Abstract
Posttransplantation lymphoproliferative disease (PTLD) associated with Epstein-Barr virus (EBV) is a life-threatening complication after allogeneic hematopoietic stem cell transplantation. PTLD is efficiently prevented by adoptive transfer of EBV-specific T cells from the donor. To make EBV-specific T cells available in urgent clinical situations, we developed a rapid protocol for their isolation by overnight stimulation of donor blood cells with peptides derived from 11 EBV antigens, interferon-γ surface capture, and immunomagnetic separation. Six patients with PTLD received 1 transfusion of EBV-specific T cells. No response was seen in 3 patients who had late-stage disease with multiorgan dysfunction at the time of T-cell transfer. In 3 patients who received T cells at an earlier stage of disease, we observed complete and stable remission of PTLD. Two patients have remained free from EBV-associated disease for more than 2 years. CD8+ T cells specific for EBV early antigens rapidly expanded after T-cell transfer, temporarily constituted greater than 20% of all peripheral blood lymphocytes, and were maintained throughout the observation period. Thus, a rapid and sustained reconstitution of a protective EBV-specific T-cell memory occurred after the infusion of small numbers of directly isolated EBV-specific T cells.
Introduction
Infection with Epstein-Barr virus (EBV) accompanies most humans throughout their life. In healthy carriers, the virus is under continuous control by EBV-specific T cells.1 If the EBV-specific T-cell response is severely impaired, EBV may promote uncontrolled B-cell proliferation, resulting in cancer. Patients after allogeneic hematopoietic stem cell transplantation (HSCT) are often severely immunosuppressed and therefore at risk of developing EBV-associated posttransplantation lymphoproliferative disease (PTLD), an aggressive and potentially life-threatening malignancy2 typically occurring within the first 6 months after transplantation.3 In this period, the patient may still receive immunosuppressive medication, lymphopoiesis has not been fully established yet, and there may be no appropriate T-cell repertoire.4 Because the malignant cells originate in most cases from donor B cells infected with EBV,2 risk of PTLD is enhanced if the transplant is not depleted of B cells or is depleted of T cells,3 a procedure often used in the context of human leukocyte antigen (HLA)–mismatched transplantations.
Up to now there has been no standardized treatment for PTLD.2,5 Reduction in immunosuppression is generally used as a first step but carries the inherent risk of triggering or aggravating graft-versus-host disease. A considerable proportion (≥ 50%) of PTLD cases responds to the anti-CD20 antibody rituximab,5,6 which is therefore widely used for treatment or prophylaxis, but may completely delete B cells for several months to years. Rituximab treatment may fail because of down-regulation of the CD20 antigen on malignant B cells or because of the absence of effector cells of antibody-mediated cellular cytotoxicity. Infusion of donor lymphocytes is an efficient treatment for PTLD but carries the risk of severe graft-versus-host disease.7 In contrast, adoptive transfer of preselected EBV-specific T cells, pioneered by Heslop et al8 and Rooney et al,9,10 was clinically efficient and safe in prophylaxis and therapy for PTLD after HSCT and led to long-lasting engraftment of the transferred T cells.8,11 In this approach, donor-derived B-cell lines transformed with EBV in vitro, lymphoblastoid cell lines (LCLs), were used to select and expand EBV-specific T cells from the donor's repertoire. However, generation of a donor-derived LCLs and the repeated restimulation of donor T cells require 2 to 3 months of processing time.10,12 This precludes the preparation and use of such T cells after PTLD has become manifest, and such a process requires substantial workforce and laboratory facilities. Therefore, more rapid methods to prepare EBV-specific T cells are required to make EBV-specific T-cell therapy more widely applicable.
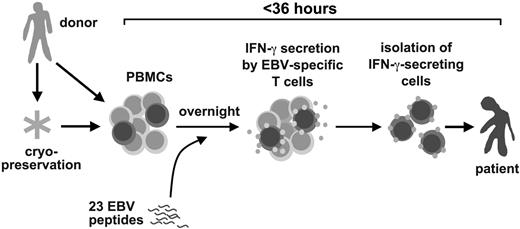
Here, we present a novel method for the rapid isolation of clinical-grade EBV-specific T cells and describe its use in PTLD therapy. EBV-specific T cells were isolated from donor blood cells in less than 36 hours by stimulation with a pool of 23 EBV peptide epitopes from 11 EBV antigens, followed by isolation of responding cells by antibody-mediated surface interferon-γ (IFN-γ) capture and immunomagnetic separation.13,14 Rapidly isolated EBV-specific T cells were used for adjuvant treatment of 6 patients with manifest PTLD. We describe clinical responses and immune reconstitution in these patients after EBV-specific T-cell transfer.
Methods
Isolation of EBV-specific T cells
EBV-specific T cells for adoptive transfer to patients after allogeneic HSCT were prepared from lymphocyte-rich leukapheresis products from the transplant donor. In each case, the donor was EBV positive (positive for immunoglobulin G to EBV nuclear antigen-1 [EBNA1] and virus capsid antigen). To stimulate EBV-specific T cells to secrete IFN-γ, leukapheresis products were resuspended at 107 cells/mL in very low endotoxin RPMI-1640 medium (Biochrom) supplemented with 10% pooled human male AB serum (Institute for Transfusion Medicine, Suhl, Germany). EBV peptide mix (1 μg/mL of each peptide) was added, and the cells were incubated for 12 hours at 37°C in a culture bag (LifeCell; Baxter). A total of 1.5 to 2.5 × 109 cells were used for stimulation. The EBV peptide mix consisted of 23 peptides of 8 to 15 amino acids in length (> 95% purity; purchased from JPT), listed in Table 1. Peptides were individually dissolved at 10 μg/mL in dimethyl sulfoxide, pooled, and stored at −20°C.
After the 12-hour stimulation period, IFN-γ–secreting cells were isolated with the use of the IFN-γ Cytokine Capture System and a CliniMACS separation device, according to the manufacturer's recommendations (Miltenyi Biotec). Briefly, bulk cells were loaded with a capture antibody specific for IFN-γ. During the following secretion phase (45 minutes) at high cell dilution (106/mL), specific cells secrete IFN-γ, which is predominantly bound to the capture antibodies on the secreting cell itself. Finally, cells that bound IFN-γ were labeled with paramagnetic particles conjugated with an IFN-γ–specific antibody recognizing a nonoverlapping epitope, and the labeled cells were isolated by repeated passage over a paramagnetic column.
Of the cells recovered as IFN-γ–positive fraction (0.3-8.4 × 106 viable cells), 10% to 15% were used for quality control; the remainder was used for therapeutic T-cell transfer. Quality control consisted of assessment of viability in an EasyCyte Mini cell counter (Guava) and analysis in a FACSCanto flow cytometer (Becton Dickinson) after staining with the following monoclonal antibodies in different combinations: anti–CD8-FITC, anti–CD4-PE-Cy7, anti–CD14-APC-Cy7 (all from Becton Dickinson Immunocytometry Systems), anti–IFN-γ-PE (Miltenyi Biotec), and 7-amino-actinomycin D (Sigma-Aldrich).
Patients
Between January and December 2007, 6 patients after allogeneic HSCT who developed biopsy-proven EBV-positive PTLD that showed signs of progression during short-term (3-14 days) therapy (reduction in immunosuppression, rituximab and cidofovir) received a single transfusion of EBV-specific T cells directly prepared from peripheral blood of their donor. All patients gave informed consent. Patient data are provided in Table 4. T cells were intravenously infused through a central line over a period of 10 minutes in 35 to 37 mL of phosphate-buffered saline/EDTA buffer with 0.5% human AB serum. The protocol was approved by the institutional review board of Ludwig-Maximilians-Universität, Munich.
Quantification of EBV DNA by polymerase chain reaction in peripheral blood cells or throat lavage was performed at least weekly with the use of primers detecting a sequence in EBV's DNA polymerase gene, BALF5.34 Levels of EBV DNA (Figures 2,Figure 3–4) refer to 20 000 peripheral blood mononuclear cells (PBMCs) or to 1 mL of throat lavage or endotracheal suctioning.
Analysis of immune reconstitution
For analysis of T-cell responses in patients after T-cell transfer, 30-mL samples of heparinized blood were obtained by venipuncture. PBMCs were isolated by centrifugation on Ficoll (Biochrom).
