In this issue of Blood, Mumford and colleagues report a D304N variant of the thromboxane A2 receptor (TxA2 R) in a patient with a bleeding diathesis.1 In their report, they describe the exciting path of a research journey initiated by a clinical observation, and leading to a discovery of a novel mechanism of a bleeding disorder: a mutation in the TxA2 R gene, leading to a D304N substitution. This mutation leads to a loss of function of the receptor due to reduced ligand binding.
Searching for the cause of a bleeding disorder may become quite frustrating in patients who have normal screening tests of coagulation and platelet function. Many such cases are left with the presumptive diagnosis of an “undefined bleeding disorder,” with general hemostatic support as the only therapeutic modality.
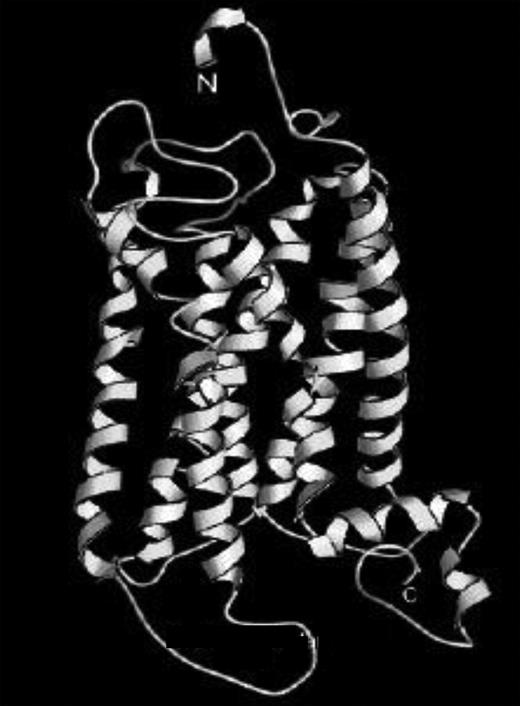
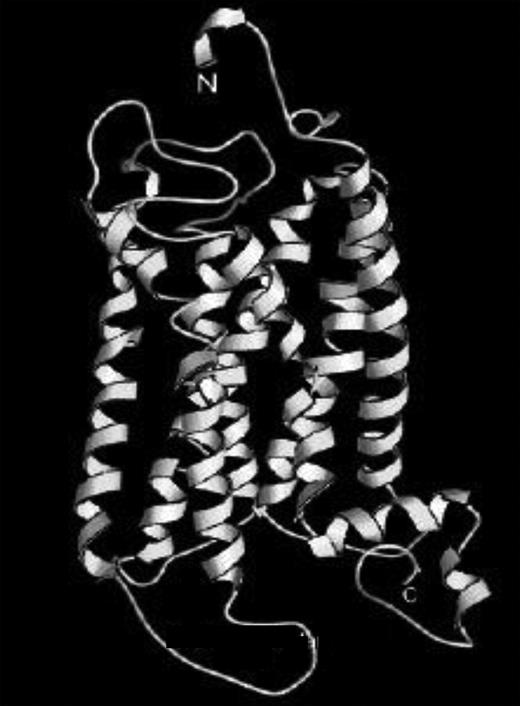
Computed 3-dimensional structure of the human TxA2 receptor. Adapted from Chou.6
Computed 3-dimensional structure of the human TxA2 receptor. Adapted from Chou.6
Our current screening tools for investigating primary hemostasis-related bleeding disorders are limited to platelet aggregometry (usually induced by ADP, epinephrine, collagen, ristocetin, and arachidonic acid), as well as testing von Willebrand factor antigen and activity. This limitation explains the quite frequent event of a “final” diagnosis of undefined primary hemostatic disorder. Only highly qualified centers where more specific tests are available may take on the task of further evaluation. The platelet physiology subcommittee of the International Society of Thrombosis and Haemostasis (ISTH) has dedicated its work during recent years to searching for ways to improve and standardize platelet function testing, applying platelet aggregometry, the PFA-100 device, and a proteomics approach.2-4
This report also raises the issue of the gap between genotype and phenotypic expression in hereditary diseases. The D304N substitution was observed in the index patient, who presented with mild bleeding symptoms, and in his father, who had no history of bleeding. As in other cases, a search for modifier genes and for other factors might shed light on this differential phenotypic expression. Thus, the genetic background of a disease does not always explain all phenotypes, and a search for genetic and environmental modifiers should be considered for a more comprehensive understanding of this entity.
Improved understanding of the complex, multiple pathways of platelet activation is significantly contributing to the development of new antiplatelet drugs. Antiplatelet drug therapy is currently undergoing a dramatic revolution, including novel observations on platelet drug resistance, more refined dose adjustment, and development of more potent and safer new drugs.5 One such example is the development of new TxA2 R inhibitors. Such inhibitors are potentially more effective than aspirin because of their inhibitory effect on endothelial cell TxA2 R as well.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Authorship
Acknowledgment: The author thanks Dr D. Rund for helpful suggestions.