-E) were performed in the presence of eptifibatide to prevent platelet aggregation. (B) Whole-cell lysates were prepared from control and rhodocytin-stimulated platelets with or without secondary mediators and indicated inhibitors. (C) CLEC-2 was immunoprecipitated from basal-, U46619/ADP-, and rhodocytin-stimulated platelet lysates, and tyrosine phosphorylation was assessed. (D) CLEC-2 was immunoprecipitated from rhodocytin-stimulated platelets pretreated with inhibitors, and tyrosine phosphorylation was assessed. (E) CLEC-2 was immunoprecipitated from rhodocytin-stimulated platelets pretreated with the indicated inhibitors with or without the addition of ADP/U46619, and tyrosine phosphorylation was assessed. Data shown are representative of 3 independent experiments.
Role of secondary mediators in rhodocytin induced aggregation and protein phosphorylation. (Ai,iii) Platelets pretreated with or without inhibitors were stimulated with 30nM rhodocytin. Addition of agonist is indicated by an arrowhead. (ii) Dose responses to rhodocytin. Platelets pretreated with vehicle or 2 U/mL apyrase/10μM indomethacin are shown as mean and standard error of 3 independent experiments. Protein phosphorylation measurements (B-E) were performed in the presence of eptifibatide to prevent platelet aggregation. (B) Whole-cell lysates were prepared from control and rhodocytin-stimulated platelets with or without secondary mediators and indicated inhibitors. (C) CLEC-2 was immunoprecipitated from basal-, U46619/ADP-, and rhodocytin-stimulated platelet lysates, and tyrosine phosphorylation was assessed. (D) CLEC-2 was immunoprecipitated from rhodocytin-stimulated platelets pretreated with inhibitors, and tyrosine phosphorylation was assessed. (E) CLEC-2 was immunoprecipitated from rhodocytin-stimulated platelets pretreated with the indicated inhibitors with or without the addition of ADP/U46619, and tyrosine phosphorylation was assessed. Data shown are representative of 3 independent experiments.
As the presence of secondary mediators lead to a substantial amplification in CLEC-2 tyrosine phosphorylation we assessed the effect of ADP and a TxA2 mimetic (U46619) on CLEC-2 phosphorylation. ADP and U46619 had no significant effect on CLEC-2 phosphorylation (Figure 4C). Thus, the role of secondary mediators in regulating CLEC-2 tyrosine phosphorylation and downstream signaling is indirect. We further assessed the effect ADP and U46619 had on rhodocytin-mediated CLEC-2 phosphorylation after inhibition of actin polymerization. We found that the 2 secondary mediators were able to partially restore phosphorylation of CLEC-2 in the presence of cytochalasin D (Figure 4E) demonstrating that actin polymerization lies upstream of secondary mediator generation and further CLEC-2 phosphorylation.
Further studies were designed to investigate whether the effect of apyrase and indomethacin was due to blockade of the P2Y1, P2Y12, or TxA2 receptors, or a combination of these effects (Figure 4Aiii,D). The antagonists MRS2179 and Cangrelor are selective to P2Y1 and P2Y12 receptors, respectively, and indomethacin prevents the formation of TxA2 by blocking cyclooxygenase. Inhibition of the P2Y1 receptor had no effect on rhodocytin-induced platelet aggregation or CLEC-2 phosphorylation. Inhibition of the P2Y12 receptor caused a slight inhibition in rhodocytin-induced aggregation and CLEC-2 phosphorylation. In contrast, the greatest inhibition in rhodocytin-induced aggregation was seen when TxA2 formation was inhibited. This also resulted in a near loss in CLEC-2 phosphorylation. These data suggest that the major feedback mechanism that leads to an increase in CLEC-2 phosphorylation is downstream of the TxA2 receptor, but that there is limited synergy with signaling downstream of the P2Y12 and P2Y1 receptors, as a combination of apyrase and indomethacin further reduces CLEC-2 phosphorylation.
CLEC-2 phosphorylation requires Rac1
Studies on platelets from Rac1-deficient mice have shown that Rac1, a member of the Rho family of small GTPases, plays a critical role in mediating PLCγ2 activation by GPVI in platelets leading to inhibition of Ca2+ mobilization.30,31 Consistent with this, we observed concentration-dependent inhibition of Rac activation and aggregation of human platelets in response to CRP in the presence of the novel and selective Rac1 inhibitor, EHT 186432 (Figure 5Ai-ii). This demonstrates that Rac1 also plays a similar role in GPVI signaling in human platelets.
Role of Rac1 in rhodocytin-induced aggregation and signaling. (Ai) Platelets were pretreated with indicated amounts of EHT 1864 and platelet aggregation was measured after addition of 3 μg/mL CRP. Addition of agonist is indicated by an arrowhead. (ii) Lysates were made from platelets pretreated with indicated amounts of EHT 1864 stimulated with or without CRP. Active Rac was pulled-down with a GST-fusion protein containing the Rac binding domain of PAK1 and detected by immunoblotting for Rac. (B) Time course of Rac activation in human platelets stimulated with 100nM rhodocytin. Platelets were stimulated with rhodocytin; at the indicated times, activation was stopped by the addition of ice-cold lysis buffer. Active Rac was pulled-down and detected by immunoblotting for Rac. (C) Lysates were made from platelets pretreated with indicated amounts of EHT 1864 and stimulated with or without rhodocytin. Active Rac was pulled-down and detected by immunoblotting for Rac. (D) Platelets pretreated with decreasing concentrations of EHT 1864 were stimulated with 300nM rhodocytin. (i) Aggregation traces are shown for control and EHT 1864–treated platelets stimulated with 300nM rhodocytin. (ii) CLEC-2 was immunoprecipitated from platelets pretreated with indicated concentrations of EHT 1864 and stimulated with vehicle or 30nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of 3 independent experiments.
Role of Rac1 in rhodocytin-induced aggregation and signaling. (Ai) Platelets were pretreated with indicated amounts of EHT 1864 and platelet aggregation was measured after addition of 3 μg/mL CRP. Addition of agonist is indicated by an arrowhead. (ii) Lysates were made from platelets pretreated with indicated amounts of EHT 1864 stimulated with or without CRP. Active Rac was pulled-down with a GST-fusion protein containing the Rac binding domain of PAK1 and detected by immunoblotting for Rac. (B) Time course of Rac activation in human platelets stimulated with 100nM rhodocytin. Platelets were stimulated with rhodocytin; at the indicated times, activation was stopped by the addition of ice-cold lysis buffer. Active Rac was pulled-down and detected by immunoblotting for Rac. (C) Lysates were made from platelets pretreated with indicated amounts of EHT 1864 and stimulated with or without rhodocytin. Active Rac was pulled-down and detected by immunoblotting for Rac. (D) Platelets pretreated with decreasing concentrations of EHT 1864 were stimulated with 300nM rhodocytin. (i) Aggregation traces are shown for control and EHT 1864–treated platelets stimulated with 300nM rhodocytin. (ii) CLEC-2 was immunoprecipitated from platelets pretreated with indicated concentrations of EHT 1864 and stimulated with vehicle or 30nM rhodocytin and blotted with an antiphosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti–CLEC-2. Data are representative of 3 independent experiments.
To investigate a possible role of Rac1 in rhodocytin-signaling in human platelets, we first monitored Rac activation using a GST-PAK1–binding assay that selectively precipitates GTP-bound Rac.31 Formation of GTP-bound Rac after stimulation by rhodocytin was detected at 30 seconds and peaked at 45 seconds before declining to a plateau (Figure 5B). The delay in activation corresponds to the characteristic lag seen for rhodocytin-induced platelet aggregation (data not shown). Activation of Rac1 by rhodocytin was inhibited in the presence of cytochalasin D and apyrase/indomethacin, suggesting that it may lie downstream of actin polymerization or second messenger generation (data not shown).
The Rac1 inhibitor, EHT 1864, completely inhibited formation of GTP-bound Rac1 by rhodocytin at 50μM and caused partial inhibition at 10μM (Figure 5C), with a similar concentration-response relationship for inhibition of aggregation (Figure 5Di). EHT 1864 also markedly inhibited whole-cell tyrosine phosphorylation (data not shown) and phosphorylation of CLEC-2 (Figure 5Dii) over the same concentration range. The residual increase in whole-cell tyrosine phosphorylation observed in the presence of EHT 1864 was retained in the presence of apyrase and indomethacin (Figure 4B). On the other hand, the inhibition of phosphorylation of CLEC-2 by rhodocytin in the presence of EHT 1864 was only weakly restored in the presence of U46619 and ADP (Figure 4E). These results therefore support a model in which rhodocytin stimulates weak tyrosine phosphorylation of CLEC-2 and whole-cell phosphorylation independent of Rac1, but that activation of the GTPase, most likely by ADP and TxA2, is required for robust tyrosine phosphorylation of CLEC-2 and whole-cell phosphorylation.
Podplanin-induced aggregation signals in a similar way to rhodocytin
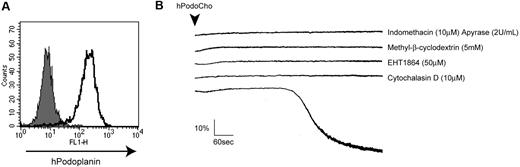
Podoplanin-expressing but not mock-transfected CHO cells have previously been shown to induce platelet aggregation.13 CHO cells that stably express human podoplanin (hPodoCHO) were generated, and surface expression of human podoplanin was assessed by flow cytometry (Figure 6A). The addition of hPodoCHO cells to platelets induces a similar pattern of aggregation to that seen with rhodocytin in that it is preceded by a characteristic lag phase (Figure 6B). Pretreatment of platelets with the lipid raft–disrupting agent MβCD, the actin-disrupting drug cytochalasin D, inhibitors of secondary mediators, or the Rac inhibitor EHT1864 all inhibited platelet aggregation in response to hPodoCHO. These results therefore demonstrate a similar profile of platelet activation by the endogenous ligand podoplanin to that induced by rhodocytin.
Signaling via podoplanin displays strong similarities to rhodocytin. (A) The level of surface expression of CHO cells stably expressing human podoplanin (hPodoCHO) was confirmed by flow cytometry using the anti-podoplanin antibody NZ-1 (infill: CHO cells; overlay: hPodoCHO cells). (B) Human washed platelets (6 × 108/mL) pretreated with the indicated inhibitors were stimulated by 4 × 106/mL hPodoCHO cells, and platelet aggregation was monitored by aggregometry. Data are representative of 3 independent experiments.
Signaling via podoplanin displays strong similarities to rhodocytin. (A) The level of surface expression of CHO cells stably expressing human podoplanin (hPodoCHO) was confirmed by flow cytometry using the anti-podoplanin antibody NZ-1 (infill: CHO cells; overlay: hPodoCHO cells). (B) Human washed platelets (6 × 108/mL) pretreated with the indicated inhibitors were stimulated by 4 × 106/mL hPodoCHO cells, and platelet aggregation was monitored by aggregometry. Data are representative of 3 independent experiments.
Discussion
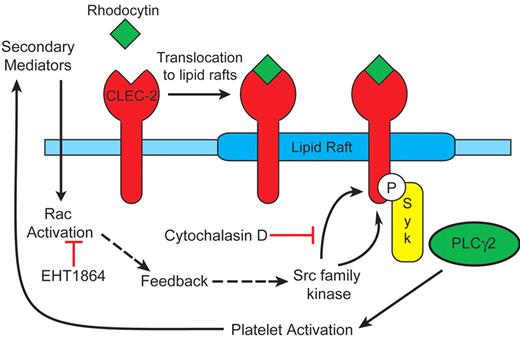
The present data support a model in which rhodocytin-mediated platelet activation occurs in membrane rafts through activation of Src and Syk family kinases and actin polymerization, and is reinforced by the release of the secondary mediators, ADP and TxA2, and Rac activation. The following observations form the basis of the model shown in Figure 7: (1) CLEC-2 translocates to lipid rafts independently of activation of Src kinases, actin polymerization, and release of ADP and TxA2; (2) tyrosine phosphorylation of CLEC-2 occurs in lipids rafts; (3) CLEC-2 tyrosine phosphorylation is dependent on actin polymerization as demonstrated by complete inhibition by cytochalasin D; (4) tyrosine phosphorylation of CLEC-2 and downstream signaling events are reinforced by the release of secondary mediators, with TxA2 formation playing the predominant role; (5) the combination of ADP and TxA2 partially restores phosphorylation of CLEC-2; and (6) Rac1 activation, which is presumably mediated downstream of the secondary mediators, is essential for this feedback phosphorylation of CLEC-2 to occur.
Model for CLEC-2 signaling. Our observations support the following model: (1) CLEC-2 translocates to lipid rafts independently of Src kinases, actin polymerization and release of ADP and TxA2; (2) Src kinase phosphorylation of CLEC-2 occurs in lipids rafts; (3) CLEC-2 tyrosine phosphorylation is dependent on actin polymerization; (4) tyrosine phosphorylation of CLEC-2 and downstream signaling events are reinforced by generation of the second mediators ADP and TxA2, which in turn activate Rac.
Model for CLEC-2 signaling. Our observations support the following model: (1) CLEC-2 translocates to lipid rafts independently of Src kinases, actin polymerization and release of ADP and TxA2; (2) Src kinase phosphorylation of CLEC-2 occurs in lipids rafts; (3) CLEC-2 tyrosine phosphorylation is dependent on actin polymerization; (4) tyrosine phosphorylation of CLEC-2 and downstream signaling events are reinforced by generation of the second mediators ADP and TxA2, which in turn activate Rac.
The involvement of lipid rafts in mediating platelet activation through CLEC-2 is not surprising given that 6 of the 7 Src family kinases that have been described in platelets are palmitoylated (Src is the exception) in their unique N-terminal regions. This targets them to specialized cholesterol-rich membrane domains, which are also enriched with many other signaling proteins.33,34 This includes the membrane adapter LAT, which has previously been shown to mediate platelet activation by CLEC-2.3,17 Furthermore, signaling by ITAM receptors in platelets and in other cells is known to occur in lipid rafts, as illustrated by studies on the platelet ITAM receptors GPVI and FcγRIIA.19,26,35,36 The ITAM-like receptor, dectin-1, another hemITAM-containing receptor, has also been shown to be recruited to lipid rafts upon activation.23 Thus, it would appear that lipid rafts play a critical role in supporting cell activation by both ITAM- and hemITAM-containing receptors.
It can be speculated that clustering of CLEC-2 by rhodocytin, which is a tetramer, causes movement into the cholesterol-enriched membrane domains. The molecular basis of this movement is unclear, although the present study has shown that this is independent of actin polymerization. Within the lipid raft environment, CLEC-2 becomes phosphorylated through a pathway that is dependent on actin polymerization, although this can be bypassed by the addition of ADP and U46619 to mimic secondary mediator formation. It is this step that contrasts markedly with signaling by ITAM receptors, as phosphorylation of Syk and PLCγ2 is not dependent on actin polymerization. The critical role of actin polymerization in the proximal events in the CLEC-2 signaling cascade could be explained by the retention or presentation of key signaling molecules in the vicinity of the C-type lectin receptor such as Src or Syk kinases. Actin polymerization, however, does not appear to be required for dimerization/oligomerization of CLEC-2 as shown using the cross-linking reagent Sulfo-EGS (supplemental Figure 2). Further, dimerization/oligomerization of CLEC-2 is also observed in the presence of apyrase and indomethacin.
In contrast to the complete inhibition of CLEC-2 tyrosine phosphorylation seen in the presence of Src kinase or actin polymerization inhibitors, a residual level of phosphorylation is seen in the presence of apyrase/indomethacin or Rac1 inhibition. The ability of ADP and the TxA2 mimetic U46619 to partially rescue CLEC-2 phosphorylation of platelets treated with cytochalasin D but not the Rac1 inhibitor EHT1864 supports this hypothesis. This suggests a role for the secondary mediators and Rac activation in amplification of an initial receptor signal and is consistent with the ability of ADP and the TxA2 mimetic U46619 to partially rescue CLEC-2 phosphorylation of platelets treated with cytochalasin D but not the Rac1 inhibitor EHT1864. The molecular basis of the action of Rac in supporting signaling by CLEC-2 is unclear, although a number of possibilities can be put forward. Rac1 has been shown to support activation of PLCγ2 through a direct interaction at the level of the split PH domain in the lipase.37,38 Rac1 also regulates a number of other targets, including phosphatidylinositol 5-kinase,39 which generates the PLCγ2 substrate, phosphatidylinositol 4,5-bisphosphate. Rac also regulates actin polymerization and remodeling, although the observation that ADP and TxA2 can overcome the inhibitory effect of cytochalasin D argues against this playing a major role downstream of Rac.
The present results report a unique series of proximal events that underlie phosphorylation of CLEC-2 by rhodocytin and platelet activation. Phosphorylation of CLEC-2 requires actin polymerization and is reinforced by release of secondary mediators and activation of Rac. This feedback pathway has significant implications for our recent observation that the selective inhibitor of Syk kinase, R406, abrogates phosphorylation of CLEC-2 by rhodocytin but has no effect on phosphorylation on the FcRγ-chain ITAM.10 Although these results are consistent with a model in which Syk mediates phosphorylation of the CLEC-2 hemITAM, with the role of Src kinases to mediate activation of Syk, they could also be explained by the loss of the feedback pathway mediated through actin polymerization and generation of secondary mediators.
In summary, we describe a novel feedback pathway that leads to signal amplification of the hemITAM-containing receptor CLEC-2. This mechanism is critically dependent on actin polymerization, secondary mediators, and Rac1 activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Craig Hughes for useful discussions.
This work was supported by the British Heart Foundation (BHF; PG/07/116) and the DFG (grant SFB/TR23 project A8 of J.A.E.). S.P.W. holds a BHF Chair.
Authorship
Contribution: A.Y.P. designed and performed experiments, analyzed and interpreted the results, made the figures, and wrote the manuscript; B.G. performed experiments; B.L., L.D., and J.A.E. contributed vital reagents and commented on the manuscript; and S.P.W. designed the research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alice Y. Pollitt, Centre for Cardiovascular Sciences, Institute for Biomedical Research, The College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: a.y.pollitt@bham.ac.uk.