Flow cytometric analysis was performed in a 4-color FACSCalibur flow cytometer (Becton Dickinson). For HLA/peptide pentamer staining, unlabeled pentamers and phycoerythrin (PE)–labeled Fluorotag were purchased from Proimmune. The following HLA/peptide pentamers were used (epitope designation/antigen/HLA restriction element): CLG/LMP2/A*0201, GLC/BMLF1/A*0201, YVL/BRLF1/A*0201, RPP/EBNA3A/B*0702, RRIY/EBNA3C/B*2705, RLR/EBNA3A/HLA-A*0301. Peptide sequences are identical to those shown in Table 1. PBMCs were stained with the respective pentamer reagent at 20°C for 10 minutes, washed, and counterstained with a mixture of Fluorotag, anti–CCR7-FITC (R&D Systems), anti–CD45RA-PE-Cy5, and anti–CD8-APC (both BD PharMingen). In negative control stainings, buffer without HLA/peptide pentamer was used in the first step.
A parallel staining was performed with anti–CD4-FITC, anti–CD8-PE, anti–CD3-PE-Cy5 (all BD PharMingen), and anti–CD56-APC (BioLegend). For analysis of their T-cell receptor (TCR) Vβ subclass composition, cells were stained with each of the 8 antibody sets contained in the IOTest BetaMark kit (Beckman-Coulter), which contains 24 antibodies against specific Vβ regions, labeled with fluorescein, PE, or both, and counterstained with anti–CD4-PECy5 (ImmunoTools) and CD8-APC (BD PharMingen).
IFN-γ enzyme-linked immunospot (ELISPOT) analysis was performed in 96-well mixed cellulose ester plates (Millipore) with antibody sets from Mabtech. PBMCs were stimulated with relevant peptides or peptide mixes (1 μg/mL for each peptide), 12-O-tetradecanoylphorbol-13-acetate (100 ng/mL; Sigma-Aldrich), and ionomycin (1μM/mL; Calbiochem) for a positive control, or medium for a negative control.
T-cell clones were established from peripheral blood cells by distributing PBMCs at 0.7 or 3 cells per well to 96-well round-bottom plates in the presence of mixed irradiated (50 Gy) pooled PBMCs from 3 anonymous donors (together 1.5 × 106/mL), irradiated LCL from an irrelevant donor (105/mL), and interleukin-2 (1000 IU/mL; Proleukin; Chiron). After 2.5 to 3 weeks, outgrowing clones were screened for antigen-specific reactivity in an enzyme-linked immunoabsorbent assay (Mabtech) by stimulation with relevant EBV peptides. For further expansion, clones were restimulated every 2 weeks with the same set of stimuli (allogeneic PBMCs, LCL, interleukin-2).
Results
Rapid isolation of EBV-specific T cells
For therapy for PTLD, we developed a rapid method to isolate EBV-specific T cells from donor peripheral blood leukaphereses by stimulation with a pool of EBV-derived peptides and subsequent immunomagnetic isolation of IFN-γ–secreting cells (Figure 1). We chose 23 EBV peptide epitopes from the published record1,33 (Table 1), including epitopes derived from 5 latent antigens (LMP2, EBNA1, EBNA3A, EBNA3B, EBNA3C), 4 immediate early/early antigens (BZLF1, BRLF1, BMLF1, BHRF1), and 2 late/structural antigens (BLLF1, BNRF1). Nineteen HLA class I–restricted epitopes restricted through frequent HLA allotypes were chosen. HLA-A1 was not covered because no immunodominant epitopes are known for this allotype.1,35 A small set of 4 HLA class II–restricted T-cell epitopes was also included. The full set of 23 peptides was used as a generic pool for each donor. We calculated retrospectively that 97% of donors for HSCT at our institution are matched in at least 1 HLA allotype with the HLA restrictions covered by this set of EBV peptides (not shown). For isolation of EBV-specific T cells, donors' leukapheresis products were stimulated for 12 hours with the EBV peptides, and reactive cells were isolated by IFN-γ–based cytokine capture technology (Miltenyi Biotec).
A rapid method to prepare Epstein-Barr virus–specific T cells for adoptive T-cell therapy for posttransplant lymphoproliferative disease.
A rapid method to prepare Epstein-Barr virus–specific T cells for adoptive T-cell therapy for posttransplant lymphoproliferative disease.
The 23 EBV peptides used for T-cell stimulation and isolation
| Type . | Short name . | Amino acid sequence . | Antigen . | HLA restriction . |
|---|---|---|---|---|
| Class I | CLG | CLGGLLTMV15 | LMP2 | A*0201 |
| GLC | GLCTLVAML16,17 | BMLF1 | A*0201 | |
| YVL | YVLDHLIVV18 | BRLF1 | A*0201 | |
| RLR | RLRAEAQVK19 | EBNA3A | A*0301 | |
| RPP | RPPIFIRRL20 | EBNA3A | B*0702 | |
| QPR | QPRAPIRPI20 | EBNA3C | B*0702 | |
| QAK | QAKWRLQTL21 | EBNA3A | B*0801 | |
| RAK | RAKFKQLL22 | BZLF1 | B*0801 | |
| IVT | IVTDFSVIK23 | EBNA3B | A*1101 | |
| DEV | DEVEFLGHY17 | BMLF1 | B*1801 | |
| RYS | RYSIFFDYM21 | EBNA3A | A*2402 | |
| TYS | TYSAGIVQI24 | EBNA3B | A*2402 | |
| TYP | TYPVLEEMF25 | BRLF1 | A*2402 | |
| RRIY | RRIYDLIEL26 | EBNA3C | B*2702/04/05 | |
| YPL | YPLHEQHGM27 | EBNA3A | B*3501 | |
| EPL | EPLPQGQLTAY18 | BZLF1 | B*3501 | |
| IED | IEDPPFNSL27 | LMP2 | B*4001 | |
| KEH | KEHVIQNAF28 | EBNA3C | B*4402 | |
| VEI | VEITPYKPTW24 | EBNA3B | B*44 | |
| Class II | PYYV | PYYVVDLSVRGM29 | BHRF1 | DRB1*04 |
| FGQL | FGQLTPHTKAVYQPR30 | BLLF1 | DRB1*13 | |
| IPQC | IPQCRLTPLSRLPFG31,32 | EBNA1 | DRB1*13/14 | |
| TDAW | TDAWRFAMNYPRNPT33 | BNRF1 | DRB1*15 |
| Type . | Short name . | Amino acid sequence . | Antigen . | HLA restriction . |
|---|---|---|---|---|
| Class I | CLG | CLGGLLTMV15 | LMP2 | A*0201 |
| GLC | GLCTLVAML16,17 | BMLF1 | A*0201 | |
| YVL | YVLDHLIVV18 | BRLF1 | A*0201 | |
| RLR | RLRAEAQVK19 | EBNA3A | A*0301 | |
| RPP | RPPIFIRRL20 | EBNA3A | B*0702 | |
| QPR | QPRAPIRPI20 | EBNA3C | B*0702 | |
| QAK | QAKWRLQTL21 | EBNA3A | B*0801 | |
| RAK | RAKFKQLL22 | BZLF1 | B*0801 | |
| IVT | IVTDFSVIK23 | EBNA3B | A*1101 | |
| DEV | DEVEFLGHY17 | BMLF1 | B*1801 | |
| RYS | RYSIFFDYM21 | EBNA3A | A*2402 | |
| TYS | TYSAGIVQI24 | EBNA3B | A*2402 | |
| TYP | TYPVLEEMF25 | BRLF1 | A*2402 | |
| RRIY | RRIYDLIEL26 | EBNA3C | B*2702/04/05 | |
| YPL | YPLHEQHGM27 | EBNA3A | B*3501 | |
| EPL | EPLPQGQLTAY18 | BZLF1 | B*3501 | |
| IED | IEDPPFNSL27 | LMP2 | B*4001 | |
| KEH | KEHVIQNAF28 | EBNA3C | B*4402 | |
| VEI | VEITPYKPTW24 | EBNA3B | B*44 | |
| Class II | PYYV | PYYVVDLSVRGM29 | BHRF1 | DRB1*04 |
| FGQL | FGQLTPHTKAVYQPR30 | BLLF1 | DRB1*13 | |
| IPQC | IPQCRLTPLSRLPFG31,32 | EBNA1 | DRB1*13/14 | |
| TDAW | TDAWRFAMNYPRNPT33 | BNRF1 | DRB1*15 |
For a comprehensive review of EBV-derived T-cell epitopes, see Hislop et al.1
We used this method to isolate EBV-specific T cells from stem cell transplant donors of 6 patients with PTLD. From a starting population of 1.5 to 2.5 × 109 cells, we obtained an IFN-γ–positive fraction of, on average, 4.5 × 106 cells (range, 0.3-8.4 × 106 cells). Of these cells, 88% to 90% were transferred to the patient; the remainder was available for analysis. Cell numbers were sufficient to analyze the phenotypic composition of the isolated IFN-γ–positive fraction (Table 2). In each case we detected CD8+ and CD4+ cells. The proportion of detectably IFN-γ–positive cells was higher in CD8+ than in CD4+ cells; 38% to 80% of CD8+ T cells stained with the IFN-γ–specific antibody (Table 2). In some preparations, high numbers of CD14+ monocytes were also present. A large proportion of the cells could not be stained with any of the antibodies used; possibly, transient down-regulation of phenotypic markers after peptide stimulation contributed to this effect. Concordantly, in control isolations of EBV-specific T cells we observed a 2- to 3-fold increase in the proportion of CD8+ and CD4+ T cells after 20 or 40 hours of rest in culture (Table 3). Likewise, antibody staining of cell-bound IFN-γ (Table 2) might have been partially blocked, because the microbeads used for cell isolation and the antibody used for analysis recognize the same epitope on IFN-γ.
Characteristics of EBV-specific T-cell preparations used for therapy
| Patient . | Total viable cells administered . | Administered cells/kg . | CD8+, %* . | CD8+ IFN-γ+, %* . | CD4+, %* . | CD4+ IFN-γ+, %* . | CD14+, %* . | CD14−CD8−CD4−, %* . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total . | CD8+ . | CD4+ . | ||||||||
| 1 | 1.3 × 106 | 1.8 × 104 | 1.5 × 103 | 2.0 × 103 | 8.2 | 3.1 | 10.9 | 1.9 | 41.4 | 39.5 |
| 2 | 3.9 × 106 | 7.1 × 104 | 15.5 × 103 | 14.9 × 103 | 21.8 | 12.4 | 21.0 | 1.5 | 13.0 | 44.2 |
| 3 | 5.2 × 106 | 7.4 × 104 | 3.0 × 103 | 3.1 × 103 | 4.0 | 3.2 | 4.2 | 2.9 | 74.9 | 16.8 |
| 4 | 0.3 × 106 | 0.4 × 104 | 0.6 × 103 | 0.9 × 103 | 14.7 | 7.5 | 21.2 | 5.1 | 25.5 | 38.6 |
| 5 | 6.1 × 106 | 8.6 × 104 | 0.7 × 103 | 2.9 × 103 | 0.8 | 0.3 | 3.4 | 0.5 | 34.7 | 61.1 |
| 6 | 7.4 × 106 | 9.7 × 104 | 0.5 × 103 | 1.3 × 103 | 0.5 | 0.2 | 1.3 | 0.3 | 69.8 | 28.4 |
| Patient . | Total viable cells administered . | Administered cells/kg . | CD8+, %* . | CD8+ IFN-γ+, %* . | CD4+, %* . | CD4+ IFN-γ+, %* . | CD14+, %* . | CD14−CD8−CD4−, %* . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total . | CD8+ . | CD4+ . | ||||||||
| 1 | 1.3 × 106 | 1.8 × 104 | 1.5 × 103 | 2.0 × 103 | 8.2 | 3.1 | 10.9 | 1.9 | 41.4 | 39.5 |
| 2 | 3.9 × 106 | 7.1 × 104 | 15.5 × 103 | 14.9 × 103 | 21.8 | 12.4 | 21.0 | 1.5 | 13.0 | 44.2 |
| 3 | 5.2 × 106 | 7.4 × 104 | 3.0 × 103 | 3.1 × 103 | 4.0 | 3.2 | 4.2 | 2.9 | 74.9 | 16.8 |
| 4 | 0.3 × 106 | 0.4 × 104 | 0.6 × 103 | 0.9 × 103 | 14.7 | 7.5 | 21.2 | 5.1 | 25.5 | 38.6 |
| 5 | 6.1 × 106 | 8.6 × 104 | 0.7 × 103 | 2.9 × 103 | 0.8 | 0.3 | 3.4 | 0.5 | 34.7 | 61.1 |
| 6 | 7.4 × 106 | 9.7 × 104 | 0.5 × 103 | 1.3 × 103 | 0.5 | 0.2 | 1.3 | 0.3 | 69.8 | 28.4 |
Subpopulations were quantified immediately after IFN-γ capture–based cell isolation by staining with fluorescent monoclonal antibodies specific for CD4, CD8, CD14, and IFN-γ. Staining was evaluated by flow cytometry. Because of transient down-regulation of T-cell markers after activation and partial blockade of IFN-γ epitopes, this analysis may underrepresent the true proportions of T cells and IFN-γ+ cells (see text and Table 3).
Percentage of total viable cells.
Reexpression of T-cell markers after IFN-γ–based isolation of EBV-specific T cells
| Donor . | CD8+, %* . | Increase in CD8+ cells 40 vs 0 h . | CD4+, %* . | Increase in CD4+ cells 40 vs 0 h . | ||||
|---|---|---|---|---|---|---|---|---|
| 0 h . | 20 h . | 40 h . | 0 h . | 20 h . | 40 h . | |||
| A | 5.4 | 11.8 | 11.7 | 2.2 | 3.6 | 9.5 | 8.4 | 2.3 |
| B | 6.4 | 13.1 | 16.3 | 2.5 | 8.4 | 30.5 | 41.7 | 5.0 |
| C | 16.0 | ND | 36 | 2.3 | 6.9 | ND | 17.4 | 2.5 |
| D | 7.1 | ND | 11 | 1.5 | 6.5 | ND | 17 | 2.6 |
| Mean ± SD | 2.1 ± 0.4 | 3.1 ± 1.2 | ||||||
| Donor . | CD8+, %* . | Increase in CD8+ cells 40 vs 0 h . | CD4+, %* . | Increase in CD4+ cells 40 vs 0 h . | ||||
|---|---|---|---|---|---|---|---|---|
| 0 h . | 20 h . | 40 h . | 0 h . | 20 h . | 40 h . | |||
| A | 5.4 | 11.8 | 11.7 | 2.2 | 3.6 | 9.5 | 8.4 | 2.3 |
| B | 6.4 | 13.1 | 16.3 | 2.5 | 8.4 | 30.5 | 41.7 | 5.0 |
| C | 16.0 | ND | 36 | 2.3 | 6.9 | ND | 17.4 | 2.5 |
| D | 7.1 | ND | 11 | 1.5 | 6.5 | ND | 17 | 2.6 |
| Mean ± SD | 2.1 ± 0.4 | 3.1 ± 1.2 | ||||||
Subpopulations were stained for FACS analysis immediately after IFN-γ capture–based cell isolation (0 hours) or after 20 hours or 40 hours of cultivation.
ND indicates not done.
Percentage of total viable cells.
Transfusion of EBV-specific T cells to patients with PTLD
Six patients with PTLD after allogeneic HSCT (Table 4) received EBV-specific T cells rapidly isolated from peripheral blood cells of their stem cell donor. All patients had biopsy-proven, EBV-positive PTLD. Five of the 6 patients had received an HLA-haploidentical family donor transplant (bone marrow, followed by CD6+-depleted peripheral blood stem cells 6 days later). Prophylaxis for graft-versus-host disease consisted of cyclosporine A and a short course of methotrexate. Patient 2, however, had only received mobilized peripheral blood stem cells from a matched unrelated donor. For 3 to 19 days before T-cell transfer, all 6 patients had been receiving first-line therapy, including reduction of immunosuppression, anti-CD20 antibody (rituximab), and cidofovir (Table 4), and had shown clinical signs of progression during this treatment. At the time of EBV-specific T-cell transfer, symptoms were comparatively mild in patients 1, 2, and 3 and included lymphadenopathy, fever, and tonsillitis. Patients 4, 5, and 6, however, had advanced PTLD with multiorgan dysfunction (Table 4) at the time of T-cell transfer. Each of the patients received a single dose of, on average, 4.0 × 106 cells (range, 0.3-7.4 × 106 cells) enriched for EBV-specific T cells (Table 2).
Characteristics of patients who received EBV-specific T cells for therapy for acute PTLD after allo-SCT
| Patient . | Age, y, sex . | Primary disease . | Transplant donor . | Time of transplantation* . | Time of diagnosis of PTLD* . | Time of RI* . | Rituximab, d* . | Cidofovir, d* . | Symptoms at time of T-cell transfer . | PTLD response . | Outcome, d* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24, F | AML | MMR | −62 | −5 | −5 | −5, −1, 5, 12 | −5, −2, 5 | Lymphadenopathy | CR | Alive & well (657) |
| 2 | 19, F | SAA | MU | −47 | −6 | −6 | −5, 0 | −5, 9 | Lymphadenopathy, tonsillitis, fever | CR | Alive & well (643) |
| 3 | 24, M | SAA | MMR | −63 | −6 | −6 | −6, −3 | −6, 2, 9 | Lymphadenopathy, fever | CR | Died (96), lung failure |
| 4 | 36, M | T-NHL | MMR | −71 | −5 | −3 | −3, 0 | −19,† −12, −3, −1 | Lymphadenopathy, fever, MOF‡ | NR | Died (1), MOF |
| 5 | 44, M | t-AML, after immunocytoma | MMR | −93 | −15 | −19† | −14, −8, 0 | −22,† 0, 3, 7, 10 | Lymphadenopathy, fever, MOF‡ | PD | Died (11), MOF |
| 6 | 46, M | Recurrent AML | MMR | −81 | −9 | −9 | −9, −7, −2, 4 | −9, −6, −1 | Lymphadenopathy, fever, MOF‡ | PD | Died (7), MOF |
| Patient . | Age, y, sex . | Primary disease . | Transplant donor . | Time of transplantation* . | Time of diagnosis of PTLD* . | Time of RI* . | Rituximab, d* . | Cidofovir, d* . | Symptoms at time of T-cell transfer . | PTLD response . | Outcome, d* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24, F | AML | MMR | −62 | −5 | −5 | −5, −1, 5, 12 | −5, −2, 5 | Lymphadenopathy | CR | Alive & well (657) |
| 2 | 19, F | SAA | MU | −47 | −6 | −6 | −5, 0 | −5, 9 | Lymphadenopathy, tonsillitis, fever | CR | Alive & well (643) |
| 3 | 24, M | SAA | MMR | −63 | −6 | −6 | −6, −3 | −6, 2, 9 | Lymphadenopathy, fever | CR | Died (96), lung failure |
| 4 | 36, M | T-NHL | MMR | −71 | −5 | −3 | −3, 0 | −19,† −12, −3, −1 | Lymphadenopathy, fever, MOF‡ | NR | Died (1), MOF |
| 5 | 44, M | t-AML, after immunocytoma | MMR | −93 | −15 | −19† | −14, −8, 0 | −22,† 0, 3, 7, 10 | Lymphadenopathy, fever, MOF‡ | PD | Died (11), MOF |
| 6 | 46, M | Recurrent AML | MMR | −81 | −9 | −9 | −9, −7, −2, 4 | −9, −6, −1 | Lymphadenopathy, fever, MOF‡ | PD | Died (7), MOF |
RI indicates reduction in immunosuppression; AML, acute myeloid leukemia; SAA, severe aplastic anemia; T-NHL, non-Hodgkin lymphoma of T-cell type; t-AML, treatment-related AML; MMR, HLA-haploidentical mismatched related donor; MU, matched unrelated donor; CR, complete remission; NR, no response assessable; PD, progressive disease; and MOF, multiorgan failure.
Times are indicated in days relative to adoptive transfer of EBV-specific T cells (day 0).
For patients 4 and 5, antiviral treatment was initiated on days −19 and −22, respectively, after diagnosis of lytic EBV infection.
Additional symptoms of patient 4 at the time of T-cell transfer included pneumonitis, hepatitis, pericarditis, hemorrhagic cystitis, and consecutive renal, respiratory, liver, and cardiac failures. Additional symptoms of patient 5 included pneumonitis with pleural effusion, hepatitis, and consecutive liver, respiratory, and hemodynamic failures. Additional symptoms of patient 6 were pneumonitis with pleural effusion, hepatitis, and consecutive respiratory, liver, renal, and hemodynamic failures. Leukemic spread of lymphoma cells was observed in patients 4 and 5 at the time of T-cell transfer.
Remission of PTLD and expansion of EBV-specific T cells after T-cell transfer
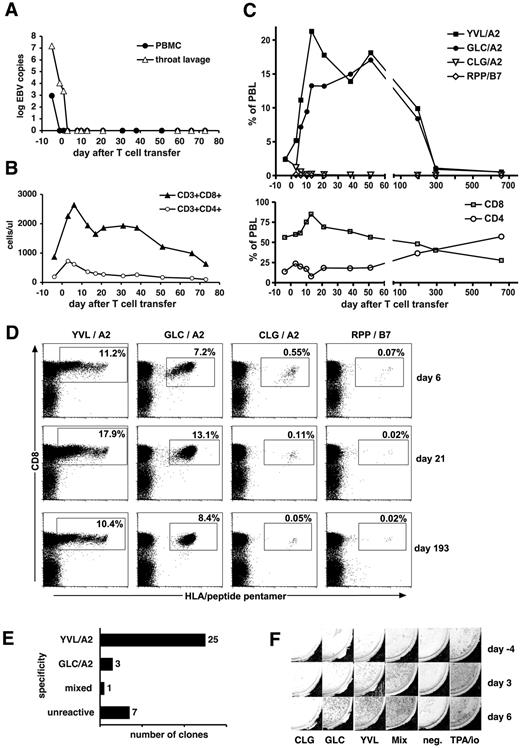
After T-cell transfer, we observed a rapid and complete remission of PTLD in patients 1, 2, and 3. Patients 1 and 2 have remained alive and healthy ever since (for > 2 years). In patient 1, lymphadenopathy and clinical symptoms had completely resolved by day 16 after T-cell transfer, and a biopsy was negative for malignant cells and for EBV DNA. EBV DNA in PBMCs and throat lavage had become undetectable earlier (Figure 2A). Before T-cell transfer, this patient had low CD4+ T-cell counts, but CD8+ T-cell counts in the upper normal range. After T-cell transfer, total CD8+ T cells rapidly expanded 3-fold and remained elevated for 70 days (Figure 2B). Staining with HLA/peptide pentamers showed a rapid increase of EBV-specific CD8+ T cells recognizing EBV epitopes in peripheral blood of this patient (Figure 2C). The dominant T-cell populations recognized 2 HLA-A2–restricted epitopes from antigens of EBV's lytic cycle, the epitope YVL from immediate-early protein BRLF1, and the epitope GLC from early protein BMLF1. YVL-specific T cells peaked on day 13 at 21% of the patient's total lymphocytes. For the next 6 months, T cells of both specificities were maintained at high frequencies greater than 8% of total lymphocytes (Figure 2C-D). Subsequently, specific T-cell populations contracted and were then maintained at levels of 1.9% to 2.8% of CD8+ T cells. T cells recognizing 2 epitopes from proteins of the latent program (CLG from LMP2, and RPP from EBNA3A) were also continuously detected, but at much smaller numbers, peaking very early after T-cell transfer (Figure 2C-D). Direct single-cell cloning of peripheral blood cells from patient 1 produced a majority of T-cell clones recognizing 1 of the 2 lytic-cycle epitopes YVL and GLC (Figure 2E). In agreement with pentamer staining, EBV-specific T cells recognizing the GLC and YVL peptides in ELISPOT assays rapidly increased in the patient after T-cell transfer (Figure 2F).
Expansion of EBV-specific T cells in patient 1 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of CD8+ T cells specific for EBV epitopes in total peripheral blood lymphocytes (PBLs) were assessed by staining with specific HLA-peptide multimers. Epitopes from immediate-early and early lytic cycle antigens (epitopes YVL and GLC) and from latent antigens (epitopes CLG and RPP) were included in the analysis. (D) Examples of HLA-peptide multimer stainings of PBL samples obtained at 3 different times after adoptive cell therapy as indicated. Cell populations within boxes were counted as multimer-positive. Percentages indicate their proportion in total lymphocytes. (E) Frequencies and specificities of antigen-specific T-cell clones obtained by direct single-cell cloning of PBLs on day 37 after adoptive T-cell transfer. (F) Assessment of interferon-γ–secreting EBV-specific T cells by an ELISPOT assay before (day −4) and after adoptive T-cell transfer (days 3 and 6). PBMCs (200 000 per well) were stimulated, as indicated, with individual EBV peptides (CLG, GLC, YVL), a mix of all 23 peptides, no stimulator (“neg”), or with TPA (12-O-tetradecanoylphorbol-13-acetate) and ionomycin for a positive control.
Expansion of EBV-specific T cells in patient 1 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of CD8+ T cells specific for EBV epitopes in total peripheral blood lymphocytes (PBLs) were assessed by staining with specific HLA-peptide multimers. Epitopes from immediate-early and early lytic cycle antigens (epitopes YVL and GLC) and from latent antigens (epitopes CLG and RPP) were included in the analysis. (D) Examples of HLA-peptide multimer stainings of PBL samples obtained at 3 different times after adoptive cell therapy as indicated. Cell populations within boxes were counted as multimer-positive. Percentages indicate their proportion in total lymphocytes. (E) Frequencies and specificities of antigen-specific T-cell clones obtained by direct single-cell cloning of PBLs on day 37 after adoptive T-cell transfer. (F) Assessment of interferon-γ–secreting EBV-specific T cells by an ELISPOT assay before (day −4) and after adoptive T-cell transfer (days 3 and 6). PBMCs (200 000 per well) were stimulated, as indicated, with individual EBV peptides (CLG, GLC, YVL), a mix of all 23 peptides, no stimulator (“neg”), or with TPA (12-O-tetradecanoylphorbol-13-acetate) and ionomycin for a positive control.
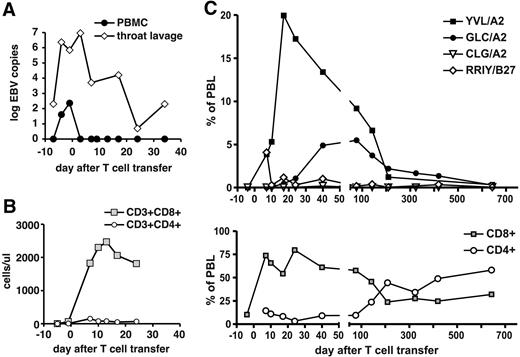
Patient 2 had initially extremely low numbers of T cells; after T-cell transfer, there was complete remission of PTLD, CD8+ T cells rapidly expanded (Figure 3B), and EBV load was reduced (Figure 3A). EBV-specific T-cell reconstitution (Figure 3C) was dominated by T cells specific for lytic-cycle epitopes YVL and GLC. YVL-specific T cells rapidly expanded after transfer and peaked at 20% of lymphocytes. T cells specific for each of the 2 epitopes were maintained at high numbers for several months, followed by a contraction, and were maintained at lower numbers later; T cells specific for 2 latent epitopes (in patient 2, CLG and RRIY) were detected, but at lower numbers than lytic antigen–specific T cells. Overall, patterns of T-cell reconstitution in patient 1 and patient 2 were very similar.
Expansion of EBV-specific T cells in patient 2 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of CD8+ T cells specific for EBV epitopes in total PBLs were assessed by HLA-peptide multimer staining. Immediate-early and early lytic cycle antigens (epitopes YVL and GLC) and latent antigens (epitopes CLG and RRIY) were included in the analysis.
Expansion of EBV-specific T cells in patient 2 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of CD8+ T cells specific for EBV epitopes in total PBLs were assessed by HLA-peptide multimer staining. Immediate-early and early lytic cycle antigens (epitopes YVL and GLC) and latent antigens (epitopes CLG and RRIY) were included in the analysis.
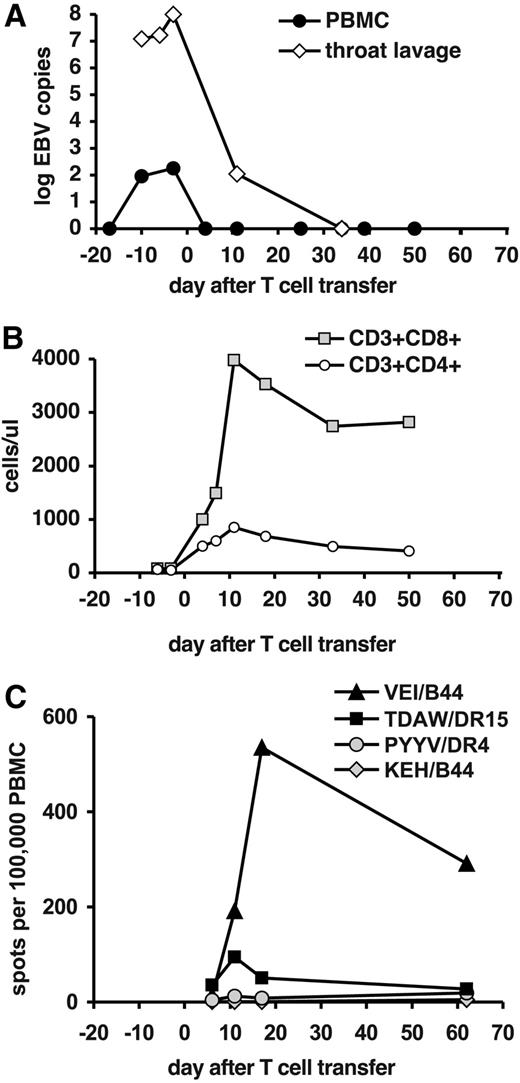
Rapid remission of PTLD after T-cell transfer was also observed in patient 3 and was associated with elimination of detectable EBV DNA in blood cells and throat (Figure 4A). This patient had very low levels of T cells before transfer (Figure 4B). No HLA/peptide pentamers were available for the donor's HLA type. In ELISPOT analyses, we observed a rapid increase of T cells recognizing the B44-restricted epitope VEI from the latent antigen EBNA3B, and against the DR15-restricted epitope TDAW from the structural protein BNRF1 (Figure 4C). The dominant VEI specificity reached a peak on day 17 after transfer, reminiscent of the kinetics of T-cell expansion in patients 1 and 2. After T-cell transfer, patient 3 remained free of EBV-associated disease, and EBV DNA remained undetectable. However, 2 months after T-cell transfer the patient developed idiopathic pneumonitis. A lung biopsy showed infiltration of monocytic cells but not T cells. Patient 3 ultimately died of lung failure 96 days after T-cell transfer.
Expansion of EBV-specific T cells in patient 3 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of T cells specific for EBV epitopes in total PBLs were assessed by an ELISPOT assay for IFN-γ. Responses against CD8+ T-cell epitopes from latent antigens (epitopes VEI and KEH), a CD4+ T-cell epitope from an early antigen (PYYV), and a CD4+ T-cell epitope from a late antigen (TDAW) were included.
Expansion of EBV-specific T cells in patient 3 after T-cell transfer. (A) EBV DNA levels in peripheral blood cells (copies per 20 000 cells) and in throat lavage fluid (copies per milliliter). (B) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. (C) Frequencies of T cells specific for EBV epitopes in total PBLs were assessed by an ELISPOT assay for IFN-γ. Responses against CD8+ T-cell epitopes from latent antigens (epitopes VEI and KEH), a CD4+ T-cell epitope from an early antigen (PYYV), and a CD4+ T-cell epitope from a late antigen (TDAW) were included.
Taken together, in all of 3 patients with early-stage PTLD we witnessed a complete remission of PTLD after transfer of small numbers of EBV-specific T cells. No side effects associated with T-cell infusion and no graft-versus-host disease were observed. In each of these patients, PTLD remission was associated with a strong expansion of EBV-specific T cells in the periphery. Antigen-specific T cells were found for a majority of EBV epitopes in the peptide pool that were restricted through the donors' HLA allotypes (Table 5): T cells from patient 1 recognized 4 of 7 matched epitopes; patient 2 recognized 5 of 6 epitopes; patient 3 recognized 2 of 3 epitopes. All were CD8+ T-cell epitopes, with 1 exception: in patient 3, some T cells recognized TDAW, a recently identified EBV epitope33 from a structural protein, BNRF1, which is recognized by CD4+ T cells in most healthy EBV carriers and often dominates their CD4+ T-cell response.36
Donor and recipient HLA types and donor-matched EBV epitopes for patients 1 through 3
| Patient . | HLA-A . | HLA-B . | HLA-DRB1 . | Class I peptides matched to the donor . | Class II peptides matched to the donor . |
|---|---|---|---|---|---|
| Donor 1 | *0201 | *1501, *0702 | *1301, *1501 | CLG,†GLC,†YVL,† RPP† | FGQL, IPQC, TDAW |
| Patient 1 | *01, *02 | *1501, *51 | *1301, *0401 | ||
| Donor 2 | *0201 | *2702, *4402 | *0301, *1601 | CLG,†GLC,†YVL,†RRIY,†VEI,†‡KEH | None |
| Patient 2 | *0201 | *2702, *4402 | *0301, *1601 | ||
| Donor 3 | 1, 29 | 44, 51 | *1501, *07xx | VEI,†KEH | TDAW† |
| Patient 3 | 28, 29 | 44, 51 | *1501, *1101 | ||
| Donor 4 | *0101, *2402 | *0702, *3701 | *11, *15 | RYS, TYS, TYP | TDAW |
| Patient 4 | *01, *02 | *37, *55 | *03, *11 | ||
| Donor 5 | *0301, *2501 | *1501, *1801 | *0101, *0401 | RLR,† DEV | PYYV |
| Patient 5 | *0301, *2501 | *1501, *1518 | *0401, *0404 | ||
| Donor 6 | *2301, *2601 | *2705, *3801 | *0301, *1202 | RRIY | |
| Patient 6 | *0301, *2301 | *0702, *3801 | *0301, *0701 |
| Patient . | HLA-A . | HLA-B . | HLA-DRB1 . | Class I peptides matched to the donor . | Class II peptides matched to the donor . |
|---|---|---|---|---|---|
| Donor 1 | *0201 | *1501, *0702 | *1301, *1501 | CLG,†GLC,†YVL,† RPP† | FGQL, IPQC, TDAW |
| Patient 1 | *01, *02 | *1501, *51 | *1301, *0401 | ||
| Donor 2 | *0201 | *2702, *4402 | *0301, *1601 | CLG,†GLC,†YVL,†RRIY,†VEI,†‡KEH | None |
| Patient 2 | *0201 | *2702, *4402 | *0301, *1601 | ||
| Donor 3 | 1, 29 | 44, 51 | *1501, *07xx | VEI,†KEH | TDAW† |
| Patient 3 | 28, 29 | 44, 51 | *1501, *1101 | ||
| Donor 4 | *0101, *2402 | *0702, *3701 | *11, *15 | RYS, TYS, TYP | TDAW |
| Patient 4 | *01, *02 | *37, *55 | *03, *11 | ||
| Donor 5 | *0301, *2501 | *1501, *1801 | *0101, *0401 | RLR,† DEV | PYYV |
| Patient 5 | *0301, *2501 | *1501, *1518 | *0401, *0404 | ||
| Donor 6 | *2301, *2601 | *2705, *3801 | *0301, *1202 | RRIY | |
| Patient 6 | *0301, *2301 | *0702, *3801 | *0301, *0701 |
Only EBV peptide epitopes that are included in the peptide pool used for T-cell isolation (Table 1) were tested and are represented in the table. HLA allotypes shared by donor and patient, and epitopes restricted through these allotypes, are printed in boldface.
Epitopes that were recognized by antigen-specific T cells in the patients after T-cell transfer. An explanation of epitope designations and HLA restrictions is found in Table 1.
Responses against this epitope in patient 2 were seen in ELISPOT assays (data not shown).
No remission of PTLD in patients with late-stage disease
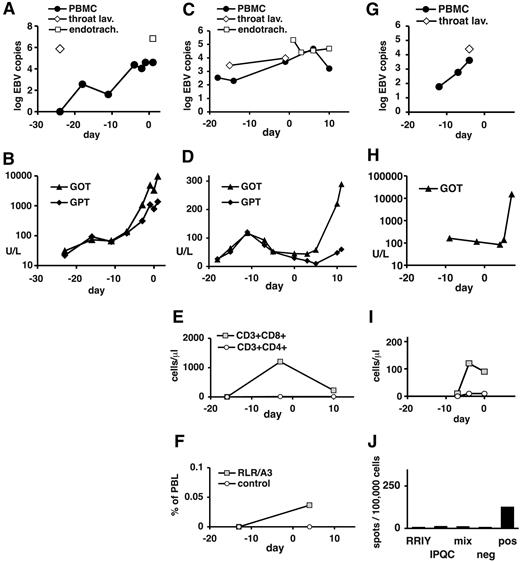
In contrast to these patients, patients 4 to 6 had late-stage PTLD with multiorgan dysfunction at the time of T-cell transfer (Table 4). After transfer, all 3 patients continued to have progressive disease (Table 4; Figure 5). Immunoreconstitution could not be analyzed in patient 4, who died of multiorgan failure (MOF) 1 day later (Table 4; Figure 5A-B). In patient 5, CD8+ T cells but no CD4+ T cells were transiently detected in peripheral blood before T-cell transfer (Figure 5E). Low numbers of CD8+ T cells specific for the HLA-A3–restricted RLR epitope from EBNA3A were seen by pentamer staining only after T-cell transfer (Figure 5F). However, no recognition of EBV epitopes was seen in IFN-γ ELISPOT assays before or after transfer (day −13, day 4; not shown). Patient 5 died of MOF 11 days after T-cell transfer. Patient 6 had low CD8+ and no CD4+ T cells in peripheral blood at the time of T-cell transfer (Figure 5I). HLA/peptide pentamer staining could not be performed because of low available cell numbers. In ELISPOT assays, low reactivity to polyclonal stimulation and no reactivity to EBV epitopes were seen (Figure 5J). Patient 6 died of MOF on day 7 after T-cell transfer. Taken together, in all 3 patients with advanced PTLD there was progression of disease after T-cell transfer, and EBV-specific T-cell responses were weak or absent (Table 5).
Clinical course and immune status in patients 4, 5, and 6 before and after EBV-specific T-cell transfer. Data are shown for patient 4 (A-B), patient 5 (C-F), and patient 6 (G-J). (A,C,G) EBV DNA levels in peripheral blood cells (copies per 20 000 cells), in throat lavage fluid (copies per milliliter), and in endotracheal suctioning (copies per milliliter). (B,D,H) Serum levels of transaminases (GOT indicates glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase) are expressed in U/L. (E,I) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. For patient 4, no data near the time of T-cell transfer were available. (F) Peripheral blood cells from patient 5 (day −13 and day 4) were stained with HLA-peptide pentamers for the EBNA3A epitope RLR or the irrelevant (mismatched) epitope EPL (see Table 5). In analysis, cells were gated on large lymphocytes. The proportion of pentamer-staining cells is shown. (J) Peripheral blood cells from patient 6 (day 6 after T-cell transfer) were tested in an IFN-γ ELISPOT assay for recognition of the matched EBV peptide RRIY, the mismatched peptide IPQC, or the mix of 23 EBV peptides. Negative control was without peptide, positive control was TPA (12-O-tetradecanoylphorbol-13-acetate) and ionomycin. (A-I) Indicated days are relative to T-cell transfer (day 0).
Clinical course and immune status in patients 4, 5, and 6 before and after EBV-specific T-cell transfer. Data are shown for patient 4 (A-B), patient 5 (C-F), and patient 6 (G-J). (A,C,G) EBV DNA levels in peripheral blood cells (copies per 20 000 cells), in throat lavage fluid (copies per milliliter), and in endotracheal suctioning (copies per milliliter). (B,D,H) Serum levels of transaminases (GOT indicates glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase) are expressed in U/L. (E,I) Absolute numbers of CD8+ and CD4+ T-cell subsets in peripheral blood. For patient 4, no data near the time of T-cell transfer were available. (F) Peripheral blood cells from patient 5 (day −13 and day 4) were stained with HLA-peptide pentamers for the EBNA3A epitope RLR or the irrelevant (mismatched) epitope EPL (see Table 5). In analysis, cells were gated on large lymphocytes. The proportion of pentamer-staining cells is shown. (J) Peripheral blood cells from patient 6 (day 6 after T-cell transfer) were tested in an IFN-γ ELISPOT assay for recognition of the matched EBV peptide RRIY, the mismatched peptide IPQC, or the mix of 23 EBV peptides. Negative control was without peptide, positive control was TPA (12-O-tetradecanoylphorbol-13-acetate) and ionomycin. (A-I) Indicated days are relative to T-cell transfer (day 0).
T-cell repertoire after T-cell transfer and remission of PTLD
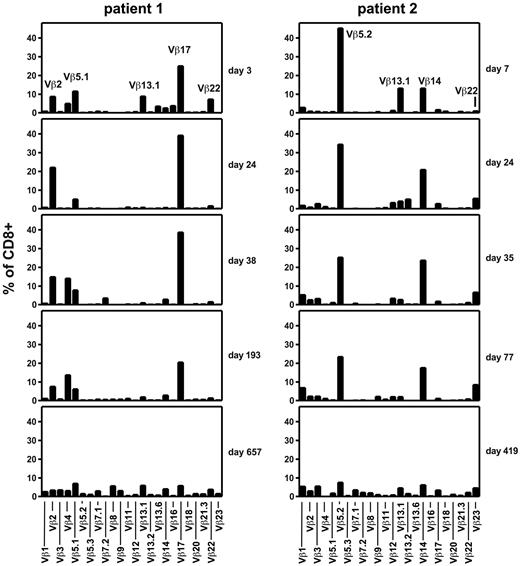
In patients 1 and 2, who have remained alive and well for more than 2 years after T-cell transfer, we investigated the diversity of the patients' T-cell repertoire by TCR β chain variable domain (TCR Vβ) staining and flow cytometry. For several months, both patients maintained a highly restricted TCR repertoire with only approximately 4 dominant TCR Vβ classes, indicating the presence of a similarly limited number of expanded clonotypes (Figure 6). In contrast, when such analyses were performed more than a year after T-cell transfer, a diversified repertoire of TCR Vβ classes was found in both donors. Total T-cell diversification (Figure 6) was broadly coincident with contraction of EBV-specific T-cell reactivities and inversion of the CD8+ to CD4+ T-cell ratio (Figures 2C and 3C).
Evolution of the TCR repertoire of CD8+ T cells after transfer of EBV-specific T cells. Peripheral blood samples from patients 1 (left) and 2 (right) were obtained at the indicated times after EBV-specific T-cell transfer and stained with a panel of 24 antibodies specific for individual TCR Vβ domains.
Evolution of the TCR repertoire of CD8+ T cells after transfer of EBV-specific T cells. Peripheral blood samples from patients 1 (left) and 2 (right) were obtained at the indicated times after EBV-specific T-cell transfer and stained with a panel of 24 antibodies specific for individual TCR Vβ domains.
Memory phenotypes of EBV-specific T cells after transfer
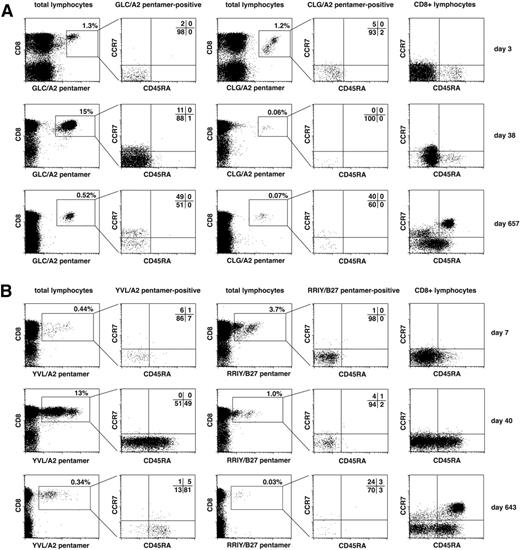
Expression of lymphocyte homing markers, chemokine receptors, and isoforms of CD45 is linked to the functional role of T-cell subsets at different stages of differentiation.37-39 We analyzed differentiation stages of EBV-specific T cells in patients 1 and 2. Figure 7 shows analyses of a representative T-cell specificity for a lytic-cycle antigen and a latency antigen in each patient. We analyzed EBV-specific T cells during the early expansion phase (day 3 or 7 after transfer), during maintenance at high levels (day 38 or 40), and after contraction of the EBV-specific T-cell response (day 657 or 643). Both latent and lytic antigen–specific T cells had an effector memory phenotype (CCR7−CD45RA−) early after transfer in both patients, consistent with the T cells' presumed functional role at this time. In both patients, T cells specific for latent antigens partially acquired CCR7 expression after T-cell contraction, as shown for CLG-specific T cells in patient 1 and RRIY-specific T cells in patient 2 (Figure 7A-B). In patient 1, T cells specific for the lytic antigen–derived epitopes GLG (Figure 7A) and YVL (not shown) showed a similar pattern of partial acquisition of CCR7, indicating central memory differentiation. In patient 2, however, T cells specific for lytic antigen epitopes (YVL; Figure 7B; GLC not shown) reassumed expression of CD45RA over time and remained CCR7 negative.
Evolution of memory phenotypes of EBV-specific T cells after T-cell transfer. Peripheral blood cells from patient 1 (A) and patient 2 (B) were costained with HLA/peptide pentamer and monoclonal antibodies for CD8, CCR7, and CD45RA. CD8+ pentamer+ cells were gated as shown and analyzed for their expression of memory differentiation markers CCR7 and CD45RA. For each patient, T cells specific for 1 exemplary lytic cycle epitope (GLC or YVL, respectively) and for 1 exemplary latent antigen–derived epitope (CLG or RRIY) are shown. Expression pattern of CCR7 and CD45RA on total CD8+ T cells is shown in the rightmost column. Analyses of T cells from an early, an intermediate, and a late time point after T-cell transfer are shown.
Evolution of memory phenotypes of EBV-specific T cells after T-cell transfer. Peripheral blood cells from patient 1 (A) and patient 2 (B) were costained with HLA/peptide pentamer and monoclonal antibodies for CD8, CCR7, and CD45RA. CD8+ pentamer+ cells were gated as shown and analyzed for their expression of memory differentiation markers CCR7 and CD45RA. For each patient, T cells specific for 1 exemplary lytic cycle epitope (GLC or YVL, respectively) and for 1 exemplary latent antigen–derived epitope (CLG or RRIY) are shown. Expression pattern of CCR7 and CD45RA on total CD8+ T cells is shown in the rightmost column. Analyses of T cells from an early, an intermediate, and a late time point after T-cell transfer are shown.
Thus, contrasting differentiation patterns of EBV-specific T cells may be observed in patients who receive an allogeneic HSC tranplant after T-cell transfer. Dominant lytic antigen–specific CD8+ T cells may either assume a CCR7+CD45RA− central memory or a CCR7−CD45RA+“terminal” effector phenotype, and both phenotypes are compatible with long-term T-cell maintenance and control of viral reactivation after PTLD remission. This is consistent with the notion that CCR7−CD45RA+ T cells are functional memory cells with reduced proliferation but full direct antiviral effector function.39,40 Of note, EBV DNA consistently remained undetectable in peripheral blood cells and throat lavages of patient 1 after PTLD remission, whereas patient 2 remained negative for EBV DNA in the blood but continued to replicate EBV in the throat at low levels after T-cell transfer for 2 years (Figure 3A; not shown). Thus, continuous challenge with lytic antigen at low levels may induce a CCR7−CD45RA+ phenotype in responsive T cells.40
Discussion
We describe the clinical application of a novel rapid method to prepare EBV-specific T cells for adoptive therapy for EBV-associated PTLD after allogeneic HSCT. Six patients with manifest PTLD at different stages of progression obtained a transfusion of EBV-specific T cells that had been isolated from donor blood cells in a 36-hour procedure. In 3 patients with very advanced PTLD, the disease progressed despite T-cell transfer. However, in 3 patients with early disease we observed complete remission of PTLD and rapid reconstitution of EBV-specific T cells after T-cell transfer. Our results show the importance of an early, if possible, prophylactic or preemptive management of PTLD,5,41 and suggest that the transfer of small numbers of EBV-specific T cells might be beneficial in such a situation. This seems encouraging and demands verification in a larger study.
However, some notes of caution are warranted. First, in addition to EBV-specific T cells, all patients received additional treatments (reduction in immunosuppression, anti-CD20, and cidofovir), and these might have contributed to remission of disease after T-cell transfer, although PTLD appeared to progress before T-cell transfer. Rituximab therapy for overt PTLD after HSCT was successful in 63% of cases41 ; in patients who respond to rituximab, signs of clinical response are generally seen within 1 week of treatment.42 Because we did not observe such signs in any of our patients before T-cell transfer, it might be hypothesized that some or all of them were nonresponders to rituximab. This seems to be corroborated by our observation that EBV DNA levels did not decrease before T-cell transfer in 5 of 6 patients (patient 1 being the exception); although a rapid decrease of EBV DNA is typical in rituximab responders, it is sometimes observed in some nonresponders as well.42,43 Second, donor-derived EBV-specific T cells might already have been present in the patients before T-cell transfer and could, by coincidence, have expanded after T-cell therapy. Derived from the same donor's EBV-specific T-cell repertoire, such preexisting T cells could not be clearly distinguished from T cells transferred later as a therapeutic procedure. Gene marking of the transferred T cells9 could resolve this question but will not be available in the foreseeable future because it would involve a complex technical procedure, in the absence of a direct beneficial effect for the patient. Therefore, larger studies will be needed to show a clear association between PTLD protection and EBV-specific T-cell transfer.
Our observations are consistent with previous studies on EBV-specific T-cell therapy for PTLD after allo-HSCT that showed therapeutic and prophylactic efficacy.8-10,43-45 In these studies, a donor-derived EBV-infected B-cell line was generated and used to repeatedly restimulate donor T cells, a process that requires 2 to 3 months of time. Therefore, such T cells were in most cases used for prophylaxis rather than therapy or had to be prepared in advance for patients at high risk of PTLD from the HSCT donor11 or third-party donors.45 More rapid procedures to isolate EBV-specific T cells have been difficult to develop because the EBV-specific T-cell immunity is spread across a wide range of viral antigens and HLA restrictions.1 We addressed this problem by including immunodominant epitopes from various antigens and of various HLA restrictions in our set of EBV peptides. Rapid procedures to isolate T cells specific for other viruses that can cause disease after allo-HSCT, such as cytomegalovirus (CMV) and adenovirus, have been developed earlier, because for these viruses T-cell responses tend to be focused on a smaller set of dominant antigens. For example, CMV-specific T cells isolated with the use of HLA/peptide tetramers efficiently controlled CMV reactivation in patients at risk.46 IFN-γ secretion–based isolation has been used to isolate virus-specific T cells after stimulation with CMV protein plus peptides,14 a peptide pool covering the sequence of the CMV pp65 antigen,47 or adenovirus lysate or protein.48,49 In most of those studies, isolated T cells had to be expanded in vitro for several days to produce cell numbers sufficient for analysis. However, in a clinical study of adenovirus-specific T cells,50 small numbers of isolated T cells were directly transferred to the patients and in some cases expanded in vivo. The preparation of EBV-specific T cells can be accelerated with the use of formalin-fixed LCLs as stimulators in combination with IFN-γ secretion–based isolation.51 Adenoviral vectors expressing EBV LMP2 and CMV pp65 and IFN-γ–based isolation were used to prepare T cells specific for all 3 viruses.52 Selected epitopes from EBV and CMV were used in an alternative T-cell isolation method,53 based on induction of CD137 (4-1BB). However, a method to prepare multiepitope EBV-specific T cells in only 2 days has not been available so far. We anticipate that the ongoing development of rapid isolation methods for virus-specific T cells will help make virus-specific T-cell therapy more widely available to the patients in need.
An important goal of T-cell therapy is long-term protection by stable establishment of a functional T-cell memory. In 2 of our patients (patients 1 and 2), who have remained alive and healthy for more than 2 years by now, long-term follow-up was possible after T-cell transfer. EBV-specific T-cell reconstitution was strikingly similar between these 2 patients, who (and whose donors) happened to be HLA-A2 positive. In both patients, we observed a strong expansion of EBV-specific T cells after adoptive transfer, maintenance at high numbers for approximately 6 months, subsequent contraction, and stabilization at lower numbers reminiscent of healthy carriers. In both patients, the expansion of EBV-specific T cells was dominated by T cells recognizing the same 2 epitopes (YVL and GLC) from immediate-early and early lytic cycle antigens (Figures 2–3). Peak numbers of T cells specific for these 2 epitopes were high (21% and 20% of peripheral blood lymphocytes [PBLs] for the YVL epitope in patients 1 and 2, respectively, corresponding to 490 and 760 specific cells/μL) and much above the frequencies of these T-cell specificities in healthy EBV carriers.38 However, similarly elevated numbers of T cells specific for dominant EBV epitopes may spontaneously develop in some patients after HSCT.4 We observed that EBV-specific T cells were maintained at high numbers as long as the overall T-cell repertoire remained oligoclonal. Contraction of EBV-specific T cells coincided with restitution of polyclonality (Figure 5) and reappearance of naive CD45RA+CCR7+ T cells (Figure 6), possibly indicating resumption of thymic output. Interestingly, T cells specific for YVL often strongly dominate CD8+ T-cell expansions observed in acute primary EBV infection (infectious mononucleosis) as well.38 In both patients 1 and 2, T cells recognizing epitopes from latent antigens were present in much smaller numbers than T cells specific for lytic-cycle epitopes (Figures 2–3), although T cells recognizing EBV antigens of the transforming latency III program might appear to be the most important effectors for the control of PTLD.1 Possibly, latent antigen-specific T cells more efficiently homed to target tissues54 than lytic antigen–specific T cells and were therefore underrepresented in the blood. Although control of lytic virus replication will indirectly be beneficial to patients with PTLD, how lytic antigen–specific T cells might contribute to the eradication of the PTLD itself seems less evident. However, tumors in PTLD often contain B cells in lytic cycle,55 and early lytic factors contribute to the tumorigenicity of EBV-transformed B cells in severe combined immunodeficient mouse models by up-regulating factors of growth and angiogenesis.56,57 Therefore, CD8+ T cells specific for EBV lytic antigens might have a direct antitumor function in PTLD.
To further improve our procedure for rapid isolation of EBV-specific T cells, additional class I and class II EBV peptides might be added to increase the coverage of HLA types and epitope specificities. With increasing numbers of peptides, it should be verified that there is no cross-competition of peptide binding to HLA. The possibility that EBV-specific T cells might cross-react with alloantigens58 needs to be ruled out by careful testing of individual T-cell specificities. Finally, epitope peptides derived from CMV, adenovirus, or other pathogens relevant in the setting after HSCT can be included to produce T cells of multiple specificities,52,59 to provide for a more complete protection from infectious complications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB-Transregio 36 and SFB 455) and by the Helmholtz Alliance on Immunotherapy of Cancer funded by the Initiative and Networking Fund of the Helmholtz Association.
Authorship
Contribution: A.M., I.B., J.T., J.M., W.H., D.J.S., and H.-J.K. developed the concepts; I.B., J.T., J.K., S.T., M.L., D.P., G.L., and H.-J. K. prepared and analyzed therapeutic cell products; J.T., D.P., G.L., and H.-J.K. enrolled patients; G.J. performed virologic analyses; A.M., L.T., and J.K. analyzed immunoreconstitution; all authors discussed and interpreted data; and A.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Moosmann, CCG Molecular Oncology, Marchioninistr 25, 81377 Munich, Germany; e-mail: andreas.moosmann@helmholtz-muenchen.de.
References
Author notes
A.M., I.B., and J.T. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal