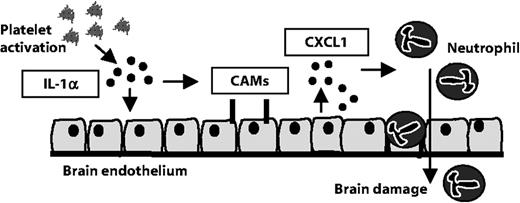
Abstract
White blood cell infiltration across an activated brain endothelium contributes to neurologic disease, including cerebral ischemia and multiple sclerosis. Identifying mechanisms of cerebrovascular activation is therefore critical to our understanding of brain disease. Platelet accumulation in microvessels of ischemic mouse brain was associated with endothelial activation in vivo. Mouse platelets expressed interleukin-1α (IL-1α), but not IL-1β, induced endothelial cell adhesion molecule expression (ICAM-1 and VCAM-1), and enhanced the release of CXC chemokine CXCL1 when incubated with primary cultures of brain endothelial cells from wild-type or IL-1α/β–deficient mice. A neutralizing antibody to IL-1α (but not IL-1β) or application of IL-1 receptor antagonist inhibited platelet-induced endothelial activation by more than 90%. Platelets from IL-1α/β–deficient mice did not induce expression of adhesion molecules in cerebrovascular endothelial cells and did not promote CXCL1 release in vitro. Conditioned medium from activated platelets induced an IL-1α–dependent activation of mouse brain endothelial cells and supported the transendothelial migration of neutrophils in vitro. Thus, we have identified platelets as a key source of IL-1α and propose that platelet activation of brain endothelium via IL-1α is a critical step for the entry of white blood cells, major contributors to inflammation-mediated injury in the brain.
Introduction
Cerebrovascular inflammation is a major contributor to diverse forms of brain injury, including cerebral ischemia, multiple sclerosis, cerebral infection, and epilepsy.1-3 Peripheral circulating cells play critical roles in cerebral inflammation. Platelets are one of the first cell types to arrive at the site of vascular dysfunction4 and can induce vascular inflammation, thus contributing to disease progression, particularly in larger vessels.
The effects of platelets on microvascular endothelial cells are poorly understood, although interactions between these cellular entities are documented in the brain in response to cerebral ischemia in several species5-9 and in cerebral malaria in humans.10 Platelet activation occurs in response to stroke in humans11,12 and in patients with multiple sclerosis.13 Platelet consumption or brain accumulation14 in the infarct area may account for the drop in platelet numbers after clinical stroke.15
Inhibition of platelet adhesion to brain endothelium may protect against diverse brain insults. Neutralizing platelet glycoprotein (GP) VI or GPIb surface adhesion receptors reduces damage in mouse focal cerebral ischemia.16 Inhibition of platelet-brain endothelial interactions, through blockade of platelet GPIIb receptor, protects mice against cerebral malaria pathogenesis.17 Mice lacking platelet GPIIb18 or mice receiving a small molecule inhibitor of GPIIb/IIIa (CD41/CD61) are also protected against cerebral ischemia.5 However, the mechanisms by which platelets contribute to brain injury are unknown.
Current therapies for the treatment of acute cerebral ischemia target platelet function, with protective effects dependent on anticoagulant or fibrinolytic actions. However, inhibition of coagulatory pathways reduces inflammation.19 Thus, the anti-inflammatory effects of platelet inhibition may mediate some of the protection afforded by these therapies in cerebral ischemia. Platelet interleukin-1α (IL-1α), IL-1β, or CD40L can activate peripheral endothelial cells,20-24 and platelets contribute to leukocyte infiltration in peripheral tissues.25,26 Similar mechanisms may contribute to brain endothelial activation.
Here, we tested the hypothesis that platelets induce inflammatory activation of brain endothelial cells. We show, for the first time, that platelet-secreted IL-1α drives mouse brain endothelial activation, leading to the induction of endothelial ICAM-1 and VCAM-1, CXCL1 release, and neutrophil transendothelial migration (TEM). This study is the first to show that actions of platelet IL-1α on brain endothelium may be critical to inflammation-mediated injury in the brain.
Methods
More details regarding the procedures outlined herein are given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Focal cerebral ischemia and immunohistochemistry
Focal cerebral ischemia in C57/BL6 or IL-1α/β−/− mice (on a C57/BL6 background27 ) induced by transient (60 minutes) middle cerebral artery occlusion and subsequent tissue processing (24 hours after onset of reperfusion) for immunohistochemistry or enzyme-linked immunosorbent assay (ELISA) was performed as described previously28 under United Kingdom Home Office personal and project licenses and protocols, which adhered to the Animals (Scientific Procedures) Act (1986).
Primary mouse brain endothelial cell culture
All mice were bred in local animal facilities and housed under a 12-hour light/dark cycle. Primary cultures of mouse brain endothelial cells (MBECs) were prepared from the brains of 4- to 12-week-old C57BL/6 mice or mice in which genes for IL-1α and IL-β were deleted (IL-1α/β−/−, on a C57/BL6 background27 ) according to the method of Song and Pachter29 with some modifications. Cultures were 95.8% plus or minus 1.6% positive for PECAM-1 and expressed endothelial markers VWF and ZO-1 (supplemental Figure 5). All cultures were grown at 37°C in a 5% CO2 humidified atmosphere. Experiments conformed to the Animals (Scientific Procedures) Act, United Kingdom (1986).
GPNT rat brain endothelial cell culture
The GPNT rat brain endothelial cell line was a generous gift from Prof John Greenwood (University College London, London, United Kingdom) and displays blood-brain barrier characteristics similar to primary brain endothelium.30
Isolation of mouse/rat platelets
Male C57/BL6, IL-1α/β−/− mice or Sprague-Dawley rats (bred in house) were anesthetized with isofluorane and blood collected via cardiac puncture. Rats were used in addition to mice as these provided a high platelet yield, which was required for the collection of platelet-conditioned medium (CM); one-tenth volume acid-citrate-dextrose was added as anticoagulant. The platelets were resuspended in Tyrode buffer with 1mM CaCl2.
