Abstract
Mutations in the iron exporter ferroportin (Fpn) result in iron overload in macrophages or hepatocytes depending upon the mutation. Patients with Fpn mutation D157G show high serum ferritin and normal to slightly elevated transferrin saturation. Here, we show that Fpn(D157G)–green fluorescent protein (GFP) is down-regulated independent of hepcidin, and that this down-regulation is due to the constitutive binding of Jak2 and Fpn phosphorylation. Expression of Fpn(D157G)-GFP in Danio rerio results in a severe growth defect, which can be rescued by iron supplementation. These results identify a hepcidin-independent regulation of Fpn that can result in alterations in iron homeostasis.
Introduction
The interaction of the peptide hormone hepcidin with the plasma iron transporter ferroportin (Fpn) coordinates iron acquisition with iron utilization and storage (for review, see Lee and Beutler1 and Nemeth2 ). Hepcidin is secreted by hepatocytes and macrophages in response to different stimuli, including inflammation and iron stores. Secretion of hepcidin by hepatocytes is decreased during hypoxia. Hepcidin released into the circulation binds to cell-surface Fpn, inducing its internalization and degradation.3 The removal of cell-surface Fpn results in decreased iron entry into plasma and increased iron retention in iron-exporting cells. The mechanism by which hepcidin mediates Fpn internalization has been elaborated in some detail. Fpn is a multitopic membrane protein, and hepcidin binds to an extracellular loop comprising amino acids 320 to 350.4 Hepcidin binding to Fpn results in the binding of the protein kinase Jak2 to Fpn.5 Jak2 binds to Fpn and bound Jak2 is autophosphorylated, resulting in Jak2 activation. Activated Jak2 then phosphorylates Fpn on either of 2 adjacent tyrosines present in a cytosolic loop of Fpn.6 These tyrosines are close to the membrane and are separated from the hepcidin-binding domain on the extracellular loop by a single transmembrane domain.
The functional unit of Fpn is a dimer,7,8 and cooperativity between the 2 monomers is required for both Jak2 binding and Jak2 activation.5 A single hepcidin bound to an Fpn dimer will not lead to Jak2 binding. Similarly, in an Fpn heterodimer, if one of the monomers is incapable of being phosphorylated by bound Jak2, then Jak2 bound to the wild-type monomer will not be activated, and Fpn will not be phosphorylated or internalized. This requirement for cooperativity between the 2 monomers provides an explanation for the dominant inheritance of the hepatocyte form of Fpn-linked iron overload disease.
We previously showed that expression of human Fpn mutant (D157G) in HEK293T cells led to impaired iron export activity.8 In analyzing this Fpn mutant, we observed that even when expressed by a high-efficiency promoter, the Fpn(D157G)–green fluorescent protein (GFP) did not accumulate in cells. We also showed that expression of Fpn(D157G)-GFP affected the accumulation of plasmid-expressed wild-type Fpn, resulting in lower levels of the wild-type protein. We show here that the lack of accumulation is the result of hepcidin independent binding and activation of Jak2 by the mutant Fpn. The activation of Jak2 results in the internalization and degradation of the mutant protein, preventing its appearance on the cell surface. We further show that when expressed in zebrafish, Fpn(D157G) results in severe growth retardation, which can be rescued by iron supplementation. These results demonstrate again that the changes in the conformation of Fpn affect Jak2 activation and phosphorylation of Fpn.
Methods
Cells and media
Human embryonic kidney HEK293T cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and transfected with Fpn-EGFP-N1 or Fpn(mutations)-EGFP-N1 and DynaminK44A-EGFP using Nucleofector technology (Amaxa), according to the manufacturer's directions. Human fibrosarcoma 2C4 and γ2A cells were maintained in DMEM with 10% FBS and transfected using Nucleofector technology.
