Abstract
The C-type lectin receptor CLEC-2 activates platelets through Src and Syk tyrosine kinases, leading to tyrosine phosphorylation of downstream adapter proteins and effector enzymes, including phospholipase-C γ2. Signaling is initiated through phosphorylation of a single conserved tyrosine located in a YxxL sequence in the CLEC-2 cytosolic tail. The signaling pathway used by CLEC-2 shares many similarities with that used by receptors that have 1 or more copies of an immunoreceptor tyrosine-based activation motif, defined by the sequence Yxx(L/I)x6-12Yxx(L/I), in their cytosolic tails or associated receptor chains. Phosphorylation of the conserved immunoreceptor tyrosine-based activation motif tyrosines promotes Syk binding and activation through binding of the Syk tandem SH2 domains. In this report, we present evidence using peptide pull-down studies, surface plasmon resonance, quantitative Western blotting, tryptophan fluorescence measurements, and competition experiments that Syk activation by CLEC-2 is mediated by the cross-linking through the tandem SH2 domains with a stoichiometry of 2:1. In support of this model, cross-linking and electron microscopy demonstrate that CLEC-2 is present as a dimer in resting platelets and converted to larger complexes on activation. This is a unique mode of activation of Syk by a single YxxL-containing receptor.
Introduction
The C-type lectin receptor CLEC-2 is expressed on platelets and on a subpopulation of other hematopoietic cells, including mouse neutrophils and dendritic cells.1-3 CLEC-2 is a receptor for the snake venom toxin rhodocytin4 and the transmembrane protein podoplanin,5,6 which is expressed on the leading edge of tumor cells and on kidney podocytes, lung type 1 alveolar cells, and lymphatic endothelium. In addition, recent evidence suggests that activated platelets express or release a ligand for CLEC-2 that supports platelet aggregation at arteriolar rates of flow.7 Mice pretreated with a specific antibody to CLEC-2 exhibit a selective loss of the C-type lectin receptor and impaired platelet activation on collagen at high shear in vitro or in vivo.7
Cross-linking of CLEC-2 by rhodocytin, podoplanin, or specific antibodies elicits powerful platelet aggregation and secretion.4,6 CLEC-2 signals through Src- and Syk-dependent tyrosine kinases, leading to phosphorylation of a series of adapter and effector proteins that culminate in activation of phospholipase-C γ2 (PLCγ2) and platelet activation.4 This mechanism of platelet activation resembles that used by the immunoglobulin collagen receptor, glycoprotein VI (GPVI), which is constitutively associated with the FcRγ chain at the platelet surface. Cross-linking of GPVI by collagen or specific agonists, such as the snake venom toxin convulxin or antibodies, leads to Src kinase-dependent phosphorylation of 2 conserved tyrosines in the FcRγ-chain immunoreceptor tyrosine-based activation motif (ITAM).8,9 ITAMs are present in a variety of hematopoietic receptors, including T- and B-cell antigen receptors and the Fc receptors, FcϵRI, FcγRIa, and FcγRIIa, and are defined by the sequence Yxx(L/I)6-12Yxx(L/I).10 Phosphorylation of the 2 conserved tyrosines on receptor activation provides docking sites for the tandem SH2 domains in the only 2 known members of the Syk family of tyrosine kinases, Syk and Zap-70, leading to their activation and initiation of downstream signaling.
Although CLEC-2 signals through a pathway similar to that of GPVI, it contains only a single YxxL sequence in its cytoplasmic tail, which, by mutation of the tyrosine to a phenylalanine, has been shown to be essential for activation of Syk.11 Two other C-type lectin receptor family members, Dectin-1 and CLEC9A, have also been shown to activate Syk through a single YxxL sequence located in their cytosolic tails.12-14 Thus, it seems probable that these 3 C-type lectin-like receptors may use a common mechanism to regulate Syk via a single YxxL sequence.
Activation of Syk by CLEC-2 is ablated by point mutations in either of the Syk SH2 domains that disrupt their ability to bind to phosphotyrosines.11 This suggests a model in which activation of Syk is mediated through cross-linking of 2 phosphorylated CLEC-2 receptors on the cell surface. This model is further supported by the observation that a CLEC-2 specific F(ab′)2 fragment but not a Fab fragment mediates activation of mouse platelets.7 Further, the related receptor, CLEC9A, is expressed as a covalent dimer on dendritic cells, consistent with the cross-linking model.14 On the other hand, these observations could be explained by the presence of a second binding site on the cytosolic tail of CLEC-2, which binds directly or via an intermediate protein to one of the Syk SH2 domains. The present study was undertaken to investigate these 2 hypotheses to establish the mode of Syk activation by CLEC-2.
Methods
Reagents
Rhodocytin was purified from Calloselasma rhodostoma venom as previously described.15 A rabbit α-Syk polyclonal antibody (pAb) was used as previously reported.16 The goat α-human CLEC-2 pAb was purchased from R&D Systems. The α-Myc monoclonal antibody was from Cell Signaling Technology. The α-mouse CLEC-2 pAb was kindly donated by Drs Katsue Suzuki-Inoue and Yukio Ozaki (University of Yamanashi, Yamanashi, Japan). The antibody to rhodocytin has been previously described.4 The polyclonal α-Src antibody was from Invitrogen. The polyclonal α-FcγRIIA tail antibody was used as previously reported.17 Horseradish peroxidase (HRP)–conjugated α-rabbit and α-goat secondary antibody and enhanced chemiluminescence reagents were purchased from GE Healthcare. GST fusion proteins corresponding to single or tandem SH2 domains of Syk were prepared as described previously.18,19 Biotinylated peptides were synthesized by Alta Bioscience and Severn Biotech. The Sulfo-EGS cross-linking compound was from Pierce (Thermo Fisher Scientific). All other reagents were purchased from Sigma-Aldrich or from previously described sources.20
Platelet preparation
Venous blood from healthy drug-free volunteers was taken into 10% sodium citrate. Washed platelets were obtained by centrifugation using prostacyclin to prevent activation during the isolation procedure.21 Platelets were resuspended in modified-Tyrode buffer (134mM NaCl, 0.34mM Na2HPO4, 2.9mM KCl, 12mM NaHCO3, 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM glucose, 1mM MgCl2; pH 7.3) as previously described.21 Platelets were used at a cell density of 5 × 108/mL unless stated.