ELISAs
Levels of murine ICAM-1, VCAM-1, CXCL1, or rat or murine IL-1α or IL-1β in cell culture supernatants or cell lysates were quantified using commercially available ELISA kits according to the manufacturer's instructions (R&D Systems).
Immunocytochemistry
MBECs or platelets on LabTek slides were fixed in 4% paraformaldehyde, 4% sucrose in phosphate-buffered saline. Cells were permeabilized with 0.1% Triton X-100, blocked in 5% bovine serum albumin, and incubated with primary antibodies, followed by secondary fluorescent antibodies in 1% bovine serum albumin. Slides were mounted and analyzed by fluorescent microscopy.
Western blot analysis
Lysed platelet pellets were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (GE Healthcare). Membranes were blocked and probed with a mouse anti–rat IL-1α antibody (2 μg/mL; R&D Systems). Antibody-antigen complexes were detected by incubating membranes with horseradish peroxidase-conjugated anti–mouse secondary antibodies (Dako United Kingdom).
Flow cytometry
Freshly isolated rat platelets or fixed/permeabilized platelets were incubated with mouse anti–rat IL-1α (40 μg/mL, R&D Systems) or mouse IgG1 isotype control (R&D Systems) for 10 minutes at room temperature, and then with donkey anti–mouse allophycocyanin-conjugated secondary antibody (10 μg/mL; eBioscience) for 10 minutes at room temperature. Platelets were then counterstained with hamster anti–rat CD61-fluorescein isothiocyanate (FITC; 25 μg/mL, BD Biosciences) for 10 minutes at room temperature. A hamster IgG1-FITC isotype control antibody did not label platelets. Flow cytometric analysis was performed on a FACSCalibur (BD Biosciences).
Neutrophil TEM assay
Murine bone marrow-derived neutrophils were isolated and added to the luminal (top) compartment of each transwell. Twenty-four hours later, migrated neutrophils were collected and cell number counted by 2 individual analyzers, blinded to treatment conditions using a hemocytometer.
Statistical analysis
Data are expressed as mean plus or minus SEM from at least 3 independent cultures and analyzed using Student t test or 1- or 2-way analysis of variance with a Bonferroni post hoc test. P less than .05 was considered to be statistically significant.
Results
Platelets localize to activated cerebral microvessels in ischemic mouse brain and mediate activation of brain endothelium through expression of IL-1α
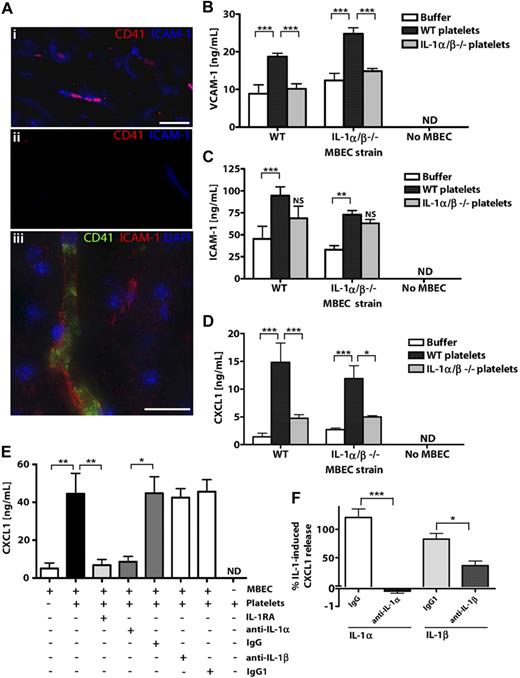
To determine whether platelets may contribute to brain endothelial activation in response to in vivo central nervous system insults, we studied platelet-endothelial interactions in mice exposed to experimental stroke using immunocytochemistry (Figure 1A). CD41-positive platelets were detected in activated ICAM-1-positive cerebral microvessels (vessel diameter ∼ 5-10 μm) of the ischemic cerebral hemisphere 24 hours after occlusion of the middle cerebral artery (Figure 1Ai). Immunodetection of CD41 or ICAM-1 was not observed in the nonischemic, contralateral cerebral hemisphere (Figure 1Aii). When visualized at higher resolution, CD41-positive platelet membranes were seen to line the lumen of the cerebral microvessels (Figure 1Aiii). Ischemic cerebral microvessels containing CD41 platelets also expressed the neutrophil chemoattractant CXCL1 (supplemental Figure 1A). We also noted that IL-1α/β–deficient mice had markedly reduced cerebral CXCL1 levels in response to cerebral ischemia (supplemental Figure 1B). This suggested a potential role for IL-1α/β and platelets in mediating cerebral endothelial activation in mice exposed to cerebral ischemia.
Platelets localize to microvessels in response to cerebral ischemia and activate brain endothelium via IL-1α. CD41-positive platelets localized to ICAM-1-positive brain microvessels (Ai,Aiii) in ischemic cerebral hemispheres, but not in nonischemic, contralateral tissue (Aii) of mice exposed to focal, transient middle cerebral artery occlusion. Scale bar represents 40 μm (Ai-Aii) or 20 μm (iii). Images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and Texas Red/DAPI filter sets and captured using a CoolSNAP ES camera (Photometrics) through MetaVue Software (Molecular Devices; Ai-Aii) or on a Delta Vision RT (Applied Precision) restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (89000, Chroma; Aiii). The images were collected using a CoolSNAP HQ (Photometrics) camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using the softWoRx software (Applied Precision), and maximum intensity projections of these deconvolved images are shown (Aiii). VCAM-1 (B), ICAM-1 (C), or CXCL1 (D-E) levels in lysates (B-C) or supernatants (D-E) of MBECs after incubation with mouse WT or IL-1α/β −/− platelets (B-D) or rat platelets (E) were quantified by ELISA. The effects of antagonizing IL-1 actions with IL-1RA or neutralizing antibodies to IL-1α/β (or isotype controls) on the release of CXCL1 from MBECs in response to rat platelets (E) or recombinant IL-1α or IL-1β (F) were determined. B, from left to right: n = 4, 6, 4, 4, 6, 3, 5, 5, 4; C, n = 6, 8, 4, 7, 8, 3, 5, 5, 4; D, n = 3; E, n = 6, 6, 5, 4, 4, 4, 4, 3; F, n = 5, 5, 6, 6. ND indicates not detected. Data are mean ± SEM. (B-F) ***P < .001. **P < .01. *P < .05. NS indicates no significant difference versus respective buffer control.
Platelets localize to microvessels in response to cerebral ischemia and activate brain endothelium via IL-1α. CD41-positive platelets localized to ICAM-1-positive brain microvessels (Ai,Aiii) in ischemic cerebral hemispheres, but not in nonischemic, contralateral tissue (Aii) of mice exposed to focal, transient middle cerebral artery occlusion. Scale bar represents 40 μm (Ai-Aii) or 20 μm (iii). Images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and Texas Red/DAPI filter sets and captured using a CoolSNAP ES camera (Photometrics) through MetaVue Software (Molecular Devices; Ai-Aii) or on a Delta Vision RT (Applied Precision) restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (89000, Chroma; Aiii). The images were collected using a CoolSNAP HQ (Photometrics) camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using the softWoRx software (Applied Precision), and maximum intensity projections of these deconvolved images are shown (Aiii). VCAM-1 (B), ICAM-1 (C), or CXCL1 (D-E) levels in lysates (B-C) or supernatants (D-E) of MBECs after incubation with mouse WT or IL-1α/β −/− platelets (B-D) or rat platelets (E) were quantified by ELISA. The effects of antagonizing IL-1 actions with IL-1RA or neutralizing antibodies to IL-1α/β (or isotype controls) on the release of CXCL1 from MBECs in response to rat platelets (E) or recombinant IL-1α or IL-1β (F) were determined. B, from left to right: n = 4, 6, 4, 4, 6, 3, 5, 5, 4; C, n = 6, 8, 4, 7, 8, 3, 5, 5, 4; D, n = 3; E, n = 6, 6, 5, 4, 4, 4, 4, 3; F, n = 5, 5, 6, 6. ND indicates not detected. Data are mean ± SEM. (B-F) ***P < .001. **P < .01. *P < .05. NS indicates no significant difference versus respective buffer control.
Using in vitro cultures, we determined whether such interactions were responsible for the activation of brain endothelium and, subsequently the mechanisms involved. We isolated platelets and MBECs from wild-type (WT) and IL-1α/β–deficient (IL-1α/β−/−) mice.
Application of WT murine platelets to WT or IL-1α/β−/− MBECs induced the expression of VCAM-1 (Figure 1B) and ICAM-1 (Figure 1C) in cellular lysates and induced the release of CXCL1 (Figure 1D). However, platelets lacking IL-1α/β failed to induce the expression of VCAM-1 (Figure 1B), ICAM-1 (Figure 1C), or release of CXCL1 (Figure 1D) from WT or IL-1α/β−/− MBECs. Thus, platelet-induced activation of MBECs was dependent on platelet expression of IL-1α/β, but not on the expression of IL-1α/β in MBECs. VCAM, ICAM-1, and CXCL1 were undetectable in lysates or conditioned media of platelets in the absence of MBECs (Figure 1B-D).
We also isolated platelets from rats, which provided a greater platelet yield and allowed the collection of significant quantities of platelet-CM for later studies (Figure 3). Application of rat platelets to MBECs induced significant release of CXCL1, which was inhibited (96%) by pretreatment of MBECs with IL-1RA (Figure 1E). We used neutralizing antibodies to IL-1α or IL-1β to determine the relative contributions of IL-1α and IL-1β to platelet-induced MBEC activation. Platelet-induced MBEC activation was dramatically inhibited (91%) by coapplication of a neutralizing antibody to IL-1α (Figure 1E). The isotype control antibody or a neutralizing antibody to IL-1β had no effect on CXCL1 release, and CXCL1 was not detected in the medium from rat platelets conditioned in the absence of MBECs (Figure 1E). As a control, the neutralizing antibodies to IL-1α or IL-1β induced significant inhibition of recombinant IL-1α or IL-1β-induced CXCL1 release in MBECs (Figure 1F). These experiments demonstrate that IL-1α (and not IL-1β) is the predominant IL-1 isoform responsible for platelet-induced brain endothelial activation.
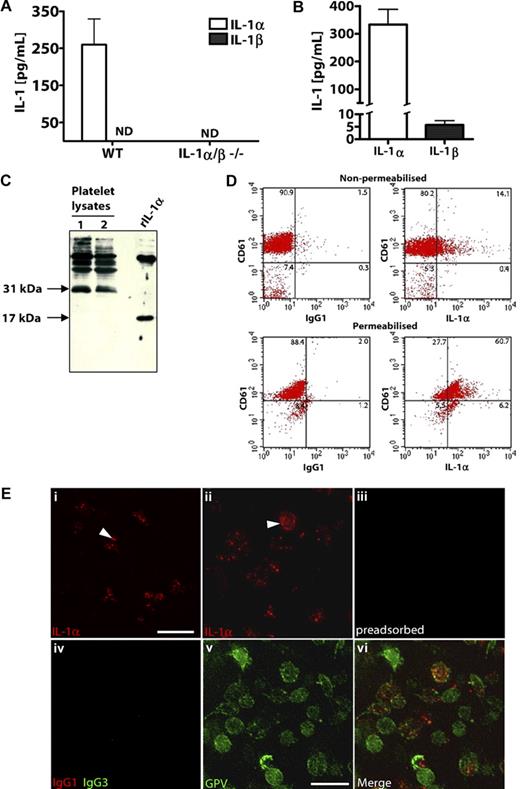
Rodent platelets express high levels of IL-1α
We studied IL-1 expression in mouse and rat platelets isolated from whole blood. Lysates from freshly isolated WT (but not IL-1α/β−/−) mouse platelets contained significant amounts of IL-1α but undetectable levels of IL-1β (Figure 2A). Similarly, rat platelets contained higher concentrations of IL-1α protein compared with IL-1β (Figure 2B). When assessed by Western blot analysis, a band at 31 kDa was detected in rat platelet lysates, corresponding to the biologically active IL-1α pro-form (Figure 2C lanes 1 and 2). Recombinant rat IL-1α was detected at 17 kDa (Figure 2C). IL-1α can exist as both an intracellular and membrane-bound, surface-associated protein.31-33 When analyzed by flow cytometry, 15% of nonpermeabilized platelets (as identified by CD61 staining) stained positively with anti–IL-1α antibodies (but not IgG1 isotype control antibodies; Figure 2D top panel). After permeabilization, 69% of platelets were IL-1α-positive (Figure 2D bottom panel). Platelet IL-1α expression was further confirmed using immunocytochemistry (Figure 2E). Punctate staining of IL-1α was visible in resting platelets (Figure 2Ei arrowhead), and we also detected some platelets with surface-associated IL-1α (Figure 2Eii arrowhead). Immunodetection of IL-1α in platelets was lost when the anti–IL-1α antibody was first preadsorbed with 50 μg/mL recombinant IL-1α (Figure 2Eiii). Furthermore, when an isotype control antibody (IgG1) was used in place of the anti–IL-1α antibody, no platelet staining was detected (Figure 2Eiv). To confirm that the IL-1α expression was specifically platelet-associated, we costained platelets with an antibody to the platelet-specific surface GPV. Platelets stained positively with an anti-GPV antibody (Figure 2Ev) but not an IgG3 isotype control antibody (Figure 2Eiv), and this localized to regions of IL-1α staining (Figure 2Evi, merge of 2Eii and 2Ev).
Platelets express higher levels of IL-1α compared with IL-1β. IL-1α and IL-1β levels in platelet lysates from WT or IL-1α/β−/− mice (A), or rats (B) were quantified by ELISA immediately after isolation. Lysates of rat platelets (lanes 1 and 2) or recombinant rat IL-1α (rIL-1α) were analyzed by Western blot for IL-1α (C). Flow cytometric dot blots of 2-color fluorescence staining of rat platelets with anti–IL-1α (or IgG1) followed by anti–mouse allophycocyanin-conjugated antibody and anti–CD61-FITC (D). Immunostaining for flow cytometry was performed on nonpermeabilized (D top) or permeabilized (D bottom) rat platelets, and numbers in corners indicate percentage of population in each quadrant (D). Platelets were immunostained with anti–IL-1α antibody, without (Ei,Eii) or with (Eiii) preadsorption of the antibody with recombinant IL-1α. Platelets were immunostained with anti-GPV antibody (Ev), which localized to regions of IL-1α staining (Evi, merge of Eii and Ev). Isotype control antibodies for anti–IL-1α (IgG1) or anti-GPV (hamster IgG3) did not stain platelets (Eiv). Scale bars represent 5 μm. Images were acquired on a Delta Vision RT restoration microscope using a 100×/1.4 Plan Apo objective, 1.6× auxiliary magnification, and the Sedat filter set (E). The images were collected using a CoolSNAP HQ (Photometrics) camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using the softWoRx software, and maximum intensity projections of these deconvolved images are shown (E). A, n = 3; B, from left to right n = 6, 7. ND indicates not detected. Data are mean ± SEM and representative of at least 3 independent cultures.
Platelets express higher levels of IL-1α compared with IL-1β. IL-1α and IL-1β levels in platelet lysates from WT or IL-1α/β−/− mice (A), or rats (B) were quantified by ELISA immediately after isolation. Lysates of rat platelets (lanes 1 and 2) or recombinant rat IL-1α (rIL-1α) were analyzed by Western blot for IL-1α (C). Flow cytometric dot blots of 2-color fluorescence staining of rat platelets with anti–IL-1α (or IgG1) followed by anti–mouse allophycocyanin-conjugated antibody and anti–CD61-FITC (D). Immunostaining for flow cytometry was performed on nonpermeabilized (D top) or permeabilized (D bottom) rat platelets, and numbers in corners indicate percentage of population in each quadrant (D). Platelets were immunostained with anti–IL-1α antibody, without (Ei,Eii) or with (Eiii) preadsorption of the antibody with recombinant IL-1α. Platelets were immunostained with anti-GPV antibody (Ev), which localized to regions of IL-1α staining (Evi, merge of Eii and Ev). Isotype control antibodies for anti–IL-1α (IgG1) or anti-GPV (hamster IgG3) did not stain platelets (Eiv). Scale bars represent 5 μm. Images were acquired on a Delta Vision RT restoration microscope using a 100×/1.4 Plan Apo objective, 1.6× auxiliary magnification, and the Sedat filter set (E). The images were collected using a CoolSNAP HQ (Photometrics) camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using the softWoRx software, and maximum intensity projections of these deconvolved images are shown (E). A, n = 3; B, from left to right n = 6, 7. ND indicates not detected. Data are mean ± SEM and representative of at least 3 independent cultures.
To ascertain the significance of IL-1α protein expression by platelets with respect to IL-1β and additional blood cell types, we measured IL-1α and IL-1β levels in unstimulated lymphocyte/monocyte- or granulocyte-enriched cell populations and compared these with IL-1α/β levels in freshly isolated platelets from naive mouse blood (supplemental Figure 2). Levels of platelet IL-1α expression were 3.6-fold higher compared with the levels expressed by lymphocyte-monocyte populations (supplemental Figure 2). We could not detect IL-1α in granulocyte-enriched cell populations (supplemental Figure 2).
Platelet-released IL-1α induces brain endothelial activation
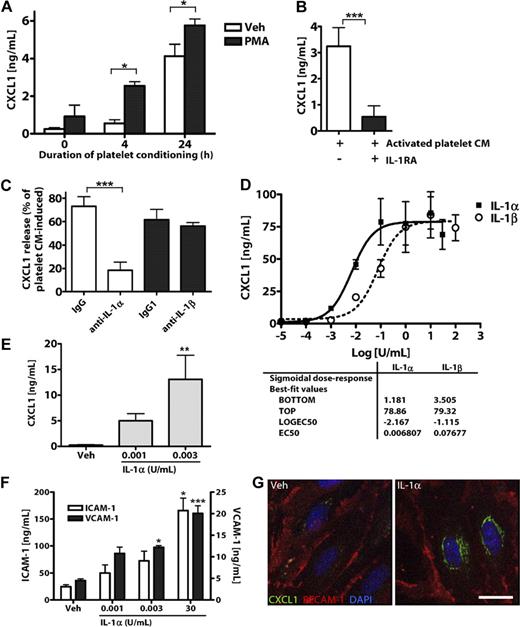
To determine whether platelet-induced endothelial activation is mediated by IL-1α secreted from platelets, we collected CM from platelets stimulated with phorbol myristate acetate (PMA) or vehicle (ethanol, 1%) for 4 to 24 hours. We then applied this CM to cultures of MBECs and used MBEC CXCL1 release as a measure of IL-1 activity. Treatment of MBECs with CM from platelets stimulated with PMA for 4 or 24 hours induced significant release of CXCL1 (Figure 3A). Buffer with or without PMA did not significantly affect CXCL1 secretion from MBECs (0 hours condition, Figure 3A).
IL-1α is released from activated platelets and potently induces brain endothelial CXCL1 release and ICAM-1 and VCAM-1 expression. (A) The effects of CM from resting (vehicle/ethanol-treated) or PMA-activated platelets on the release of CXCL1 from MBECs. The effects of IL-1RA (B) or coapplication of neutralizing antibodies to IL-1α or IL-1β on activated platelet CM-induced CXCL1 release from MBECs (C). Rat brain endothelial cell line GPNT (D), or primary cultures of MBECs (E-G) were treated with vehicle, IL-1α, or IL-1β (0-100 U/mL). Twenty-four hours later (D-F), the CM was assayed for CXCL1 (D-E) or the cells lysed and assayed for ICAM-1 and VCAM-1 (F) by ELISA. (G) Four hours after treatment, MBECs were immunostained for CXCL1 and PECAM-1 (scale bar represents 20 μm). Images were acquired on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (G). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (G). A, from left to right: n = 4, 3, 3, 3, 3, 3; B, n = 4, 3; C, n = 5, 5, 6, 6; D, n = 2; E, n = 5, 3, 3; F, ICAM-1 n = 5, 5, 5, 6; VCAM-1, n = 3, 3, 3, 4. Data are mean ± SEM. ***P < .001. **P < .01. *P < .05.
IL-1α is released from activated platelets and potently induces brain endothelial CXCL1 release and ICAM-1 and VCAM-1 expression. (A) The effects of CM from resting (vehicle/ethanol-treated) or PMA-activated platelets on the release of CXCL1 from MBECs. The effects of IL-1RA (B) or coapplication of neutralizing antibodies to IL-1α or IL-1β on activated platelet CM-induced CXCL1 release from MBECs (C). Rat brain endothelial cell line GPNT (D), or primary cultures of MBECs (E-G) were treated with vehicle, IL-1α, or IL-1β (0-100 U/mL). Twenty-four hours later (D-F), the CM was assayed for CXCL1 (D-E) or the cells lysed and assayed for ICAM-1 and VCAM-1 (F) by ELISA. (G) Four hours after treatment, MBECs were immunostained for CXCL1 and PECAM-1 (scale bar represents 20 μm). Images were acquired on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (G). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2 μm, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (G). A, from left to right: n = 4, 3, 3, 3, 3, 3; B, n = 4, 3; C, n = 5, 5, 6, 6; D, n = 2; E, n = 5, 3, 3; F, ICAM-1 n = 5, 5, 5, 6; VCAM-1, n = 3, 3, 3, 4. Data are mean ± SEM. ***P < .001. **P < .01. *P < .05.
To determine whether this response of MBECs was mediated by IL-1α, we tested the effect of IL-1 inhibitors on activated platelet CM (PMA, 4 hours)–induced CXCL1 release from MBECs. Pretreatment of MBECs with IL-1RA inhibited platelet CM-induced CXCL1 release (Figure 3B). Coapplication of a neutralizing antibody to IL-1α (but not an isotype control, or an antibody to IL-1β) caused a significant inhibition of platelet CM-induced MBEC CXCL1 release (Figure 3C). PMA induced the secretion of small amounts of IL-1α from rat platelets (compared with vehicle-treated platelets), as assessed by ELISA (supplemental Figure 4).
When analyzed by cytometric bead array assay, IL-1α (but not IL-1β) levels in MBEC supernatants increased after application of WT mouse platelets (but not IL-1α/β−/− platelets; supplemental Figure 3A-B). Increases in IL-1α in response to WT platelet addition to MBEC cultures correlated with MBEC secretion of CXCL1 (supplemental Figure 3C). No correlation was observed between levels of IL-1β in platelet-MBEC cultures and MBEC secretion of CXCL1 (supplemental Figure 3C), indicating that IL-1α is the predominant platelet-derived IL-1 isoform driving brain endothelial activation.
To determine whether brain endothelial cells respond to low concentrations of IL-1α (and explaining their response to platelet CM, Figure 3A), we calculated half-maximal effective concentration values for IL-1α– and IL-1β–induced CXCL1 secretion. Treatment of the rat brain endothelial cell line GPNT with IL-1α or IL-1β for 24 hours induced significant release of the chemokine CXCL1, as quantified by ELISA (Figure 3D). IL-1α was an extremely potent inducer of CXCL1 release from GPNT cells, with a half-maximal effective concentration of 0.007 U/mL (3.2pM). Similarly, in primary cultures of MBECs, extremely low concentrations of IL-1α (0.003 U/mL, 1.4pM) induced significant CXCL1 release (Figure 3E). IL-1α also induced MBEC expression of the cell adhesion molecules ICAM-1 and VCAM-1 at concentrations as low as 0.003 U/mL (Figure 3F). Immunofluorescent staining of MBEC cultures with an endothelial-specific anti–PECAM-1 antibody confirmed that IL-1α specifically induced endothelial CXCL1 expression (Figure 3G).
Platelet-derived IL-1α drives neutrophil TEM
Cerebrovascular activation is associated with neutrophil brain infiltration.1 We determined whether platelet-secreted IL-1α alone was sufficient to mediate neutrophil TEM across primary cultures of MBECs in vitro. CM from resting platelets induced a small, nonsignificant increase in TEM that could be reversed by pretreatment of MBECs with IL-1RA (Figure 4A). Treatment of MBECs with CM from PMA-activated platelets induced a marked increase in neutrophil TEM, which was abolished by pretreatment of MBECs with IL-1RA (Figure 4A). Addition of PMA did not affect the basal migration of neutrophils across MBECs (Figure 4A). After stimulation with platelet CM, MBEC transwells were washed twice before the addition of neutrophils. Therefore, we conclude that brain endothelial cells are the target for platelet-derived IL-1α, and their activation induces the TEM of neutrophils. As a control, recombinant IL-1α induced significant neutrophil TEM, as seen by light microscopy and cell counting (Figure 4B-C).
Platelet-derived IL-1α drives neutrophil TEM. (A) The effects of CM from resting platelets or PMA-activated platelets (± IL-1RA) on neutrophil TEM. The effects of recombinant IL-1α (0.003-30 U/mL) on neutrophil TEM (B, light microscope images of migrated neutrophils in abluminal transwell compartment of MBECs in response to treatment with vehicle or 30 U/mL IL-1α, scale bar represents 40 μm; C, quantification). Brightfield images were collected on an Olympus CKX31 cell culture microscope (Olympus) equipped with a 40×/0.55 objective and a Motic 2300 digital camera and Motic imaging software (Motic; B). Immunodetection of SJC-positive neutrophils in, or in close proximity to (arrowheads), ICAM-1-positive brain vessels in mice exposed to cerebral ischemia (Di; and Dii, high magnification). CD41-positive platelets are shown in red (scale bars represent 20 μm). Fluorescent images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and FITC, Texas Red, and DAPI filter sets and captured using a CoolSNAP ES camera through MetaVue Software (Di) or on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (Dii). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2[ mu]m, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (Dii). A, n = 3; C, from left to right, n = 6, 7, 5. Data are mean ± SEM. (A) ***P < .001, **P < .01, vs vehicle. (C) **P < .01 vs vehicle.
Platelet-derived IL-1α drives neutrophil TEM. (A) The effects of CM from resting platelets or PMA-activated platelets (± IL-1RA) on neutrophil TEM. The effects of recombinant IL-1α (0.003-30 U/mL) on neutrophil TEM (B, light microscope images of migrated neutrophils in abluminal transwell compartment of MBECs in response to treatment with vehicle or 30 U/mL IL-1α, scale bar represents 40 μm; C, quantification). Brightfield images were collected on an Olympus CKX31 cell culture microscope (Olympus) equipped with a 40×/0.55 objective and a Motic 2300 digital camera and Motic imaging software (Motic; B). Immunodetection of SJC-positive neutrophils in, or in close proximity to (arrowheads), ICAM-1-positive brain vessels in mice exposed to cerebral ischemia (Di; and Dii, high magnification). CD41-positive platelets are shown in red (scale bars represent 20 μm). Fluorescent images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and FITC, Texas Red, and DAPI filter sets and captured using a CoolSNAP ES camera through MetaVue Software (Di) or on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (Dii). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2[ mu]m, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (Dii). A, n = 3; C, from left to right, n = 6, 7, 5. Data are mean ± SEM. (A) ***P < .001, **P < .01, vs vehicle. (C) **P < .01 vs vehicle.
We next assessed whether platelet accumulation in cerebral microvessels after ischemic brain injury in vivo is associated with neutrophil infiltration into ischemic brain tissue (Figure 4D). SJC-positive neutrophils were associated with, or in close proximity to (arrowheads), activated ICAM-1-positive, CD41 (platelet)-positive cerebral vessels of the ischemic cerebral hemisphere (Figure 4Di and 4Dii, higher magnification).
Discussion
Our data reveal a previously unrecognized pathway of platelet, IL-1α-mediated activation of brain endothelium that leads to the TEM of neutrophils. In vivo, we show that platelets associate with regions of activated cerebral endothelium, which were closely associated with neutrophils in response to cerebral ischemia in mice. This pathway may contribute to inflammation-mediated brain injury (Figure 5).
Platelet IL-1α drives cerebrovascular inflammation. Our data suggest that platelet-derived IL-1α induces the activation of cerebrovascular endothelial cells. The expression of brain endothelial cell adhesion molecules (CAMs) and release of the chemokine CXCL1 are induced by platelet IL-1α. In addition, platelet-derived IL-1α supports the TEM of neutrophils. We propose that platelet IL-1α is a key contributor to cerebrovascular inflammation, permitting the brain infiltration of neurotoxic white blood cells.
Platelet IL-1α drives cerebrovascular inflammation. Our data suggest that platelet-derived IL-1α induces the activation of cerebrovascular endothelial cells. The expression of brain endothelial cell adhesion molecules (CAMs) and release of the chemokine CXCL1 are induced by platelet IL-1α. In addition, platelet-derived IL-1α supports the TEM of neutrophils. We propose that platelet IL-1α is a key contributor to cerebrovascular inflammation, permitting the brain infiltration of neurotoxic white blood cells.
Expression of inflammatory cytokines, in particular IL-1, is critical to the pathogenesis of diverse brain diseases. We and others have shown that inhibition of IL-1 actions is dramatically neuroprotective in rodent models of cerebral ischemia.34-36 In addition, we have shown that IL-1α/β–deficient mice have markedly reduced damage in response to cerebral ischemia.27 However, the cellular source and target of IL-1 in these diseases are unknown.37 Recent studies using endothelial IL-1 receptor-deficient mice demonstrate that the endothelium is key to IL-1 responses in the brain, including the induction of leukocyte infiltration.38 The strong evolutionary link between coagulation and the innate immune response is recognized in vascular pathology,19 but how these processes affect the progression of brain injury is not established. Platelet-brain endothelial interactions occur early in the onset of brain diseases, including cerebral ischemia39,40 and cerebral malaria,17 suggesting they may drive cerebrovascular activation.
We show that exposure of brain endothelial cells to mouse or rat platelets induces chemokine secretion and cell adhesion molecule expression, which are critical components of cerebrovascular inflammation. Application of murine WT platelets to brain endothelial cells from WT or IL-1α/β–deficient mice induced expression of CAMs and secretion of CXCL1. In contrast, platelets from IL-1α/β–deficient mice failed to induce CAM/CXCL1 expression. Increased IL-1α (but not IL-1β) levels in brain endothelial cell cultures after application of WT platelets correlated with the extent of endothelial activation. We show that murine platelets contained high concentrations of IL-1α but undetectable levels of IL-1β. Similarly, IL-1α levels were higher than the levels of IL-1β in rat platelets. Accordingly, a neutralizing antibody to IL-1α (but not IL-1β) completely inhibited the platelet-induced brain endothelial induction of CAM/CXCL1. We identify platelets to be a major source of IL-1α, having higher basal IL-1α protein levels compared with other blood cell types, including leukocyte-monocyte– or granulocyte-enriched cell populations. These studies show that platelet-derived IL-1α is a critical mediator of cerebrovascular inflammation.
We show that CM from activated platelets induced brain endothelial activation (as measured by CXCL1 release) and that this effect was abolished by coapplication of a neutralizing antibody to IL-1α (but not to IL-1β). The extent of endothelial activation induced by the platelet CM was lower compared with the direct application of platelets to endothelium. Indeed, levels of IL-1α in platelet CM were low, and some IL-1α was retained in platelets even after activation with PMA (supplemental Figure 4). This is in agreement with previous studies demonstrating that IL-1 activity may be retained in activated platelets or platelet membranes.21,24 We show that mouse brain endothelium is very sensitive to IL-1α, with concentrations as low as 0.003 U/mL (1.5pM) inducing CXCL1 secretion and CAM expression (Figure 3D-F). We propose that, although low concentrations of IL-1α are released from platelets on activation, the quantities are sufficient to induce significant activation of brain endothelium. This is supported by our studies showing that the activation of brain endothelium, induced by platelet-CM, is blocked by coapplication of a neutralizing antibody to IL-1α.
Platelets contain constitutive mRNA for IL-1β, rapidly translate the protein during fibrin clot formation, and release soluble and microvesicle-associated IL-1β.41 IL-1α mRNA is detectable at low levels in platelet preparations.21,42 However, it is unlikely that protein synthesis accounts for platelet IL-1α expression in our system, as our novel findings show that freshly isolated platelets possess significant IL-1α protein, which (as demonstrated by Western blot) is largely of the bioactive pro-form.
The mechanisms by which platelet-endothelial cell-cell binding occurs, particularly with respect to large-vessel disease, have been well documented.43 However, our study demonstrates that this interaction is not a prerequisite for platelet-induced brain endothelial activation. CM from activated platelets induced neutrophil TEM, suggesting that local release of platelet IL-1α could mediate cerebrovascular inflammation in vivo. Indeed, microvascular thrombosis is induced by cerebral ischemia,39,40 and platelet rolling along the cerebrovasculature precedes leukocyte adhesion in brain microvessels in response to systemic inflammatory challenge.44
Additional platelet-derived factors can induce activation of extracerebral endothelium, including CD40L.20,45 The expression of CD40L is induced by systemic inflammatory bowel disease45 or cerebral ischemia46 in humans. Thus, it is possible that the IL-1α–dependent mechanism of platelet-mediated brain endothelial activation we identify is augmented by systemic inflammatory processes or ischemia. However, we show that platelet-secreted IL-1α is capable of driving endothelial activation, whereas CD40L-mediated endothelial activation is thought to be dependent on direct platelet-endothelial cell-cell contact.20,45 Given the unique characteristics of brain endothelial expression of, and response to, inflammatory mediators,47-49 further studies are required to determine whether and how platelet CD40L influences brain endothelial inflammation.
We are the first to demonstrate that platelet-derived IL-1α (but not IL-1β) drives cerebrovascular activation leading to the TEM of neutrophils, which are known mediators of brain injury. The contribution of cerebral IL-1 to ischemic brain injury is well established in experimental studies.37 The present data suggest that interventions to target IL-1 systemically (to inhibit platelet-derived IL-1α, at the platelet-brain endothelial interface) may have significant benefit in central nervous system disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joel Pachter (University of Connecticut Health Center) for advice with the preparation of MBECs, Jane Kott and Robert Fernandez (Bioimaging Facility, Faculty of Life Sciences, University of Manchester, Manchester, United Kingdom) for their help with the microscopy, Drs Sandra Campbell and Daniel Anthony (University of Oxford, Oxford, United Kingdom) for the antineutrophil SJC antibody, and Prof John Greenwood (University College London, London, United Kingdom) for the GPNT rat brain endothelial cell line.
This work was supported by the Medical Research Council, United Kingdom.
Authorship
Contribution: P.T. designed research; P.T., B.W.M., A.G., and A.D. performed research; P.T. and A.D. analyzed data; S.M.A. and N.J.R. contributed new reagents and analytical tools and oversaw the study; and P.T., S.M.A., and N.J.R. wrote the paper.
Conflict-of-interest disclosure: N.J.R. is a nonexecutive director of AstraZeneca, but the company had no involvement in this work. The remaining authors declare no competing financial interests.
Correspondence: Stuart M. Allan, AV Hill Bldg, Oxford Rd, Manchester, M13 9PT, United Kingdom; e-mail: stuart.allan@manchester.ac.uk.


![Figure 4. Platelet-derived IL-1α drives neutrophil TEM. (A) The effects of CM from resting platelets or PMA-activated platelets (± IL-1RA) on neutrophil TEM. The effects of recombinant IL-1α (0.003-30 U/mL) on neutrophil TEM (B, light microscope images of migrated neutrophils in abluminal transwell compartment of MBECs in response to treatment with vehicle or 30 U/mL IL-1α, scale bar represents 40 μm; C, quantification). Brightfield images were collected on an Olympus CKX31 cell culture microscope (Olympus) equipped with a 40×/0.55 objective and a Motic 2300 digital camera and Motic imaging software (Motic; B). Immunodetection of SJC-positive neutrophils in, or in close proximity to (arrowheads), ICAM-1-positive brain vessels in mice exposed to cerebral ischemia (Di; and Dii, high magnification). CD41-positive platelets are shown in red (scale bars represent 20 μm). Fluorescent images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and FITC, Texas Red, and DAPI filter sets and captured using a CoolSNAP ES camera through MetaVue Software (Di) or on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (Dii). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2[ mu]m, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (Dii). A, n = 3; C, from left to right, n = 6, 7, 5. Data are mean ± SEM. (A) ***P < .001, **P < .01, vs vehicle. (C) **P < .01 vs vehicle.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/17/10.1182_blood-2009-11-252643/4/m_zh89991052110004.jpeg?Expires=1767981576&Signature=HSMMqGqrQkbVhP0V6cKHxtdN4FGt4KEZeXNOxOBe-FfSwDqlsT1Ch9anH6Ox3viwX1ld6IaZWmEu7Nx~9V9HiQyKW4Dool1ICKp6IBRJXU0WvUpBoZJstOzIi2OuEQ1LVEVauIABLPvqrg6mGmyRgSKdmF7GpLEDO8-o2882BZdoaqyctZKd3zNvXZcaH4KXIp2HlyKkbXiYwkFGu8bvCgtsrqAXfVw8MDyoeCEKpD3cVM3n9cPKDlX3K3Ur9IU9NAyAlNW2v08p2ktasTOIrWk~-JaM~nW1mC7dVbsAFbNuYZs5YoVCJsm-H71w2ffK8vmI0oSKaA6ia2H8Y3~XJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 4. Platelet-derived IL-1α drives neutrophil TEM. (A) The effects of CM from resting platelets or PMA-activated platelets (± IL-1RA) on neutrophil TEM. The effects of recombinant IL-1α (0.003-30 U/mL) on neutrophil TEM (B, light microscope images of migrated neutrophils in abluminal transwell compartment of MBECs in response to treatment with vehicle or 30 U/mL IL-1α, scale bar represents 40 μm; C, quantification). Brightfield images were collected on an Olympus CKX31 cell culture microscope (Olympus) equipped with a 40×/0.55 objective and a Motic 2300 digital camera and Motic imaging software (Motic; B). Immunodetection of SJC-positive neutrophils in, or in close proximity to (arrowheads), ICAM-1-positive brain vessels in mice exposed to cerebral ischemia (Di; and Dii, high magnification). CD41-positive platelets are shown in red (scale bars represent 20 μm). Fluorescent images were acquired on a Olympus BX51 microscope using a 40×/0.75 Plan Fln objective and FITC, Texas Red, and DAPI filter sets and captured using a CoolSNAP ES camera through MetaVue Software (Di) or on a Delta Vision RT restoration microscope using a 40×/1.3 Plan Apo objective and the Sedat filter set (Dii). The images were collected using a CoolSNAP HQ camera with a Z optical spacing of 0.2[ mu]m, raw images were deconvolved using softWoRx software, and maximum intensity projections of these deconvolved images are shown (Dii). A, n = 3; C, from left to right, n = 6, 7, 5. Data are mean ± SEM. (A) ***P < .001, **P < .01, vs vehicle. (C) **P < .01 vs vehicle.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/17/10.1182_blood-2009-11-252643/4/m_zh89991052110004.jpeg?Expires=1768691661&Signature=VqV8FM6ZIczjU~rN387I3~-qvex8bN~d5raejfwCAEUcFINPN~46tvxCpahmFnpmje1ev7oO8aCQyrU9R1LGWqp8S2uc1uF1256-jaPQN7HKsGY4yKHvNBrqngxGv5~u94QmleQvXZroG~J8nO7I75RIjCJvVwnoBR8POFOzeyxcVTSyhxmkGYBTtSTCujzl0HuId-WiVKKjaYSTxbQej3YZPDGqkBDUWuqyljv5DQhSZL4WIQvoMOidf0~4luqbH9548q2jbtsim8qzDsTwAHEaEPwtobAXI1zYJNezZ4X~4jJGlty9~vGzu7uUyUB7zPVwFOAUqtq8iKEw3fd6Yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)