Zebrafish studies
Danio rerio (zebrafish) were maintained at 28.5°C on a 14-hour light/10-hour dark cycle. Wild-type zebrafish were bred and raised according to established procedures approved by the University of Utah Institutional Animal Care and Use Committee. Procedures for embryo collection, cDNA injection, and analysis of injected embryos were performed as described previously.9
siRNA transfection
Small interfering RNA (siRNA) oligonucleotide pools matching selected regions of human epsin and nonspecific oligonucleotide pools were obtained from Dharmacon RNA Technologies. HEK293T cells were transfected with siRNA oligonucleotides at a final concentration of 100nM using OligofectAMINE reagent (Invitrogen).
Other procedures
Immunoprecipitation of Fpn-GFP was performed as described previously6 using protein A/G resin (Santa Cruz Biotechnology) and rabbit anti-Fpn. To determine whether Fpn bound Jak2 is phosphorylated, the Fpn immunoprecipitate was further immunoprecipitated using rabbit anti-Jak2. Immunoprecipitation of phosphotyrosine was performed using mouse antiphosphotyrosine antibody (1:500; Calbiochem) and protein A/G resin (Santa Cruz Biotechnology). Western analysis was performed using either rabbit anti-Fpn (1:1000), mouse antiphosphotyrosine clone 16F4 (1:500; Calbiochem), with a rabbit phosphotyrosine-specific Fpn antibody (Y302/Y303; 1:500; Cell Signaling); or mouse anti-Jak2 (1:1000; Cell Signaling), followed by either peroxidase-conjugated goat anti–mouse IgG (1:10 000; Jackson ImmunoResearch Laboratories) or peroxidase-conjugated goat anti–rabbit IgG (1:10 000; Jackson ImmunoResearch Laboratories). All Western blots were normalized for total protein concentration using the bicinchoninic acid assay (Pierce Chemical). Fluorescence images were visualized using an epifluorescence microscope (Olympus Inc) with a 60×/1.4 numeric aperture oil-immersion objective. Images were acquired using Magnafire analysis software (Optronix). All experiments were performed a minimum of 3 times.
Results
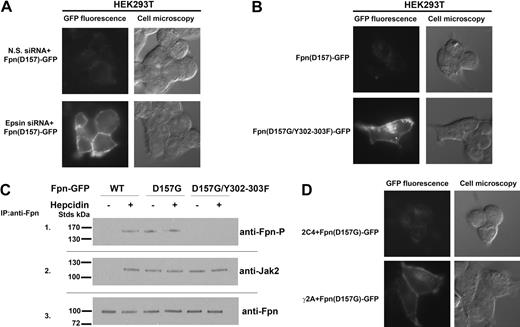
Expression of Fpn(D157G)-GFP in cells led to low levels of cellular Fpn8 (Figure 1A). In contrast, wild-type Fpn-GFP expressed using the same promoter resulted in high levels of cell-surface Fpn-GFP. We previously showed silencing of epsin inhibits hepcidin-mediated Fpn-GFP internalization.6 In cells silenced for epsin, Fpn(D157G)-GFP accumulated at the cell surface. This result indicates that the low level of Fpn(D157G) seen previously was the result of increased degradation rather than impaired synthesis. Hepcidin-mediated internalization of Fpn requires the phosphorylation of either of 2 adjacent tyrosines (Y302 or Y303) on a cytosolic loop of cell-surface Fpn.6 Mutagenesis of tyrosines 302 and 303 to phenylalanines resulted in Fpn(D157G) accumulation at the plasma membrane (Figure 1B). These data suggest that the same residues Y302 and Y303 of Fpn might be phosphorylated in the D157G Fpn mutant in the absence of hepcidin. As shown in Figure 1C (panel 1), Fpn(D157G) is phosphorylated in the absence of hepcidin. We demonstrated that hepcidin binding to Fpn led to the binding and activation of the protein kinase Jak2, which was required for phosphorylation of Fpn.5 Jak2 is not bound to wild-type Fpn-GFP in the absence of hepcidin. In contrast, Jak2 was bound to cell-surface Fpn(D157G)-GFP in the absence of hepcidin and was still bound when the Y302F and Y303F were mutated in Fpn(D157G) (Figure 1C panel 2). Phosphorylated Fpn was not detected in the immunoprecipitation containing Fpn(D157G) with the Y302F-Y303F mutations even if Jak2 was bound to Fpn.