Immunoprecipitation, pull-downs, and Western blotting
Washed platelets were pretreated with 9μM Integrilin to inhibit platelet activation and aggregation through integrin αIIbβ3. Platelets were stimulated with agonists at 37°C with stirring at 1200g in a Born lumi-aggregometer. Reactions were terminated by addition of 2 times ice-cold NP-40 lysis buffer. Platelet lysates were precleared, and detergent-insoluble debris was discarded.21 An aliquot was dissolved with sodium dodecyl sulfate (SDS) sample buffer for detection of total tyrosine phosphorylation. Lysates were incubated with either the indicated antibodies and protein G-Sepharose, or biotinylated CLEC-2 peptides and streptavidin-agarose. Precipitated proteins or whole-cell lysates were separated by reducing SDS-polyacrylamide gel electrophoresis (PAGE), electrotransferred, and Western blotted. For in vitro pull-down studies, Syk GST-SH2 domain proteins were incubated with a 50-fold excess of biotinylated CLEC-2 peptide, and the resulting complexes were precipitated with glutathione-agarose. Samples were dot-blotted onto nitrocellulose membrane and probed with HRP-conjugated streptavidin. Densitometry was performed to quantitate the amount of peptide pulled out.
Platelet surface protein cross-linking
After platelet stimulation, Sulfo-EGS (0.15mM or 1.5mM final concentration) was added and allowed to incubate at room temperature for 30 minutes. The reaction was then quenched with the addition of Tris-HCl (pH 7.5; 25mM final concentration), and allowed to incubate for a further 20 minutes at room temperature. The platelets were then lysed with the addition of an equal volume of 2 times ice-cold NP-40 lysis buffer.
Constructs
Wild-type and Y7F CLEC-2 and wild-type FcRγ-chain cloned into pEF6 have been described previously.11,22 Further mutations were generated by a 2-step polymerase chain reaction method. The mutating primers CLEC-2 Δ21-28-FWD (5′-TAA-AAC-TCG-GAA-ACC-AGC-TCT-CAT-CTG-GTG-GCG-TGT-GAT-GGC-TTT-GAT-TC-3′), CLEC-2 Δ21-28-REV (5′-GAA-TCA-AAG-CCA-TCA-CAC-GCC-ACC-AGA-TGA-GAG-CTG-GTT-TCC-GAG-TTT-TA-3′), CLEC-2 S21/27A-FWD (5′-GAA-ACC-AGC-TCT-CAT-CGC-CGT-TGG-CTC-TGC-ATC-CGC-CTC-CTG-GTG-GC-3′), CLEC-2 S21/27A-REV (5′-GCC-ACC-AGG-AGG-CGG-ATG-CAG-AGC-CAA-CGG-CGA-TGA-GAG-CTG-GTT-TC-3′), CLEC-2 T9A-FWD (5′-CAT-GCA-GGA-TGA-AGA-TGG-ATA-CAT-CGC-CTT-AAA-TAT-TAA-AAC-TCG-3′), CLEC-2 T9A-REV (5′-CGA-GTT-TTA-ATA-TTT-AAG-GCG-ATG-TAT-CCA-TCT-TCA-TCC-TGC-ATG-3′), FcRγ-chain Y66F-FWD (5′-CAG-ATG-GTG-TTT-TCA-CGG-GCC-TGA-G-3′), FcRγ-chain Y66F-REV (5′-CTC-AGG-CCC-GTG-AAA-ACA-CCA-TCT-G-3′), FcRγ-chain Y77F-FWD (5′-GGA-ACC-AGG-AGA-CTT-TCG-AGA-CTC-TGA-AGC-3′), and FcRγ-chain Y77-REV (5′-GCT-TCA-GAG-TCT-CGA-AAG-TCT-CCT-GGT-TCC-3′) were used along with vector specific primers 5147 and 4150. The nuclear factor of activated T cells (NFAT) luciferase reporter contains 3 copies of the distal NFAT site from the IL-2 promoter23 and was provided by Prof A. Weiss (University of California San Francisco). The pEF6-lacZ expression construct was from Invitrogen.
Cell culture and transfection
DT40 chicken B cells were grown in RPMI supplemented with 10% fetal bovine serum, 1% chicken serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 50μM β-mercaptoethanol, and 20mM GlutaMAX. Cells were transfected in 400 μL of cytomix by electroporation using a GenePulser II (Bio-Rad) set at 350 V and 500 μF.
Luciferase assay
Cells were transfected as described in “Cell culture and transfection” with 10 μg of the indicated Myc-tagged or FLAG-tagged-CLEC-2 construct or 2 μg/mL each of GPVI and the indicated Myc-tagged-FcRγ construct, 20 μg of the luciferase reporter construct, and 2 μg of pEF6-lacZ to control for transfection efficiency.11,22 Twenty hours after transfection, live cells were counted by trypan blue exclusion and samples divided for luciferase assay, β-galactosidase assay, and flow cytometry. Luciferase assays were carried out as described previously.24 Luciferase activity was measured with a Centro LB 960 microplate luminometer (Berthold Technologies). Data were normalized to β-galactosidase activity and expressed as percentage of the wild-type response. All luciferase data were averaged from triplicate readings. β-Galactosidase assays were performed with half of a million cells using the Galacto-Light chemiluminescent reporter assay, according to the manufacturer's instructions (Applied Biosystems). β-Galactosidase activity was measured in triplicate using a microplate luminometer.
Immunogold labeling and electron microscopy
Washed mouse platelets were prepared as previously described.25 Nonstimulated or rhodocytin (100nM for 2 minutes)–stimulated mouse platelets were used for immunogold labeling and electron microscopy performed as previously described.26 In nonstimulated platelets, CLEC-2 receptors were labeled with CLEC-2 specific monoclonal IgG and 10-nm colloidal gold particles coated with goat α-mouse IgG. This approach could not be used to label CLEC-2 in rhodocytin-stimulated platelets as the snake toxin either masks the antibody epitope or induces a conformational change, which does not allow the CLEC-2 specific antibody to bind. A rabbit polyclonal antibody to rhodocytin and 10-nm colloidal gold particles coated with goat anti–rabbit IgG was used instead.
Flow cytometry
Surface expression of Myc-tagged-CLEC-2 and the Myc-tagged-FcRγ mutants was measured by flow cytometry. Cells (5 × 105) were stained in a 25-μL volume for 20 minutes on ice with 10 μg/mL α-Myc antibody. Cells were then washed and incubated for 20 minutes on ice with 15 μg/mL fluorescein isothiocyanate–conjugated α-mouse IgG secondary antibody. Stained cells were analyzed using a FACSCalibur (BD Biosciences). Data were collected and analyzed using CellQuest software (BD Biosciences).
Surface plasmon resonance
Surface plasmon resonance experiments were performed using a Biacore T100 machine (Biacore, GE Healthcare). Biotin tagged CLEC-2 and FcRγ peptides were attached to carboxymethylated dextran-coated CM5 research grade sensor chips (Biacore) after attachment of streptavidin to the chip surface using amine coupling. All experiments were performed at 25°C in 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150mM NaCl, 1mM MgCl2, 0.005% Surfactant P-20. A range of concentrations (0-80μM) of N-SH2, C-SH2, and tandem SH2 domains were injected over all peptide surfaces to determine equilibrium dissociation constants (KD). Specific binding was identified by subtraction of signal from an appropriate reference flow cell. Equilibrium dissociation constants (KD values) were derived from global fitting to the predicted function using the Levenberg-Marquardt algorithm as implemented in the program Origin (OriginLab).
Tryptophan fluorescence titration
Fluorescence measurements were performed in a PTI spectrofluorimeter (Photon Technology International). Both Syk SH2 domains and CLEC-2 peptides were extensively dialyzed into experimental buffer (50mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, 150mM glycine, 1mM ethylenediaminetetraacetic acid, and 5mM β-mercaptoethanol; pH 7.5) before experimentation. Protein and peptide concentrations were determined by spectrophotometry using a ND-1000 spectrophotometer (Nanodrop; Thermo Scientific). The Syk SH2 domains (3 mL of 250nM) were loaded into a quartz cuvette and placed into the cell turret with constant stirring. The CLEC-2 peptide (2mM) was titrated in and allowed to mix for 2 minutes before scanning. An excitation wavelength of 295 nm was used, and emission spectra were collected over the range of 300 to 420 nm in 2.5-nm steps. Slit widths of 0.75 mm were used for both excitation and emission. The peak fluorescence of 340 nm was monitored to calculate the equilibrium dissociation constant, KD.
Statistical analysis
NFAT-luciferase data are expressed as geometric mean plus or minus SE. Statistical analysis was carried out using the unpaired Student t test. Significance was taken P less than .05.
Results
Structure-function relationship of the CLEC-2 cytoplasmic tail and Syk tandem SH2 domains
Sequence alignment of the CLEC-2 cytosolic tail both between species and with the 2 other family members, Dectin-1 and CLEC9A, was performed to highlight conserved regions that might provide a second binding site for one of the SH2 domains of Syk or for an intermediate adapter protein. Sequence alignment of 9 species of CLEC-2 using ClustalW (www.ebi.ac.uk/clustalw) highlighted conservation of the N-terminal MQDEDGYxTL sequence, a threonine at position +2 in the YxxL motif and of 2 serine residues located at positions 21 and 27 in human CLEC-2 (Figure 1A). Several positively charged amino acids and a tryptophan-arginine (WR) sequence at the juxtamembrane position were also conserved. Sequence alignment of 9 species of Dectin-1 also highlighted conservation of the DEDGYxxL sequence and of 2 serine residues (Figure 1B) but not the other amino acids that were conserved in CLEC-2 apart from the membrane WR sequence. Intriguingly, a conserved threonine residue was found at the +1 position in the Dectin-1 YxxL motif. The same relationship did not hold for CLEC9A, with the conserved DEDG sequence being replaced by the similarly charged EEEI sequence and just one of the serines and the threonine at position Y+1 being conserved. The presence of the conserved serine and threonine residues is of interest given their potential for phosphorylation. Further, the presence of a threonine group at position Y + 1 or Y + 2 is of also of interest given previous observations in mast cells and mouse platelets that phosphorylation of a conserved threonine at position Y + 1 in 1 of the 2 YxxL sequences of the FcRγ-chain by a novel isoform of protein kinase C (δ or ϵ) is required for maximal binding and activation of Syk.27-29
Sequence alignment of CLEC-2 family proteins. (A) Ten species of CLEC-2 were aligned using ClustalW web-based software, highlighting conservation of the DEDGYxTL motif and serines at positions 21 and 27. (B) Nine species of Dectin-1 were aligned, highlighting conservation of the DEDGYTxL motif and serines at positions 32 and 40. (C) The intracellular tails of CLEC-2, Dectin-1, and CLEC9A were aligned, highlighting conservation of the YxxL motif and partial conservation of a serine-rich region.
Sequence alignment of CLEC-2 family proteins. (A) Ten species of CLEC-2 were aligned using ClustalW web-based software, highlighting conservation of the DEDGYxTL motif and serines at positions 21 and 27. (B) Nine species of Dectin-1 were aligned, highlighting conservation of the DEDGYTxL motif and serines at positions 32 and 40. (C) The intracellular tails of CLEC-2, Dectin-1, and CLEC9A were aligned, highlighting conservation of the YxxL motif and partial conservation of a serine-rich region.
Based on these alignments, experiments were designed to investigate the role of the YxxL, and the conserved serine and threonine residues in the cytoplasmic tail of CLEC-2 through a combination of peptide pull-down studies using human CLEC-2 cytosolic peptides and human mutant CLEC-2 transfected into chicken DT40 B cells. The latter is a hematopoietic cell line that we have used extensively to investigate signaling by both CLEC-2 and GPVI using a sensitive NFAT reporter assay.22,30
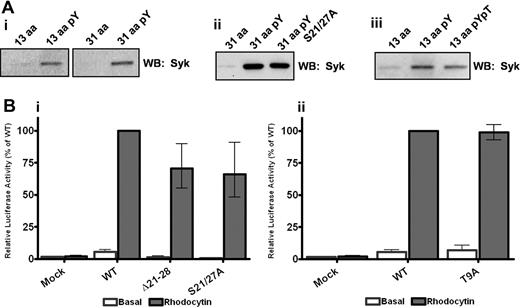
In confirmation of our previous finding,11 the snake toxin rhodocytin stimulates powerful activation of NFAT activity in CLEC-2 but not in mock-transfected DT40 cells, with the response ablated after mutation of the conserved tyrosine at position 7 to phenylalanine as shown later in Figure 3. In line with this, a phosphorylated peptide based on the first 13 amino acids of the CLEC-2 tail, which includes the conserved MQDEDGYxxL sequence, was able to effectively precipitate Syk from a platelet lysate, whereas the corresponding nonphosphorylated 13-amino acid peptide was ineffective (Figure 2Ai). Significantly, there was no increase in the amount of precipitated Syk when a tyrosine-phosphorylated peptide based on the full 31-amino acid cytoplasmic tail of CLEC-2 was used, although again the nonphosphorylated peptide was ineffective (Figure 2Ai). This demonstrates that a single phosphorylated YxxL sequence is sufficient to mediate binding to Syk and that a similar level of binding is seen with the 13 and the full-length 31-amino acid peptides based on the CLEC-2 tail in the pull-down assay. It is unclear, however, whether the interaction with Syk in this design is mediated through binding of 2 separate phosphorylated peptides to the tandem SH2 domains in the kinase, bearing in mind that multiple phosphorylated peptides are attached to the beads. These observations therefore do not rule out a possible direct or indirect interaction between the Syk tandem SH2 domains and a second site in the CLEC-2 cytosolic tail, which may support the association of Syk with a CLEC-2 monomer in an intact cell.
YxxL is essential for Syk association and signaling through CLEC-2. (A) Washed platelets (5 × 108/mL) were lysed with 2 times NP40 lysis buffer, precleared, and interacting proteins precipitated with the addition of 10 μg of the relevant biotinylated CLEC-2 peptide. Precipitated proteins were separated by SDS-PAGE and Western blotted for the presence of Syk. (B) DT40 cells were transfected with 10 μg/mL of the stated CLEC-2 construct and an NFAT-luciferase reporter plasmid. Transfected cells were stimulated with 50nM rhodocytin for 6 hours at 37°C, and then the luciferase activity was measured as an index of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 to 6 separate experiments.
YxxL is essential for Syk association and signaling through CLEC-2. (A) Washed platelets (5 × 108/mL) were lysed with 2 times NP40 lysis buffer, precleared, and interacting proteins precipitated with the addition of 10 μg of the relevant biotinylated CLEC-2 peptide. Precipitated proteins were separated by SDS-PAGE and Western blotted for the presence of Syk. (B) DT40 cells were transfected with 10 μg/mL of the stated CLEC-2 construct and an NFAT-luciferase reporter plasmid. Transfected cells were stimulated with 50nM rhodocytin for 6 hours at 37°C, and then the luciferase activity was measured as an index of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 to 6 separate experiments.
The contribution of the 2 conserved serine residues in the CLEC-2 tail to the interaction with Syk and to intracellular signaling was investigated using the same approach as for the YxxL motif. Mutation of the 2 serine residues to alanine, or deletion of the serine-rich region (21-28) had no significant effect on surface expression or the ability of the mutant CLEC-2 receptor to activate NFAT in the DT40 cell line relative to the wild-type receptor (Figure 2Bi; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Further, a 31-amino acid tyrosine-phosphorylated peptide containing alanine residues rather than serine groups at positions 21 and 27 associated with a similar level of Syk as the tyrosine-phosphorylated wild-type peptide (Figure 2Aii). These results demonstrate that neither of the conserved serine residues plays a significant role in supporting Syk association or in signaling to PLCγ2 and NFAT activation.
Using the same approach, we were also unable to find evidence for a functional role for the threonine at position 9 in signaling by CLEC-2. Transfection of DT40 cells with the T9A mutant of CLEC-2 had no significant effect on surface expression or on NFAT activation induced by rhodocytin relative to the wild-type receptor (Figure 2Bii; supplemental Figure 2). Further, a tyrosine-phosphorylated 31 amino acid peptide containing a phosphorylated threonine residue precipitated similar levels of Syk as the wild-type phosphorylated peptide (Figure 2Aiii).
These results confirm a critical role for the phosphorylated YxxL sequence in both the interaction with Syk and in mediating NFAT signaling by CLEC-2 in transfected DT40 cells but also demonstrate that the 2 conserved serines at positions 21 and 27 and the partially conserved threonine at position 9 are dispensable for these events. The functional role of these 3 residues therefore remains to be determined. The present results indirectly favor a model in which Syk cross-links 2 molecules of CLEC-2 via its 2 SH2 domains, both of which are known to be essential for CLEC-2 signaling.11
Inhibitory action of Y7F CLEC-2 on signaling by wild-type CLEC-2
To further investigate whether cross-linking of 2 CLEC-2 receptors is required for activation of Syk, we coexpressed wild-type CLEC-2 with various levels of the Y7F mutant of CLEC-2, which is unable to bind to Syk (Figure 2Ai) and does not support NFAT activation (Figure 3).11 The rationale for this experiment is that the Y7F mutant should have an inhibitory effect if the cross-linking model is correct by forming an “inactive heterodimer” with the WT receptor. On the other hand, it should have no effect if Syk is activated by a monomeric CLEC-2 receptor as the Y7F mutant is unable to support binding to Syk and activation.
The Y7F CLEC-2 mutant inhibits signaling by the wild-type receptor. DT40 cells were transfected with 10 μg/mL of wild-type CLEC-2 and/or various amounts of Y7F CLEC-2 and an NFAT-luciferase reporter gene. Transfected cells were stimulated with 50nM rhodocytin for 6 hours at 37°C, after which time the amount of luciferase activity was measured as a readout of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 to 8 separate experiments.
The Y7F CLEC-2 mutant inhibits signaling by the wild-type receptor. DT40 cells were transfected with 10 μg/mL of wild-type CLEC-2 and/or various amounts of Y7F CLEC-2 and an NFAT-luciferase reporter gene. Transfected cells were stimulated with 50nM rhodocytin for 6 hours at 37°C, after which time the amount of luciferase activity was measured as a readout of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 to 8 separate experiments.
Surface expression of wild-type CLEC-2 was unaffected by expression of the Y7F mutant in DT40 cells (supplemental Figure 3), whereas NFAT activation was dramatically inhibited when the mutant receptor was expressed at a similar or greater level than the wild-type receptor (Figure 3). Reducing the level of expression of the Y7F mutant caused a corresponding reduction in the inhibitory effect. In contrast, the weak constitutive (agonist-independent) signal induced by expression of CLEC-2, which we have previously described,22 was not inhibited in the presence of the Y7F mutant, suggesting that it may be mediated by a CLEC-2 monomer (Figure 3). These data provide evidence that ligand-activated CLEC-2 signals as a dimer/oligomer and that expression of the Y7F inactive mutant has a “dominant negative” effect by inducing a portion of the native receptors into a mixed (wild-type and Y7F) dimeric or higher-order structure that is unable to signal.
CLEC-2 is present in higher-order structures on the platelet surface
The results shown in Figures 2 and 3 provide evidence that dimerization of CLEC-2 is essential for Syk activation. To investigate whether CLEC-2 is present as a dimer or higher-order structure on platelets, we used Sulfo-EGS, a cross-linking reagent that cross-links proteins that fall within a distance of 1.6 nm (16Å) of each other. Dimerization and oligomerization of CLEC-2 were investigated by immunoprecipitation and Western blotting.
In nonstimulated or rhodocytin-stimulated platelets, CLEC-2 migrates on reducing SDS-PAGE gel electrophoresis as a characteristic doublet because of differential glycosylation (Figure 4A lanes 1 and 4).4 The molecular weights of the 2 differentially glycosylated forms of CLEC-2, 30 and 40 kDa, are consistent with both forms being a monomer, which is further supported by the fact that they are reduced to a single band of just less than 30 kDa on deglycosylation.4 After addition of a low or a high concentration of cross-linker, the presence of higher molecular weight bands of CLEC-2 can be seen together with a corresponding reduction in intensity of the 30- and 40-kDa bands. In nonstimulated platelets, a new broad band is observed at between 60 and 80 kDa, which corresponds approximately to a doubling of the molecular weight of monomeric CLEC-2 (Figure 4A, lanes 2 and 3). With the higher concentration of cross-linker, there is also the suggestion of the presence of higher-order structures in nonstimulated platelets, which corresponds to a further reduction in the monomeric form and to a reduction in the dimeric form relative to the lower concentration of Sulfo-EGS. In the presence of rhodocytin, Sulfo-EGS induces the formation of both the predicted CLEC-2 dimer and higher-order structures, most notably at the upper concentration where there is almost no detectable monomeric CLEC-2 (Figure 4A lanes 5 and 6).
CLEC-2 oligomers are present on the platelet surface. Washed platelets (5 × 108/mL) under basal or rhodocytin-stimulated (100nM) conditions had their surface proteins cross-linked with the addition of 0.15mM or 1.5mM Sulfo-EGS cross-linking reagent, with a linker length of 1.6 nm (16 Å). The cross-linking reaction was subsequently blocked, and then the platelets were lysed with 2× NP40 lysis buffer. (A) Lysates were precleared with protein G-Sepharose and then immunoprecipitated with α-CLEC-2 antibody and protein G-Sepharose. Precipitated proteins were separated by reducing SDS-PAGE and Western blotted for CLEC-2. (B) Lysates were separated by reducing SDS-PAGE and Western blotted for CLEC-2, FcγRIIA, and Src. The results are representative of 3 experiments.
CLEC-2 oligomers are present on the platelet surface. Washed platelets (5 × 108/mL) under basal or rhodocytin-stimulated (100nM) conditions had their surface proteins cross-linked with the addition of 0.15mM or 1.5mM Sulfo-EGS cross-linking reagent, with a linker length of 1.6 nm (16 Å). The cross-linking reaction was subsequently blocked, and then the platelets were lysed with 2× NP40 lysis buffer. (A) Lysates were precleared with protein G-Sepharose and then immunoprecipitated with α-CLEC-2 antibody and protein G-Sepharose. Precipitated proteins were separated by reducing SDS-PAGE and Western blotted for CLEC-2. (B) Lysates were separated by reducing SDS-PAGE and Western blotted for CLEC-2, FcγRIIA, and Src. The results are representative of 3 experiments.
The corresponding changes in the levels of monomeric, dimeric, and oligomeric forms of CLEC-2 are consistent with the interconversion of one form to another, although there is also the suggestion of loss of protein with the higher concentration of cross-linker, a conclusion supported by densitometric analysis (not shown). The loss of protein may reflect the formation of higher-order structures that are resistant to solubilization. The broad nature and smearing of bands seen in these studies are probably the consequence of oligomerization of the differentially glycosylated forms of CLEC-2 as well as possible cross-linking to other proteins, although interestingly, Western blotting studies failed to detect the presence of rhodocytin in the higher-order structures, suggesting that cross-linking to the toxin had not occurred (not shown).
A similar overall pattern of change in CLEC-2 dimerization/oligomerization was seen in whole-cell lysates prepared from basal (not shown) and rhodocytin-activated platelets, as demonstrated by Western blotting for CLEC-2 (Figure 4B). In marked contrast, there was no apparent dimerization/oligomerization of the low-affinity immune receptor, FcγRIIA, or the membrane-associated protein Src in the presence of the intermediate and high concentration of Sulfo-EGS in basal (not shown) or rhodocytin-activated platelets (Figure 4B) as shown by the similar levels of the monomeric forms of both proteins as revealed by Western blotting (Figure 4B). These results demonstrate that the oligomerization of CLEC-2 is not simply the result of a nonspecific effect of cross-linking of platelet surface proteins.
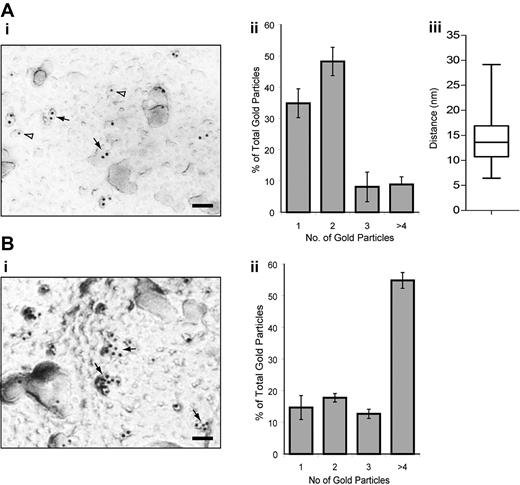
We also investigated possible dimerization/oligomerization of CLEC-2 by immunogold labeling and electron microscopy on nonstimulated and rhodocytin-stimulated mouse platelets using a specific antibody to the C-type lectin receptor (Figure 5). Under nonstimulated conditions, approximately 35% and 50% of gold particles were found alone or in pairs, respectively, with the latter separated by a mean distance of 14 nm. These results further suggest that, under basal conditions, CLEC-2 is present as a monomer and dimer on the platelet surface. Furthermore, after rhodocytin stimulation, more than 55% of gold particles are present in clusters of 4 or more gold particles demonstrating the formation of oligomers.
Electron microscopy and immunogold staining of CLEC-2 on resting and rhodocytin-stimulated mouse platelets. (Ai,Bi) Electron micrograph showing α-CLEC2 immunostaining. The majority of CLEC-2 immunogold labeling can be found within a pair (solid arrows) or alone (open arrowheads) under basal conditions (A) or as larger clusters after rhodocytin stimulation and rhodocytin immunogold labeling (B). Scale bar represents 50 nm. (Aii,Bii) Quantification of the CLEC-2 immunogold labeling distribution under basal (A) or rhodocytin-stimulated (B) conditions. The distribution of CLEC-2 immunogold labeling is depicted as a histogram. Data represent the mean plus or minus SD (n = 5). (Aiii) Quantification of the distance between CLEC-2 immunogold-labeled pairs. The intercenter distance between the gold particles within pairs was measured using ImageJ (n = 100) and presented as a box-and-whisker plot. Solid horizontal line shows the median for the data, the top of the box the 25th percentile, the bottom the 75th percentile, and the additional lines the range of the data.
Electron microscopy and immunogold staining of CLEC-2 on resting and rhodocytin-stimulated mouse platelets. (Ai,Bi) Electron micrograph showing α-CLEC2 immunostaining. The majority of CLEC-2 immunogold labeling can be found within a pair (solid arrows) or alone (open arrowheads) under basal conditions (A) or as larger clusters after rhodocytin stimulation and rhodocytin immunogold labeling (B). Scale bar represents 50 nm. (Aii,Bii) Quantification of the CLEC-2 immunogold labeling distribution under basal (A) or rhodocytin-stimulated (B) conditions. The distribution of CLEC-2 immunogold labeling is depicted as a histogram. Data represent the mean plus or minus SD (n = 5). (Aiii) Quantification of the distance between CLEC-2 immunogold-labeled pairs. The intercenter distance between the gold particles within pairs was measured using ImageJ (n = 100) and presented as a box-and-whisker plot. Solid horizontal line shows the median for the data, the top of the box the 25th percentile, the bottom the 75th percentile, and the additional lines the range of the data.
These 2 distinct approaches demonstrate that a significant proportion of CLEC-2 is present as dimer on the surface of a nonstimulated platelet and that the receptor is converted to higher oligomeric structures on activation. Furthermore, the CLEC-2 dimer is not the consequence of disulphide cross-linking as the C-type lectin receptor runs solely as a monomer under both reducing (Figure 4) and nonreducing conditions (supplemental Figure 4).
Surface plasmon resonance and tryptophan fluorescence measurements
The result in Figure 4 further supports a model in which the tandem SH2 domains in Syk bind to the phosphorylated YxxL sequence in separate CLEC-2 receptors, which are preformed into dimers. This is consistent with our previous report that point mutants that disrupt binding of the individual SH2 domains to phosphotyrosine abrogate signaling by CLEC-2.11 One prediction of this model is that both of the SH2 domains in Syk must be able to bind to the phosphorylated YxxL peptide. This was investigated using surface plasmon resonance to measure the equilibrium dissociation constants (KD) of binding of the N-terminal, C-terminal, and tandem SH2 domains of Syk. To achieve this, each protein was flowed over different surfaces coated with the nonphosphorylated or phosphorylated 13-amino acid N-terminal CLEC-2 peptide. No detectable binding was observed for any of the SH2 domain-containing proteins when flowed over the nonphosphorylated CLEC-2 peptide, confirming the requirement for tyrosine phosphorylation of the YxxL. When flowed over the tyrosine-phosphorylated peptide, a KD of 10.5μM was calculated for the Syk N-SH2 domain and 2.4μM for the Syk C-SH2 domain, demonstrating that both SH2 domains are able to bind to the phosphorylated CLEC-2 cytoplasmic tail. Moreover, when the tSH2 domain protein was flowed over tyrosine-phosphorylated CLEC-2 peptide, a KD of 256nM was calculated, thereby demonstrating cooperativity between the 2 SH2 domains. This is consistent with each SH2 domain binding to separate molecules of the phosphorylated CLEC-2 peptides on the surface of the chip. As a comparison, the individual and tandem Syk SH2 domains were also flowed over a dually phosphorylated peptide based on the human FcRγ-chain ITAM. A similar cooperativity was observed when the tandem SH2 domains were flowed over the peptide with a calculated KD of 20nM compared with the individual KD values of the N- and C-terminal SH2 domains of 19.7 and 1.7μM, respectively (Figure 6A). These data demonstrate that the Syk tandem SH2 domain protein binds with higher affinity than the single Syk SH2 domains to a surface containing multiple copies of a phosphorylated YxxL-containing peptide. This has important implications for the binding of Syk to a dually phosphorylated ITAM protein or to the phosphorylated CLEC-2 tail when presented as a dimer. Consistent with these data, quantitative Western blotting was used to show that twice the amount of a CLEC-2 peptide was able to associate with the tandem-SH2 domains of Syk compared with the single SH2 domains alone, confirming a 2:1 stoichiometry of CLEC-2 to Syk (Figure 6B).
Surface plasmon resonance measurements and quantitative Western blotting of the CLEC-2-Syk SH2 interaction. (A) Biotinylated CLEC-2 and FcRγ chain peptides were bound to streptavidin-coated biosensor chip surfaces. The N-SH2, C-SH2, or the tandem SH2 domains of Syk were purified and flowed over the chip at a range of concentrations. Nonlinear regression was used to analyze the data and calculate KD values. The results are representative of 3 experiments. (B) GST-tagged Syk SH2 domain proteins were incubated with a 50-fold excess of biotinylated phospho-CLEC-2 peptide and precipitated with glutathione-agarose beads. The precipitated proteins were dot-blotted, and the amount of associated CLEC-2 was measured using HRP-streptavidin and densitometric analysis. The result is representative of 4 experiments.
Surface plasmon resonance measurements and quantitative Western blotting of the CLEC-2-Syk SH2 interaction. (A) Biotinylated CLEC-2 and FcRγ chain peptides were bound to streptavidin-coated biosensor chip surfaces. The N-SH2, C-SH2, or the tandem SH2 domains of Syk were purified and flowed over the chip at a range of concentrations. Nonlinear regression was used to analyze the data and calculate KD values. The results are representative of 3 experiments. (B) GST-tagged Syk SH2 domain proteins were incubated with a 50-fold excess of biotinylated phospho-CLEC-2 peptide and precipitated with glutathione-agarose beads. The precipitated proteins were dot-blotted, and the amount of associated CLEC-2 was measured using HRP-streptavidin and densitometric analysis. The result is representative of 4 experiments.
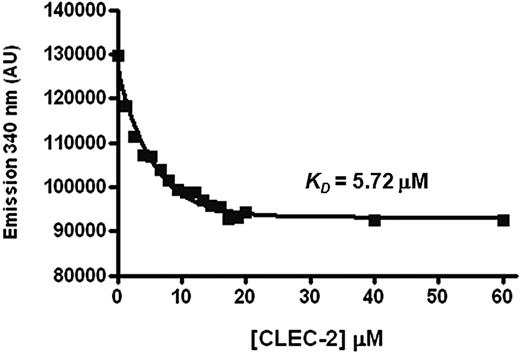
Tryptophan fluorescence titration was used to derive a second estimate of the affinity of the tandem Syk SH2 domains for the phosphorylated CLEC-2 peptide.19,31 This technique is based on the principle that binding to the peptide causes a change in fluorescence of 1 or more tryptophan groups in the tandem Syk SH2 domain. The N-terminal 13-amino acid CLEC-2 peptide was used for these studies as this supports a similar level of binding of Syk to that of the 31-amino acid phosphorylated peptide and does not contain a tryptophan residue. Addition of the peptide resulted in a saturatable decrease in peak tryptophan fluorescence of the tandem Syk SH2 domain at 340 nm, indicative of ligand binding (Figure 7). The resulting dissociation constant (KD) of 5.7μM for the Syk-tandem SH2/CLEC-2 interaction was of a similar order as the KD derived by surface plasmon resonance of CLEC-2 peptide binding to either the N- or C-terminal SH2 domain of Syk. In contrast, the fluorescence data recorded a 20-fold weaker binding to the tSH2 than was observed by surface plasmon resonance. This discrepancy may reflect the influence of avidity in the binding between the Syk tandem SH2 domains and a surface-immobilized peptide, whereas the tryptophan fluorescence measurements were made in solution. In contrast, we were unable to detect binding of the CLEC-2 peptide to the single C-terminal SH2 domain of Syk over the same concentration range. The equivalent experiment is not valid for the N-terminal SH2 domain as this does not contain a tryptophan residue. This increased affinity of the tSH2 domain for a CLEC-2 peptide compared with the single C-terminal SH2 domain mirrors the result seen using surface plasmon resonance. It should be noted, however, that there is also 1 tryptophan residue in the linker region that is present only in the tSH2 domain. It is therefore possible that ligand binding is causing only the linker region tryptophan to change its fluorescence and this too would explain the lack of binding to the C-terminal SH2 domain.
Tryptophan fluorescence titration measurements of the CLEC-2-Syk SH2 interaction. The purified tandem SH2 domains of Syk were placed into a quartz cuvette in a Photon Technology International spectrofluorimeter. After excitation at 295 nm, emission at 340 nm was plotted during titration of a CLEC-2 peptide. Nonlinear regression was used to analyze the data and calculate KD values. The graph is representative of 3 experiments.
Tryptophan fluorescence titration measurements of the CLEC-2-Syk SH2 interaction. The purified tandem SH2 domains of Syk were placed into a quartz cuvette in a Photon Technology International spectrofluorimeter. After excitation at 295 nm, emission at 340 nm was plotted during titration of a CLEC-2 peptide. Nonlinear regression was used to analyze the data and calculate KD values. The graph is representative of 3 experiments.
GPVI requires both FcRγ-chain YxxL sequences to signal
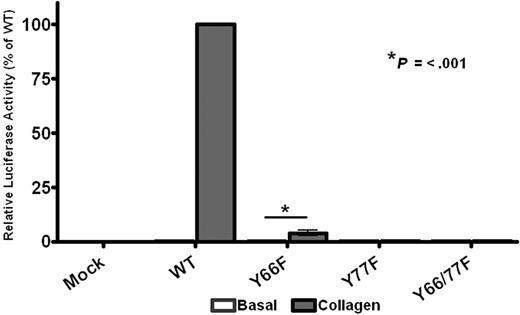
The results in Figures 2 to 7 support a dimerization model of CLEC-2 signaling. This raises the question as to whether ITAM receptors, which contain a tandem YxxL sequence, are able to activate Syk through intermolecular binding between adjacent phosphorylated YxxL sequences or whether intramolecular binding to the doubly phosphorylated YxxLs of a single ITAM is essential for activation. This question was addressed through the generation of single point mutations (Y-F) of the conserved ITAM tyrosines in the FcRγ-chain, which associates with GPVI. An N-terminal Myc-tagged version of the FcRγ-chain, which supports a similar level of signaling to that of the wild-type protein,22 was used in these studies to enable measurement of the level of surface expression. As shown in Figure 8, mutation of the C-terminal ITAM tyrosine abolished NFAT activation by collagen in DT40 cells transfected with GPVI and the mutant FcRγ-chain, whereas mutation of the N-terminal ITAM tyrosine suppressed the response by more than 95%, despite similar expression levels of both GPVI and FcRγ-chain as measured by flow cytometry (supplemental Figure 5). Thus, these results demonstrate that both ITAM tyrosines are required for robust signaling by the GPVI-FcR γ-chain complex and that the FcRγ-chain is unable to function efficiently with phosphorylation of only a single YxxL.
GPVI signaling requires both ITAM tyrosines. DT40 cells were transfected with 2 μg/mL stated FcRγ-chain construct, 2 μg/mL GPVI, along with an NFAT-luciferase reporter gene. Transfected cells were then stimulated with 10 μg/mL collagen for 6 hours at 37°C, after which time the amount of luciferase activity was measured as a readout of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 separate experiments.
GPVI signaling requires both ITAM tyrosines. DT40 cells were transfected with 2 μg/mL stated FcRγ-chain construct, 2 μg/mL GPVI, along with an NFAT-luciferase reporter gene. Transfected cells were then stimulated with 10 μg/mL collagen for 6 hours at 37°C, after which time the amount of luciferase activity was measured as a readout of signaling. Results were normalized for transfection efficiency and plotted as a percentage of the wild-type response. Error bars represent the geometric mean ± SE of 3 separate experiments.
Discussion
The results presented in the previous section support a model in which CLEC-2 regulates Syk through dimerization with each of the 2 Syk SH2 domains binding to a phosphorylated YxxL sequence in the individual cytosolic tails of CLEC-2, which itself is present as a dimer on the platelet surface. The evidence in support of this model is as follows: (1) it has previously been reported that both SH2 domains of Syk are essential for activation by CLEC-211 ; (2) the CLEC-2 cytosolic tail contains a single YxxL sequence that, when phosphorylated, is essential for activation of NFAT; (3) a significant proportion of CLEC-2 is present as a dimer in nonstimulated platelets and converted to higher-order structures on stimulation; (4) coexpression of FxxL mutant CLEC-2 inhibits signaling by the wild-type receptor; (5) there is no evidence for a second (direct or indirect) binding site for Syk in the CLEC-2 cytoplasmic tail; (6) surface plasmon resonance has shown that both SH2 domains of Syk have micromolar affinity for the tyrosine-phosphorylated CLEC-2 and that they interact in a cooperative manner to facilitate binding; and (7) quantitative Western blotting confirms a stoichiometry of 2:1 for the interaction between CLEC-2 and Syk. The other C-type lectin receptors, which have been shown to regulate Syk through a single YxxL sequence, Dectin-1, and CLEC9A, may also function as dimers. An alignment of the cytoplasmic tails of all 3 receptors emphasizes their overall similarity in organization but fails to identify a conserved or recognized sequence that probably confers binding, either directly or indirectly, to the second SH2 domain of Syk. Further, CLEC9A is expressed as a covalently linked dimer, adding weight to the proposed dimerization model.14 These data therefore suggest that the minimum signaling unit for CLEC-2 and the 2 related C-type lectin receptors is a phosphorylated dimer, enabling recruitment of a single molecule of Syk. An alternative model in which Syk cross-links 2 CLEC-2 dimers is also possible.
The cross-linking experiments in stimulated platelets also indicate the presence of oligomeric forms of CLEC-2, and it is possible that these contribute to the proposed model for activation of Syk by creating a critical density of phosphorylated YxxLs. Alternatively, the apparent formation of CLEC-2 oligomers may be the result of binding to rhodocytin, which is a tetramer and so could therefore cross-link multiple receptors,32 although the presence of the snake toxin itself in these higher-order structures was not detected. It is also possible that the higher molecular structures could represent binding to other membrane proteins, although no specific binding partners of CLEC-2 have been reported.
Oligomerization of CLEC-2 on the platelet surface is also supported by electron microscopy, which clearly demonstrates couplets of CLEC-2 on the surface of resting platelets with a separation of only 14 nm. In the crystal structure of monomeric CLEC-2, the shortest axis of the monomer is approximately 20 nm and the longest axis is approximately 40 nm.33 Therefore, the epitope that is bound by the antibody used in these studies must lie on a region of the surface of each CLEC-2 monomer that is near the equivalent region on the paired monomer. This is probably near the dimer interface. Although the crystal structure of CLEC-2 fails to predict a dimerization motif, C-type lectin-like molecules can form dimers with a reasonably large interface without a precise dimerization motif. In the related C-type lectin-like molecule NKG2D, for example, large patches of surface on the monomers interact to produce the dimer, and the interface includes 19 hydrogen bonds and 72 van der Waals contacts.34 The surface buried within the dimer interface of NKG2D accounts for approximately 15% of the total monomer surface. The size of the CLEC-2 dimer interface is unclear, but the interface is sufficiently strong to hold the 2 monomers together in the absence of disulfide bond formation. It will be particularly interesting to establish whether or not the distance between the labeled antibodies is altered in the presence of ligand, which could in principle introduce a conformational change in the dimer configuration. If this occurs, then the interface itself may undergo a significant change on ligand binding, which might be of significance with respect to CLEC-2 phosphorylation and intracellular signaling.
The dimerization model represents a unique mode of regulation of Syk and contrasts with that used by ITAM receptors, which require phosphorylation of the 2 conserved tyrosines in the ITAM for optimal signaling. Given the dimerization model, it is of interest to consider why the FcRγ-chain does not function in this way as shown by the dramatic inhibition of NFAT activation after mutation of the individual FcRγ-chain YxxL sequences. One possible explanation is that the FcRγ-chain, which is a covalent dimer, is expressed in a conformation that does not favor cross-linking of YxxL groups on separate chains, possibly to minimize constitutive signaling in the absence of agonist binding. On the other hand, the presence of 2 YxxL groups on the same chain facilitates binding to proteins with tandem SH2 domains without a need for receptor dimerization/oligomerization. This is illustrated by comparison of the KDs derived for binding of single and tandem SH2 domains of Syk to immobilized tyrosine-phosphorylated peptides in the surface plasmon resonance experiments described in Figures 6 and 7. Dual YxxL groups that form an ITAM are found in separate exons (http://www.ensembl.org), suggesting that ITAM-like receptors may have preceded ITAM receptors. The greater number of ITAM receptors over ITAM-like receptors may reflect an evolutionary advantage in having 2 YxxL groups on the same protein, although this may also reflect the fact that the majority of ITAM receptors are composed of multiple chains and therefore unable to undergo dimerization/oligomerization. Indeed, it is noteworthy that the low-affinity immune receptor, FcγRIIA, which contains 2 YxxL groups in a classic ITAM sequence, does not appear to be present as a dimer in resting platelets as shown in the cross-linking studies.
In conclusion, the present observations support a novel dimerization model of activation of Syk by the C-type lectin receptor CLEC-2 in which the tyrosine kinase cross-links 2 CLEC-2 receptors through binding of its tandem SH2 domains to the conserved YxxL motif in each receptor. Confirmation of this model in an intact cell will require the application of specialist fluorescence-based imaging techniques, such as Förster resonance energy transfer. Such studies are ongoing in the laboratory.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Katsue Suzuki-Inoue and Yukio Ozaki for the antibody to mouse CLEC-2.
This work was supported by The Wellcome Trust (073107 and 088410), the British Heart Foundation (BHF; PG/07/116 and PG/05/134), the Medical Research Council, and the Deutsche Forschungsgemeinschaft (grant SFB/TR23 project A8; J.A.E.).
C.E.H. is a PhD student at the University of Birmingham. M.G.T. is a BHF Senior Research Fellow (FS/08/062). C.A.O. is a Medical Research Council Senior Clinical Research Fellow (G116/165). S.P.W. holds a BHF Chair (CH/03/003).
Authorship
Contribution: C.E.H. designed and performed research, collected, analyzed, and interpreted data, made the figures, and wrote the manuscript; A.Y.P., J.M., and J.H.H. designed and performed research and collected, analyzed, and interpreted data; J.A.E. contributed vital reagents; M.G.T. designed research and interpreted data; C.A.O. and K.F. designed research and analyzed and interpreted data; and S.P.W. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig E. Hughes, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: c.e.hughes@bham.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal