To the editor:
The JAK2 617V>F point mutation is an important diagnostic tool for Philadelphia chromosome–negative myeloproliferative disorders. JAK2 mutation analysis has been endorsed by the World Health Organization for diagnosing polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis.1
On March 30, 2006, Ipsogen announced a license agreement for the worldwide and exclusive rights on a mutation in the JAK2 gene.2 Options for clinical laboratories performing JAK2 617V>F mutation testing are to use commercial kits from Ipsogen or to pay Ipsogen an upfront fee plus royalty payments for each test performed if their kits are not used. We compared the JAK2 Ipsogen MutaScreen kit & Reference Scale kit (MSPP-03), which is a TaqMan allelic discrimination assay that contains fluorescent probes specific for wild-type (617V) and mutant (617F) alleles, with results previously obtained using a laboratory-developed melt-curve assay,3 and found agreement in 58 of 60 cases.
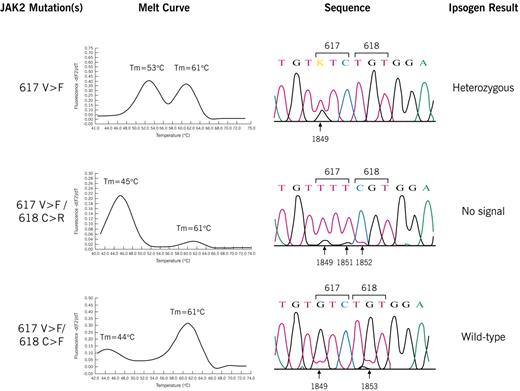
The 2 discordant samples (Figure 1) had atypical melt-curves, suggestive of novel mutation(s). Sequence analysis from patient no. 1 with PV showed homozygosity for 2 mutations in codon 617 (1849G>T, 1851C>T) and 1 in codon 618 (1852T>C) resulting in 617V>F and 618C>R mutations.4 Patient no. 2 with ET and on Hydrea was heterozygous for 2 mutations: 617V>F (1849G>T) and 618C>F (1853G>T). Patient no. 1 failed to give a fluorescent signal using the Ipsogen kit; patient no. 2 incorrectly genotyped as “wild-type.” To differentiate nonamplification (patient no. 1) from failure of the mutant probe bind to the mutant allele (patients no. 1 and no. 2), PCR products obtained using the Ipsogen kit were subject to agarose gel electrophoresis. Both samples had single prominent bands which were the same size as control samples (data not shown). These results are consistent the mutant probe not binding to the mutant allele in both patients and are consistent with codon 617 and 618 mutations being on the same allele.
Melt-curve, sequence, and Ipsogen MutaScreen kit results. Results of heterozygous control (top) and patients no. 1 (middle) and no. 2 (bottom). Melting temperatures (Tm) of wild-type and JAK2 mutations are indicated. Sequence analysis of 617V>F heterozygote compared with patients no. 1 (homozygous 617V>F/618C>R; 1849G>T, 1851C>T, 1852T>C) and no. 2 (heterozygous 617V>F/618C>F; 1849G>T, 1853G>T). Using the Ipsogen kit, no signal is obtained in patient no. 1, who is homozygous mutant, due to failure of the mutant probe to bind to the mutant allele. Patient no. 2, who is heterozygous, genotypes as wild-type because the mutant probe does not bind to the mutant allele.
Melt-curve, sequence, and Ipsogen MutaScreen kit results. Results of heterozygous control (top) and patients no. 1 (middle) and no. 2 (bottom). Melting temperatures (Tm) of wild-type and JAK2 mutations are indicated. Sequence analysis of 617V>F heterozygote compared with patients no. 1 (homozygous 617V>F/618C>R; 1849G>T, 1851C>T, 1852T>C) and no. 2 (heterozygous 617V>F/618C>F; 1849G>T, 1853G>T). Using the Ipsogen kit, no signal is obtained in patient no. 1, who is homozygous mutant, due to failure of the mutant probe to bind to the mutant allele. Patient no. 2, who is heterozygous, genotypes as wild-type because the mutant probe does not bind to the mutant allele.
In conclusion, the Ipsogen kit failed to identify 2 samples with JAK2 617V>F mutations which had additional mutations. The Ipsogen kit would likely miss other 617V>F samples which have a second mutation and other exon 14 mutations.5-8 Since the JAK2 617V>F mutation (or other functionally similar mutations) are part of the revised WHO criteria for myeloproliferative disorders,1 failure to detect this mutation may result in misdiagnosis.
These findings highlight the broader issue patents impose upon clinical laboratories and the potential for inferior diagnostic testing and missed mutations. This issue came to a forefront on May 12, 2009, when the American Civil Liberties Union filed a lawsuit against Myriad Genetics, which has exclusive rights to perform BRCA1 and BRCA2 mutation testing.9 The lawsuit claims patents on these genes are unconstitutional and invalid. Moreover, BRCA1/2 mutations have been missed.10 We evaluated the Ipsogen kit because it was cost-prohibitive to pay the substantial upfront fee and additional royalty on test sales using a “home-brew” assay. It is unfortunate that such restrictions can compromise patient care, and it will be interesting to see the outcome of the Myriad Genetics lawsuit and whether this paves the way to prevention of monopolies created by gene patenting.
Authorship
Acknowledgment: We thank Dr Matthew Kalp for obtaining the history of patient no. 2 and reviewing the literature.
Contribution: I.W. wrote the article; and F.M., C.H., and M.J. performed the laboratory work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ilka Warshawsky, Cleveland Clinic Foundation, Dept of Clinical Pathology – L30, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: warshai@ccf.org.