Fpn(D157G)-GFP binds Jak2 and is phosphorylated in the absence of hepcidin. (A) HEK293T Fpn-GFP cells were transfected with nonspecific (N.S.) or epsin-specific siRNA oligonucleotide pools. At 48 hours after silencing, cells were transfected with plasmid containing Fpn(D157G)-GFP. After 18 hours, the presence of Fpn(D157G)-GFP was assessed by epifluorescence. (B) HEK293T cells were transiently transfected with Fpn(D157G)-GFP or Fpn(D157G)/(Y302-303F)-GFP. After 18 hours, the presence of both Fpn-GFP mutants was assessed by epifluorescence. (C) HEK293T cells were cotransfected with plasmids containing Fpn-GFP or DynaminK44A and incubated in the presence or absence of 1.0 μg/mL hepcidin for 30 minutes. Cells were placed at 0°C and solubilized. Samples were immunoprecipitated with rabbit anti-Fpn antibodies as described in “Other procedures.” Immunoprecipitated samples were analyzed by Western blots probed using rabbit anti-Fpn–P (panel 1), rabbit anti-Jak2 (panel 2), or rabbit anti-Fpn (panel 3), followed by a peroxidase-conjugated goat anti–rabbit IgG or peroxidase-conjugated goat anti–mouse IgG. (D) Wild-type (2C4) and Jak2-deficient cells (γ2A) were transfected with Fpn(D157G)-GFP. At 18 hours after transfection, Fpn(D157G)-GFP localization was determined by epifluorescence microscopy.
Fpn(D157G)-GFP binds Jak2 and is phosphorylated in the absence of hepcidin. (A) HEK293T Fpn-GFP cells were transfected with nonspecific (N.S.) or epsin-specific siRNA oligonucleotide pools. At 48 hours after silencing, cells were transfected with plasmid containing Fpn(D157G)-GFP. After 18 hours, the presence of Fpn(D157G)-GFP was assessed by epifluorescence. (B) HEK293T cells were transiently transfected with Fpn(D157G)-GFP or Fpn(D157G)/(Y302-303F)-GFP. After 18 hours, the presence of both Fpn-GFP mutants was assessed by epifluorescence. (C) HEK293T cells were cotransfected with plasmids containing Fpn-GFP or DynaminK44A and incubated in the presence or absence of 1.0 μg/mL hepcidin for 30 minutes. Cells were placed at 0°C and solubilized. Samples were immunoprecipitated with rabbit anti-Fpn antibodies as described in “Other procedures.” Immunoprecipitated samples were analyzed by Western blots probed using rabbit anti-Fpn–P (panel 1), rabbit anti-Jak2 (panel 2), or rabbit anti-Fpn (panel 3), followed by a peroxidase-conjugated goat anti–rabbit IgG or peroxidase-conjugated goat anti–mouse IgG. (D) Wild-type (2C4) and Jak2-deficient cells (γ2A) were transfected with Fpn(D157G)-GFP. At 18 hours after transfection, Fpn(D157G)-GFP localization was determined by epifluorescence microscopy.
The hepcidin-independent binding of Jak2 by Fpn(D157G) suggests that Jak2 might be responsible for the constitutive degradation of Fpn(D157G). To determine whether Jak2 is required for hepcidin-independent Fpn(D157G)-GFP phosphorylation, we utsed a human fibrosarcoma cell line (γ2A) that does not express Jak2 mRNA or protein.5 Transfection of Fpn(D157G)-GFP into these cells resulted in the accumulation of Fpn on the cell surface, while no such accumulation was seen in the wild-type parental cell line (2C4; Figure 1D).
Clinical reports of patients expressing Fpn(D157G) indicated high levels of ferritin and normal to modestly increased transferrin saturation.10 A report of a pedigree with a Fpn(D157N) mutation showed that the probands had high serum ferritin and normal to increased serum iron levels.11 Based on the low surface expression of Fpn(D157G)-GFP, we predicted that patients would show the macrophage form of ferroportin disease with macrophage iron loading and low serum transferrin saturation. We reported that human or mouse mutant Fpn, which leads to macrophage iron retention, expressed in zebrafish, resulted in impaired iron delivery to erythrocyte precursors affecting red cell hemoglobinization.9 To test whether Fpn(D157G)-GFP is dominant, we injected mouse pCMVFpn(D157G) into wild-type zebrafish embryos and examined its effects on embryonic development. Expression of wild-type Fpn-GFP in zebrafish embryos resulted in normal development (Figure 2A). Expression of Fpn(D157G)-GFP in zebrafish embryos resulted in severe developmental abnormalities, giving rise to high mortality. As shown in Figure 2B, a large yolk fills most of the center of the image and the embryo itself arcs along the dorsal side, stretching from the head to the tailbud. This phenotype was rescued by coinjection of iron (Figure 2C). Coinjection of iron-dextran did not alter Fpn(D157G)-GFP levels (Figure 2D). These results suggest that the increased mortality was due to decreased iron export from yolk sac to embryo. Because zebrafish have endogenous Fpn, this result indicates that Fpn(D157G) affects the ability of wild-type zebrafish Fpn to function properly.
Expression of Fpn(D157G)-GFP in zebrafish results in embryonic iron deficiency. (A) Zebrafish embryos were injected with plasmid containing wild-type Fpn-GFP. (B) Zebrafish embryos were injected with plasmid containing Fpn(D157G)-GFP. At 24 hours postfertilization (hpf), embryo development was analyzed using an Olympus Microscope U-CMAD-Z camera and a 10× objective. (C) Zebrafish embryos were injected with Fpn(D157G)-GFP and with iron-dextran. At 24 hpf, embryos development was analyzed as in panel A. The figures are representative of 5 different experiments in which 200 embryos were injected for each condition. (D) Embryos injected with Fpn(D157G)-GFP with or without iron-dextran were solubilized in lysis buffer, and Fpn-GFP levels were assayed by Western blot analysis.
Expression of Fpn(D157G)-GFP in zebrafish results in embryonic iron deficiency. (A) Zebrafish embryos were injected with plasmid containing wild-type Fpn-GFP. (B) Zebrafish embryos were injected with plasmid containing Fpn(D157G)-GFP. At 24 hours postfertilization (hpf), embryo development was analyzed using an Olympus Microscope U-CMAD-Z camera and a 10× objective. (C) Zebrafish embryos were injected with Fpn(D157G)-GFP and with iron-dextran. At 24 hpf, embryos development was analyzed as in panel A. The figures are representative of 5 different experiments in which 200 embryos were injected for each condition. (D) Embryos injected with Fpn(D157G)-GFP with or without iron-dextran were solubilized in lysis buffer, and Fpn-GFP levels were assayed by Western blot analysis.
Discussion
The binding of hepcidin to Fpn is the critical step in the regulation of vertebrate systemic iron homeostasis. Hepcidin-induced Fpn degradation is mediated by Jak2-induced binding to Fpn, leading to Jak2 activation and Fpn phosphorylation.5 Hepcidin-resistant iron overload disease may result from an inability of hepcidin to bind to mutant Fpn or an inability of mutant Fpn to respond to bound hepcidin. In contrast, the macrophage form of Fpn-linked iron overload disease is due to reduced levels of cell-surface Fpn. Most of the Fpn mutations found in this form of the disorder prevent the appropriate cell-surface targeting of Fpn, resulting in Fpn that accumulates within cells.8,12,13 There are Fpn mutants that are targeted to the cell surface but are unable to transport iron. Fpn(D157G) is the first Fpn mutant that is targeted to the cell surface but is then rapidly degraded. Fpn mutations that lead to defective iron transport predominately show lower than normal transferrin saturation due to the defect in macrophage iron export. We previously reported that expression of Fpn(D157G) in cultured cells induced the degradation of wild-type Fpn.8 Clinical data on patients expressing Fpn (D157G) are limited. One adult middle-aged man identified as having this missense mutation showed high serum ferritin but normal transferrin saturation.10 Decreased transferrin saturation in adults with the macrophage form or ferroportin disease may only be seen under conditions of increased erythropoiesis driving iron demand. Clinical data on a second Fpn mutation Fpn(D157N) are harder to interpret. Two young siblings showed high serum ferritin and normal transferrin saturation, but one of the siblings had higher than normal liver iron as assessed by a superconducting quantum interference device (SQUID).11 At steady state, most plasma iron results from macrophage recycling of red blood cell heme. Iron acquisition by the intestine represents a much smaller fraction of the steady-state transferrin-bound iron. We suggest that iron-loaded macrophages are maximally induced for Fpn; consequently, decreased Fpn activity would have a significant effect on iron export and steady-state plasma iron. Under normal conditions, the expression level of Fpn in intestine is low but can be increased by iron demand. Defective iron transporter activity due to one mutant allele of Fpn can be compensated for by increased Fpn expression. Thus, iron deficiency in Fpn disease would only be seen under conditions of iron demand. The developing zebrafish embryo has a high iron demand and offers a facile system to study the effects of Fpn mutations on iron transport activity. The lack of a clear clinical phenotype in patients with the Fpn(D157G) mutation was the stimulus for us to express Fpn(D157G) in zebrafish because developing zebrafish show high iron demand, permitting the identification of iron-related phenotypes.
Data presented here show that expression of Fpn(D157G) leads to severely impaired iron export in zebrafish embryos. We point out that the human Fpn is overexpressed, which exaggerates its affect on the endogenous Fpn. Expression of other Fpn mutants that affect macrophage iron delivery in zebrafish leads to iron-limited erythropoiesis; the iron-limited erythropoiesis is rescued by coinjection of iron.9 Overexpression of those mutant Fpns did not lead to as severe a developmental effect as does expression of Fpn(D157G). Iron supplementation of Fpn(D157G)-expressing zebrafish, however, similar to other mutants, rescues the phenotype. The rescue of the phenotype supports the view that the developmental defect is due to iron limitation. It may be possible that a different amino acid substitution at D157, such as D157N, may lead to a different phenotype.
Fpn is a dimer and binding of Jak2 to Fpn and the activation of Jak2 bound to Fpn is highly cooperative, requiring participation of both monomers.5 Mutations in one Fpn monomer can prevent binding of Jak2 to both monomers or, if Jak2 is bound, mutations in one monomer can prevent activation of bound Jak2. The data presented here provide support for the importance of conformational changes in Fpn that affect Jak2 binding. The discovery that a mutant Fpn leads to Jak2 activation in a hepcidin-independent manner provides additional evidence for the importance of Jak2 in Fpn down-regulation. The D157G mutation occurs in the third cytosolic loop of Fpn (based on the model of Fpn published by Liu et al13 ). This loop is also the site of 2 Fpn mutations that affects Jak2-mediated Fpn phosphorylation. Fpn mutant Q182H, which is in the same cytosolic loop, shows delayed phosphorylation and internalization of Fpn in response to hepcidin.6 Mutation N144H, which may be in the transmembrane domain adjacent to the third cytosolic loop, results in Jak2 binding but no Jak2 activation or Fpn phosphorylation.5 The location of these 3 human Fpn mutations that affect Fpn phosphorylation, although in very different ways, suggests the importance of this cytosolic domain in Jak2 binding and activation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to express their appreciation to the Kaplan lab for critical reading of the manuscript.
This work was supported by National Institutes of Health (NIH) grant no. DK070947 to J.K. and by Center of Excellence in Molecular Hematology (CEMH) grant no. SP30 DK072437.
National Institutes of Health
Authorship
Contribution: I.D.D. performed experiments, analyzed the data and wrote the paper; E.L. performed experiments and edited the paper; and D.M.W. and J.K. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerry Kaplan, Department of Pathology, School of Medicine, University of Utah, 50 N Medical Dr, Salt Lake City, UT 84132; e-mail: jerry.kaplan@path.utah.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal