Abstract
The role of c-Jun NH2-terminal kinase 1 (JNK1) in hemostasis and thrombosis remains unclear. We show here, with JNK1-deficient (JNK1−/−) mice, that JNK1 plays an important role in platelet biology and thrombus formation. In tail-bleeding assays, JNK1−/− mice exhibited longer bleeding times than wild-type mice (396 ± 39 seconds vs 245 ± 32 seconds). We also carried out in vitro whole-blood perfusion assays on a collagen matrix under arterial shear conditions. Thrombus formation was significantly reduced for JNK1−/− platelets (51%). In an in vivo model of thrombosis induced by photochemical injury to cecum vessels, occlusion times were 4.3 times longer in JNK1−/− arterioles than in wild-type arterioles. Moreover, in vitro studies carried out in platelet aggregation conditions demonstrated that, at low doses of agonists, platelet secretion was impaired in JNK1−/− platelets, leading to altered integrin αIIbβ3 activation and reduced platelet aggregation, via a mechanism involving protein kinase C. JNK1 thus appears to be essential for platelet secretion in vitro, consistent with its role in thrombus growth in vivo. Finally, we showed that ERK2 and another isoform of JNK affect platelet aggregation through 2 pathways, one dependent and another independent of JNK1.
Introduction
Platelet adhesion at the site of vessel damage is the first step in thrombus formation. It involves interactions of von Willebrand factor (VWF) with platelet glycoprotein (GP) Ib-IX-V, and of collagen with GPVI and integrin α2β1. The adhesion of platelets to subendothelial thrombogenic proteins initiates platelet activation. This activation is associated with a change in platelet shape, granule secretion, thromboxane A2 (TXA2) synthesis, and intracellular “inside-out” signaling events leading to integrin αIIbβ3 activation. The activation of this integrin leads to a change in its conformation, enabling it to bind to ligands, such as fibrinogen and VWF in particular.1 This ligand binding promotes platelet-platelet interactions (platelet aggregation) and thrombus formation. Adenosine 5′-diphosphate (ADP) secretion and TXA2 generation are key events in thrombus dynamics, constituting positive feedback loops that amplify the initial stimulus and stabilize the growing thrombus. In addition, the coordination of several signaling pathways involving protein kinase C (PKC),2 phosphatidylinositol 3-kinase,3 Rap1b,4 talin,5,6 and kindlin-2/37,8 is required for full activation of the integrin αIIbβ3.
Interest has recently focused on mitogen-activated protein kinase (MAP kinase) pathways because of their role in integrin activation in platelets and thrombus formation. MAP kinases constitute a family of serine/threonine protein kinases. Three subgroups of MAP kinases have been defined: extracellular signal-regulated kinases (ERKs), p38, and c-Jun NH2-terminal kinases (JNKs).9,10 ERK2, p38, and JNK1/2 have been shown to be present and activated in platelets.11–14 Previous studies from several groups using MEK and p38 inhibitors have shed light on the roles of ERK and p38 pathways in platelet aggregation, adhesion, thrombus formation, GPIb signaling, and integrin signaling.15–18 In contrast, little is known about either the activation or the role of JNK1 and JNK2 in platelets.19 In human platelets, the JNK1 isoform is activated by thrombin,12 VWF,20 and collagen,21 and JNK1 activation by thrombin is down-regulated by αIIbβ3.12 Our previous studies with inhibitors of all JNK isoforms (JNK1, JNK2, and JNK3) suggested a role for JNKs in platelet adhesion and thrombus formation.21 Chen et al observed the CD36-dependent activation of JNK by oxidized low-density lipoprotein in platelets.14 Thus, although some knowledge has been gained in the role of JNK MAP kinases in platelet activation, the underlying mechanisms remain unclear.
The present study in JNK1-deficient (JNK1−/−) mice provides evidence for a key role of JNK1 in platelet function and thrombosis. These mice display features of unstable hemostasis, including impaired thrombus formation in vitro and in vivo. Further, we demonstrate that (1) JNK1 mediates platelet secretion via a PKC-dependent mechanism, and (2) JNK1 plays an indirect role in integrin αIIbβ3 activation in vitro, consistent with its role in thrombus growth in vivo. We also show that ERK2 and another JNK isoform are complementary to JNK1 in collagen- and thrombin-induced platelet aggregation.
Methods
Reagents and antibodies
Fibrillar collagen (equine type I) and ADP were obtained from Kordia. Bovine serum albumin fraction V was obtained from Euromedex. Apyrase (grade VII), prostaglandin E1, rhodamine 6G, bovine thrombin, MRS2179, indomethacin, and Rose Bengal were obtained from Sigma-Aldrich. We purchased d-Phe-Pro-Arg chloromethylketone dihydrochloride (PPACK), Ro-31-8220, and the pan-JNK inhibitor SP600125 from Calbiochem-VWR. Calcein-AM and the adenosine triphosphate (ATP) determination kit were purchased from Invitrogen. The MEK inhibitor U0126 was purchased from Promega. The protease-activated receptor (PAR4) agonist peptide (AYPGKF-NH2; PAR4-AP) was purchased from Bachem. AR-C69931MX was kindly provided by Dr Christian Gachet (Inserm U949). Phycoerythrin-labeled rat anti–mouse integrin αIIbβ3 monoclonal antibody (mAb; JON/A), fluorescein isothiocyanate (FITC)–labeled rat anti–mouse CD62P (P-selectin) mAb (Wug.E9), FITC-labeled rat anti–mouse GPIbα mAb (Xia.B2), FITC-labeled rat anti–mouse GPVI mAb (JAQ1), and rat anti–mouse integrin αIIbβ3 mAb (Leo.H4) were obtained from Emfret Analytics. FITC-labeled rat anti–mouse integrin αIIb chain (CD41) mAb (MWReg30) was purchased from BD Biosciences. Antiphospho-(Ser) PKC substrate antibody and polyclonal antibodies directed against the phosphorylated form of JNK1/2 and total JNK1/2 were purchased from Cell Signaling Technology. A polyclonal antibody directed against total ERK1/2 was obtained from Upstate Biotechnology. A polyclonal antibody directed against the phosphorylated form of ERK1/2 was obtained from Invitrogen.
Mouse strains
JNK1-deficient (JNK1−/−) mice were generated in the laboratory of Dr R. J. Davis.22 The original line was backcrossed onto a C57BL/6 background for more than 10 generations, yielding congenic C57BL/6 JNK1−/− mice. All experimental procedures were carried out in accordance with European legislation concerning the use of laboratory animals and were approved by the ethical committee of Inserm.
Isolation of mouse platelets
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (60 mg/kg). Xylocaine (0.5% vol/vol) was used as a local analgesic. Whole blood was collected by cardiac puncture and mixed with 80μM PPACK and 10% (vol/vol) ACD-C buffer (124mM sodium citrate, 130mM citric acid, 110mM dextrose, pH 6.5) to prevent coagulation. Platelet-rich plasma was obtained by centrifuging whole blood for 7 minutes at 160g. Platelets were obtained from platelet-rich plasma by centrifugation for 10 minutes at 670g and washed in the presence of apyrase (100 mU/mL) and prostaglandin E1 (1μM) to minimize platelet activation. The numbers of wild-type (WT) and JNK1−/− platelets were adjusted to similar levels in modified Tyrode-HEPES buffer (137mM NaCl, 2mM KCl, 0.3mM NaH2PO4, 5.5mM glucose, 5mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 12mM NaHCO3, 2mM CaCl2, pH 7.3) supplemented with 0.2% bovine serum albumin.
Hematologic analysis and bleeding time
Complete blood counts and hematocrit were determined with an automatic cell counter, using the standard parameters for mice. Bleeding time assays were performed by cutting off the tip of the tail (3 mm from the tip) and immediately immersing it in saline. We then recorded the time taken for the bleeding to stop. Tail bleeding was monitored for at least 60 seconds beyond this time point, to ensure that bleeding did not begin again. Tail bleeding assays were stopped at 600 seconds if the bleeding did not stop.
Electron microscopy
Washed WT or JNK1−/− platelets were incubated for 10 minutes in the presence or absence of 100μM PAR4-AP, at 37°C, without stirring. Platelets were then fixed by incubation for 1 hour at room temperature with 1.25% glutaraldhehyde in 0.1M phosphate buffer, pH 7.2, centrifuged for 10 minutes at 1100g, and washed once in phosphate buffer. Platelets were kept in 0.2% glutaraldehyde at 4°C until processing for standard electron microscopy, as previously described.23
Platelet aggregation
Platelet aggregation was monitored by measuring light transmission through the stirred suspension of washed WT or JNK1−/− platelets (3 × 108/mL) at 37°C using a Chronolog aggregometer. When required, platelets were first incubated with MRS2179 (100μM), AR-C69931MX (10μM), indomethacin (5μM), SP600125 (10μM), U0126 (10μM), Ro-31-8220 (18μM), or dimethyl sulfoxide (DMSO) for 10 minutes at 37°C. Platelet aggregation was triggered by adding fibrillar collagen, ADP, Thrombin, or PAR4-AP. Representative traces for aggregation were obtained from at least 3 independent experiments. Results are expressed as the percentage change in light transmission with respect to the blank (buffer without platelets), set at 100%.
Measurement of platelet dense granule secretion
Dense granule secretion by platelets was evaluated by measuring ATP release with the ATP determination kit. Platelet aggregation was stopped by adding ice-cold ethylenediaminetetraacetic acid (EDTA; 16mM) and centrifuging (12 000g; 1 minute). Supernatants were incubated with recombinant firefly luciferase (0.25 mg/mL) and its substrate, D-luciferin (0.5mM), according to the manufacturer's instructions. Light emission was assessed with a luminometer (Fluoroskan Ascent FL; Thermo LabSystems). Dense granule secretion was determined as a percentage of the maximum amount of ATP released by platelets.
Measurement of TXA2 generation
Thromboxane generation was determined in conditions of platelet aggregation induced by fibrillar collagen or thrombin. Platelet aggregation was stopped after 3 minutes of stimulation, by adding ice-cold EDTA (16mM) and centrifuging samples (12 000g; 1 minute). Supernatants were diluted 1:100 in the standard diluent (assay buffer). Levels of TXB2, the stable metabolite of TXA2, were determined with the Thromboxane B2 Enzyme Immunoassay Kit, following the manufacturer's instructions (Tebu-bio).
Flow cytometric analysis
Washed WT or JNK1−/− platelets (108/mL) were stimulated with a range of agonists, as indicated. After incubation for 10 minutes at 37°C without stirring, platelets were incubated with appropriate fluorophore-conjugated antibodies for 30 minutes at room temperature. The reaction was stopped by adding 2% (vol/vol) paraformaldehyde, and the samples were analyzed with a FACSCalibur flow cytometer (BD Biosciences).
Immunoblotting
Washed WT or JNK1−/− platelets (300 μL; 3 × 108/mL) were stimulated with collagen in an aggregometer. After 3 minutes of stimulation, platelets were lysed in denaturing buffer (50mM Tris, 100mM NaCl, 50mM NaF, 5mM ethylenediaminetetraacetic acid, 40mM β-glycerophosphate, 100μM phenylarsine oxide, 1% sodium dodecyl sulfate, 5 μg/mL leupeptin, 10 μg/mL aprotinin, pH 7.4). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies. Immunoreactive bands were visualized with enhanced chemiluminescence detection reagents (Perbio Science).
In vitro thrombus formation under flow conditions
Thrombus formation was evaluated in a whole-blood perfusion assay on a fibrillar collagen matrix under arterial shear conditions (shear rate of 1500 per seconds−1). Glass microcapillary tubes (Vitrocom Hollow Rectangle capillaries; Fiber Optic Center) were coated with fibrillar collagen (30 μg/mL; overnight at 4°C). Blood samples from 2 mice were collected in 80μM PPACK, pooled together, and fluorescently labeled with rhodamine 6G (10 μg/mL). Labeled whole blood was then perfused through the coated glass microcapillary, at a shear rate of 1500, with a KD Scientific syringe pump (Fisher Bioblock Scientific). Real-time thrombus formation was recorded with an inverted epifluorescence microscope (Nikon Eclipse TE2000-U), coupled to Metamorph 7.0r1 software (Universal Imaging Corporation). Thrombus formation was determined as the mean percentage of the total area covered by thrombi and as the mean integrated fluorescence intensity (IFI) per square micrometer (μm2) of each thrombus, as previously described.24
Photochemically induced thrombosis model
Cecum arterioles and venules were injured with the photoactivable dye Rose Bengal, as previously described.25 Anesthetized mice were catheterized via the jugular vein. Fluorescently labeled platelets (108 platelets) were injected through the catheter, followed by an intravenous bolus of Rose Bengal (20 mg/kg body weight), which was also administered as a continuous infusion (20 mg/kg per hour) throughout the experiment. The intestines of the mice were spread on a microscope slide, and the arterioles and venules lying over the cecum were visualized under an epifluorescence microscope. Injury was induced by exposing the vessels to a wavelength of 540 nm for 30 seconds. The recruitment of fluorescent platelets to the lesion was recorded in real time. Vessel occlusion was defined as blood flow being stopped for at least 1 minute.
Statistical analysis
Results are expressed as mean values plus or minus SEM. Statistical significance was evaluated with unpaired Student t tests or 2-tailed Mann-Whitney U tests, as indicated, using GraphPad Prism statistical software.
Results
JNK1−/− mice display features of unstable hemostasis in vivo
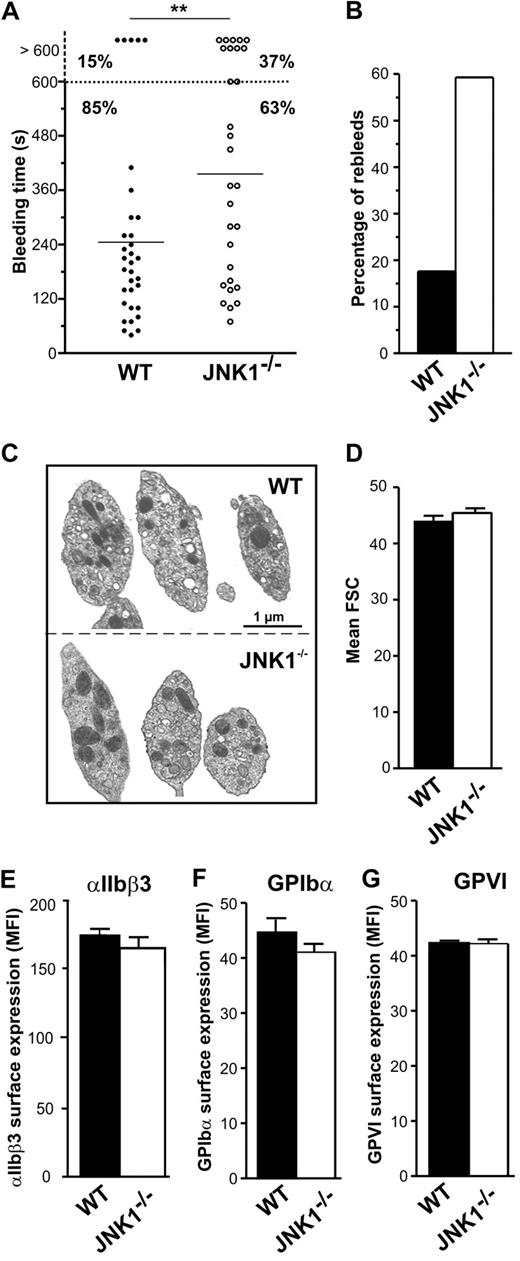
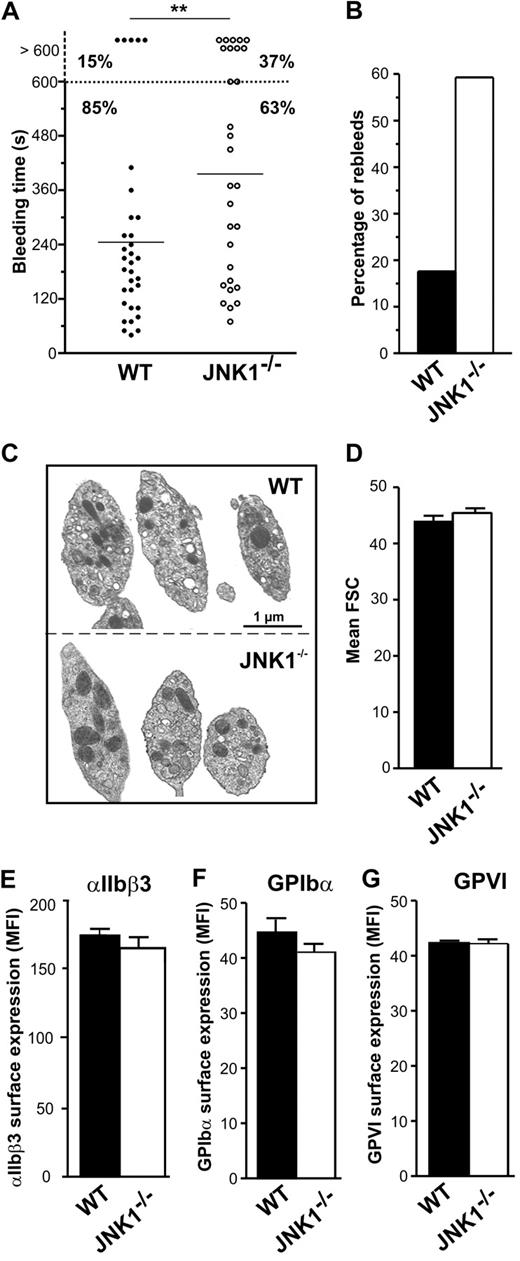
We investigated the role of JNK1 in hemostasis and thrombosis, by comparing the tail bleeding times of WT and JNK1−/− mice in an in vivo tail-bleeding assay. Bleeding times for JNK1−/− mice were significantly longer than those for WT mice (396 ± 39 seconds vs 245 ± 32 seconds; P < .01; Figure 1A). Moreover, excessive bleeding (lasting > 600 seconds) was observed in 37% of JNK1−/− mice but in only 15% of WT mice. The percentage of mice with which bleeding started again (rebleeding) was 3 times higher for JNK1−/− mice than for WT mice (59.3% vs 17.6%; Figure 1B). JNK1−/− and WT mice did not differ significantly in the number of platelets, white cell counts, hematocrit, and hemoglobin concentration, suggesting that the observed differences in bleeding were not the result of differences in hematologic parameters (Table 1).
JNK1−/− mice display features of unstable hemostasis in vivo. The role of JNK1 in hemostasis was investigated in an in vivo tail-bleeding assay. (A) Bleeding times for WT (●) and JNK1−/− mice (○). Means are indicated by horizontal lines. Statistical significance was determined in 2-tailed Mann-Whitney tests (**P < .01). (B) Percentage of WT (■) and JNK1−/− (□) mice exhibiting rebleeding. Results were obtained from 33 WT and 30 JNK1−/− mice. (C) Images of WT and JNK1−/− platelet ultrastructure, obtained by electron microscopy. Bar represents 1 μm. (D) Size of WT and JNK1−/− platelets, evaluated by flow cytometry and based on forward scatter (FSC). Data are mean plus or minus SEM (n = 12; unpaired Student t test). Surface expression of integrin αIIbβ3, GPIbα, and GPVI, as determined by flow cytometry with FITC-conjugated anti–mouse CD41 mAb (MWReg30; E), FITC-conjugated anti–mouse GPIbα mAb (Xia.B2; F), or FITC-conjugated anti–mouse GPVI mAb (JAQ1; G). Results are expressed as mean fluorescence intensities (MFI) plus or minus SEM (n = 3; unpaired Student t test).
JNK1−/− mice display features of unstable hemostasis in vivo. The role of JNK1 in hemostasis was investigated in an in vivo tail-bleeding assay. (A) Bleeding times for WT (●) and JNK1−/− mice (○). Means are indicated by horizontal lines. Statistical significance was determined in 2-tailed Mann-Whitney tests (**P < .01). (B) Percentage of WT (■) and JNK1−/− (□) mice exhibiting rebleeding. Results were obtained from 33 WT and 30 JNK1−/− mice. (C) Images of WT and JNK1−/− platelet ultrastructure, obtained by electron microscopy. Bar represents 1 μm. (D) Size of WT and JNK1−/− platelets, evaluated by flow cytometry and based on forward scatter (FSC). Data are mean plus or minus SEM (n = 12; unpaired Student t test). Surface expression of integrin αIIbβ3, GPIbα, and GPVI, as determined by flow cytometry with FITC-conjugated anti–mouse CD41 mAb (MWReg30; E), FITC-conjugated anti–mouse GPIbα mAb (Xia.B2; F), or FITC-conjugated anti–mouse GPVI mAb (JAQ1; G). Results are expressed as mean fluorescence intensities (MFI) plus or minus SEM (n = 3; unpaired Student t test).
Electron microscopy showed resting JNK1−/− platelets to have a normal discoid morphology (Figure 1C). These observations were consistent with forward scatter data (Figure 1D) and mean platelet volume (Table 1). We observed no abnormalities in the cell-surface expression of integrin αIIbβ3 (Figure 1E), GPIbα (Figure 1F), or GPVI (Figure 1G) in JNK1−/− platelets.
Involvement of JNK1 in thrombus formation in vitro and in vivo
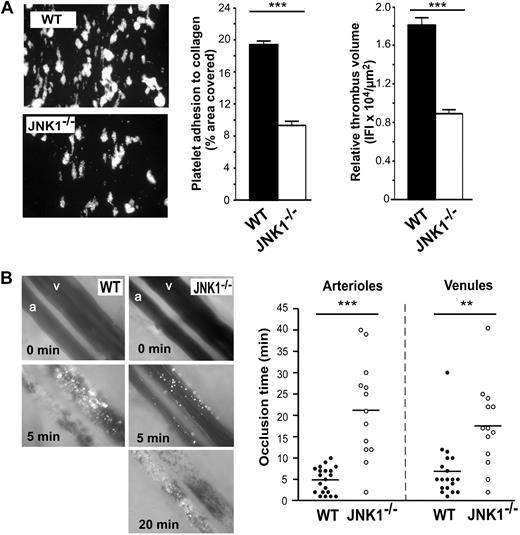
We investigated the role of JNK1 in platelet function further, by assessing thrombus formation by JNK1−/− platelets in vitro. We carried out a whole-blood perfusion assay over a fibrillar collagen matrix at an arterial shear rate of 1500 seconds−1 (Figure 2A). After 90 seconds of perfusion, the area covered by large thrombi (the formation of which is dependent on ADP secretion; results not shown), was 19.4% plus or minus 0.4% for WT platelets and 9.3% plus or minus 0.5% for JNK1−/− platelets (52% lower than WT; P < .001). Similarly, the thrombus volume obtained for JNK1−/− platelets was 51% smaller than that for WT platelets (8911 ± 412 vs 18 090 ± 774 IFI/μm2 of each thrombus; P < .001). These observations strongly suggest that platelet JNK1 is important for thrombus formation in vitro under arterial shear conditions.
JNK1−/− mice display impaired thrombus formation in vitro and in vivo. The role of JNK1 in thrombus formation was investigated in vitro (A), in a whole-blood perfusion assay carried out over a fibrillar collagen matrix at a shear rate of 1500 seconds−1 and in vivo (B), in a model of arteriolar and venular thrombosis induced by photochemical damage of the cecum. (A) Whole blood from WT and JNK1−/− mice, collected in PPACK (80μM), was fluorescently labeled by incubation with rhodamine 6G (10 μg/mL) for 10 minutes, then perfused through fibrillar collagen-coated glass microcapillaries at a shear rate of 1500 seconds−1 for 90 seconds. After perfusion, the formation of thrombi was observed under an epifluorescence microscope (original magnification ×20). The mean percentage of the total area covered by platelets plus or minus SEM was calculated in 3 independent experiments, and the extent of thrombus formation was evaluated in all 3 dimensions, by calculating relative thrombus volume, measured as the IFI per μm2 of each thrombus plus or minus SEM. The statistical significance of differences between WT and JNK1−/− mice was determined in unpaired Student t tests (***P < .001). (B) Photographs show the progression of thrombus formation induced by photochemical injury to cecum arterioles (a) and venules (v) in WT and JNK1−/− mice. Calcein-AM fluorescently labeled platelets (108 platelets) were injected into the mice, with an intravenous bolus of the photoactivated dye Rose Bengal (20 mg/kg body weight). Injury to cecum arterioles and venules was induced by exposing the vessels to a wavelength of 540 nm for 30 seconds. Thrombus formation was visualized in real time using an inverted epifluorescent microscope (Nikon Eclipse TE 2000-U) using a lens at 20×/0.5 numeric aperture (Nikon). Images were acquired using a CCD CoolSNAP HQ2 camera (Photometrics/Roper Scientific) and processed with Metamorph 7.0r1 software (Universal Imaging Corporation). Vessel occlusion was defined as the stopping of blood flow for at least 1 minute. The arterioles and venules irrigating the cecum selected for analysis had diameters of 60 plus or minus 10 μm and 107 plus or minus 15 μm, respectively. The dot plot shows occlusion times for arterioles and venules as a result of photochemically induced thrombosis in WT (n = 7) and JNK1−/− (n = 6) mice. Means are indicated by horizontal lines. Statistical differences between WT and JNK1−/− mice were evaluated in 2-tailed Mann-Whitney tests (**P < .01; ***P < .001).
JNK1−/− mice display impaired thrombus formation in vitro and in vivo. The role of JNK1 in thrombus formation was investigated in vitro (A), in a whole-blood perfusion assay carried out over a fibrillar collagen matrix at a shear rate of 1500 seconds−1 and in vivo (B), in a model of arteriolar and venular thrombosis induced by photochemical damage of the cecum. (A) Whole blood from WT and JNK1−/− mice, collected in PPACK (80μM), was fluorescently labeled by incubation with rhodamine 6G (10 μg/mL) for 10 minutes, then perfused through fibrillar collagen-coated glass microcapillaries at a shear rate of 1500 seconds−1 for 90 seconds. After perfusion, the formation of thrombi was observed under an epifluorescence microscope (original magnification ×20). The mean percentage of the total area covered by platelets plus or minus SEM was calculated in 3 independent experiments, and the extent of thrombus formation was evaluated in all 3 dimensions, by calculating relative thrombus volume, measured as the IFI per μm2 of each thrombus plus or minus SEM. The statistical significance of differences between WT and JNK1−/− mice was determined in unpaired Student t tests (***P < .001). (B) Photographs show the progression of thrombus formation induced by photochemical injury to cecum arterioles (a) and venules (v) in WT and JNK1−/− mice. Calcein-AM fluorescently labeled platelets (108 platelets) were injected into the mice, with an intravenous bolus of the photoactivated dye Rose Bengal (20 mg/kg body weight). Injury to cecum arterioles and venules was induced by exposing the vessels to a wavelength of 540 nm for 30 seconds. Thrombus formation was visualized in real time using an inverted epifluorescent microscope (Nikon Eclipse TE 2000-U) using a lens at 20×/0.5 numeric aperture (Nikon). Images were acquired using a CCD CoolSNAP HQ2 camera (Photometrics/Roper Scientific) and processed with Metamorph 7.0r1 software (Universal Imaging Corporation). Vessel occlusion was defined as the stopping of blood flow for at least 1 minute. The arterioles and venules irrigating the cecum selected for analysis had diameters of 60 plus or minus 10 μm and 107 plus or minus 15 μm, respectively. The dot plot shows occlusion times for arterioles and venules as a result of photochemically induced thrombosis in WT (n = 7) and JNK1−/− (n = 6) mice. Means are indicated by horizontal lines. Statistical differences between WT and JNK1−/− mice were evaluated in 2-tailed Mann-Whitney tests (**P < .01; ***P < .001).
Next, we assessed the role of JNK1 in thrombosis in vivo, in a model of arteriolar and venular thrombosis in the cecum. Thrombosis was induced by photochemical damage and monitored in real time, by intravital microscopy (Figure 2B). Occlusion times were 4.3 times longer for JNK1−/− arterioles than for WT arterioles (21.2 ± 3.2 minutes vs 4.9 ± 0.7 minutes, respectively; P < .001). Occlusion times were also longer (by a factor of 2.5) in JNK1−/− venules than in WT venules (17.5 ± 2.9 minutes vs 6.9 ± 1.5 minutes; P < .01), providing evidence for the involvement of JNK1 in hemostasis and thrombosis in vivo.
JNK1 is involved in platelet aggregation
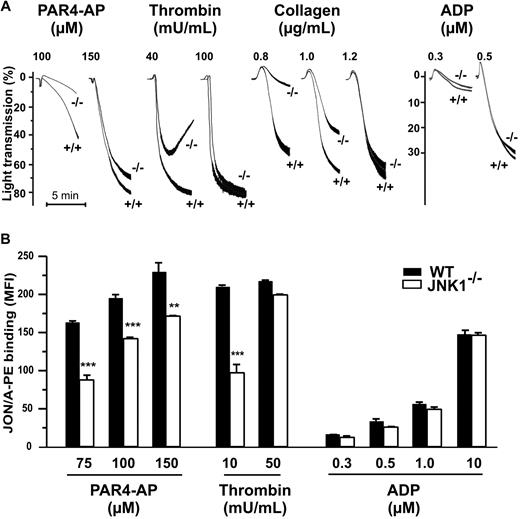
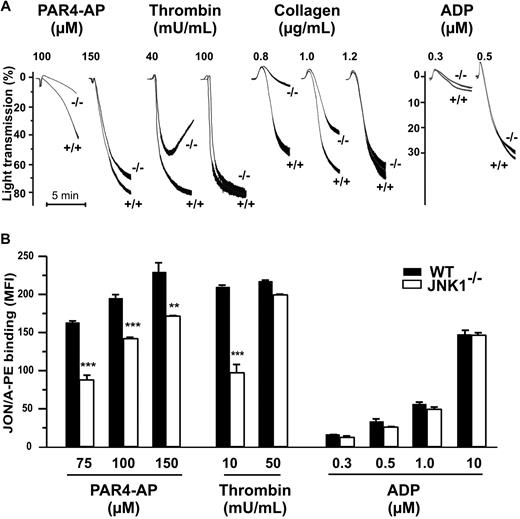
Thrombus formation and platelet function, including integrin αIIbβ3 engagement, are closely associated. We studied the role of JNK1 in the aggregation of washed platelets induced by various agonists. The levels of platelet aggregation induced by PAR4-AP (100μM), thrombin (40 mU/mL), and collagen (0.8 μg/mL), applied separately, were 78%, 64%, and 90% lower, respectively, for JNK1−/− than for WT platelets (Figure 3A). Apyrase, when added, completely inhibited platelet aggregation in response to these low doses of agonist (data not shown). However, at higher doses of agonists (150μM PAR4-AP, 100 mU/mL thrombin, or 1.2 μg/mL collagen), JNK1−/− and WT platelets displayed similar levels of aggregation. ADP also induced similar levels of aggregation in JNK1−/− and WT platelets, for all doses tested.
JNK1−/− platelets show impaired aggregation responses, associated with defective activation of αIIbβ3. (A) The effect of JNK1 deletion on platelet aggregation. Aggregation of washed platelets (108), monitored by measuring light transmission through stirred suspensions of WT (+/+) or JNK1−/− (−/−) platelets, was initiated by adding the PAR4 agonist peptide (PAR4-AP; 100 and 150μM), thrombin (40 and 100 mU/mL), collagen (0.8-1.2 μg/mL), or ADP (0.3 and 0.5μM). Aggregation was measured and expressed as the percentage change in light transmission, with the value for the blank (buffer without platelet) set at 100%. Traces are representative of at least 3 independent experiments. (B) Integrin αIIbβ3 activation assessed by flow cytometry of WT platelets or JNK1−/− platelets, activated by PAR4-AP (75-150μM), thrombin (10 and 50 mU/mL), or ADP (0.3-10μM). Platelets were incubated with a phycoerythrin-labeled rat anti–mouse integrin αIIbβ3 mAb (JON/A) specific for the activated conformation of the mouse integrin. Flow cytometry was performed, without stirring, to prevent platelet aggregation. The level of activated integrin is indicated by the mean fluorescence intensities (MFI) plus or minus SEM, in arbitrary units, from at least 3 experiments. **P < .01, ***P < .001 (unpaired Student t test).
JNK1−/− platelets show impaired aggregation responses, associated with defective activation of αIIbβ3. (A) The effect of JNK1 deletion on platelet aggregation. Aggregation of washed platelets (108), monitored by measuring light transmission through stirred suspensions of WT (+/+) or JNK1−/− (−/−) platelets, was initiated by adding the PAR4 agonist peptide (PAR4-AP; 100 and 150μM), thrombin (40 and 100 mU/mL), collagen (0.8-1.2 μg/mL), or ADP (0.3 and 0.5μM). Aggregation was measured and expressed as the percentage change in light transmission, with the value for the blank (buffer without platelet) set at 100%. Traces are representative of at least 3 independent experiments. (B) Integrin αIIbβ3 activation assessed by flow cytometry of WT platelets or JNK1−/− platelets, activated by PAR4-AP (75-150μM), thrombin (10 and 50 mU/mL), or ADP (0.3-10μM). Platelets were incubated with a phycoerythrin-labeled rat anti–mouse integrin αIIbβ3 mAb (JON/A) specific for the activated conformation of the mouse integrin. Flow cytometry was performed, without stirring, to prevent platelet aggregation. The level of activated integrin is indicated by the mean fluorescence intensities (MFI) plus or minus SEM, in arbitrary units, from at least 3 experiments. **P < .01, ***P < .001 (unpaired Student t test).
In vitro aggregation involves integrin αIIbβ3 activation. We therefore evaluated the activation of this integrin, using a mAb (JON/A) specific for the activated conformation of the mouse integrin (Figure 3B). Levels of JON/A mAb binding to JNK1−/− platelets stimulated by PAR4-AP (75μM) or thrombin (10 mU/mL) were 48% (P < .001) and 57% (P < .001) lower, respectively, than those for WT platelets. By contrast, as expected, αIIbβ3 activation levels were either slightly lower in JNK1−/− platelets (26%; P < .01) or not significantly different between JNK1−/− and WT platelets, after stimulation with higher concentrations of PAR4 (150μM), thrombin (50 mU/mL) or ADP.
JNK1 is involved in platelet secretion
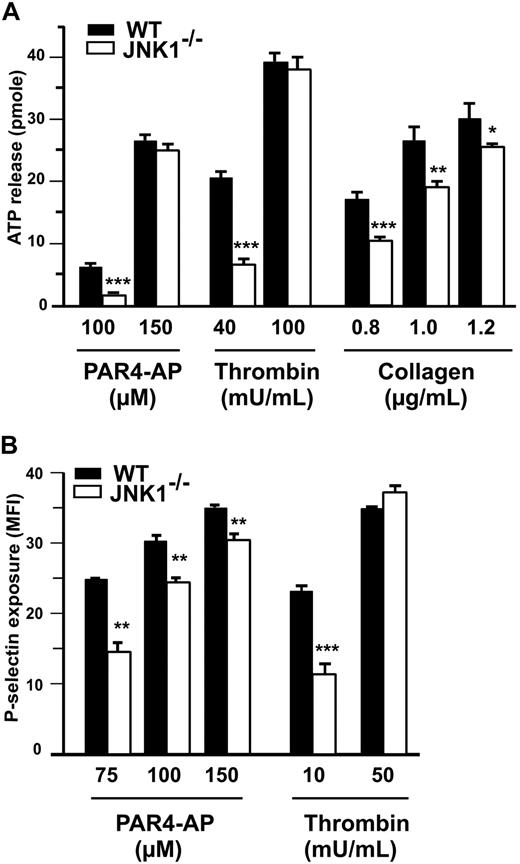
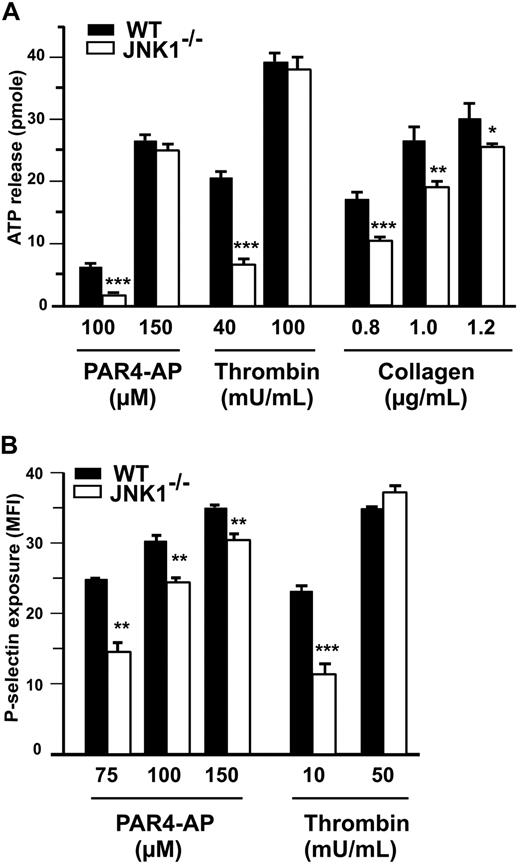
At low agonist concentrations, granule secretion plays a key role in platelet aggregation and integrin αIIbβ3 activation. We therefore investigated the role of JNK1 in the secretion of dense granules by platelets, by measuring ATP release (Figure 4A), and in the secretion of α-granules, by detecting P-selectin exposure (Figure 4B). At low doses of PAR4-AP (100μM), thrombin (40 mU/mL), and collagen (0.8 μg/mL), with stirring, 61%, 68%, and 53%, less ATP, respectively, was released by JNK1−/− than by WT platelets (Figure 4A). P-selectin exposure was evaluated by flow cytometry, without stirring, after platelet stimulation with PAR4-AP (75μM) or thrombin (10 mU/mL). P-selectin levels were 48% (P < .01) and 62% (P < .001) lower after stimulation with PAR4-AP and thrombin, respectively, in JNK1−/− platelets than in WT platelets (Figure 4B). At higher doses of agonists, ATP release and P-selectin exposure differed little, between JNK1−/− and WT platelets. Altogether, these results demonstrate that JNK1 is involved in platelet secretion required for platelet aggregation.
JNK1−/− platelets display abnormal platelet granule secretion. (A) Dense granule secretion was evaluated by measuring ATP release after the aggregation of WT or JNK1−/− platelets induced by PAR4-AP (100 and 150μM), thrombin (40 and 100 mU/mL), or collagen (0.8-1.2 μg/mL) with stirring. Results are expressed as the amount of ATP released (picomoles) by 108 platelets. (B) α-Granule secretion was determined by flow cytometry evaluation of P-selectin exposure in WT or JNK1−/− platelets. Platelets were activated with PAR4-AP (75-150μM) or thrombin (10 and 50 mU/mL) and labeled with FITC-labeled rat anti–mouse P-selectin mAb (Wug.E9). Flow cytometry experiments were performed without stirring to prevent platelet aggregation. The levels of P-selectin exposed at the surface are expressed as mean fluorescence intensities (MFI) plus or minus SEM, in arbitrary units, from at least 3 experiments. *P < .05, **P < .01, ***P < .001 (unpaired Student t test).
JNK1−/− platelets display abnormal platelet granule secretion. (A) Dense granule secretion was evaluated by measuring ATP release after the aggregation of WT or JNK1−/− platelets induced by PAR4-AP (100 and 150μM), thrombin (40 and 100 mU/mL), or collagen (0.8-1.2 μg/mL) with stirring. Results are expressed as the amount of ATP released (picomoles) by 108 platelets. (B) α-Granule secretion was determined by flow cytometry evaluation of P-selectin exposure in WT or JNK1−/− platelets. Platelets were activated with PAR4-AP (75-150μM) or thrombin (10 and 50 mU/mL) and labeled with FITC-labeled rat anti–mouse P-selectin mAb (Wug.E9). Flow cytometry experiments were performed without stirring to prevent platelet aggregation. The levels of P-selectin exposed at the surface are expressed as mean fluorescence intensities (MFI) plus or minus SEM, in arbitrary units, from at least 3 experiments. *P < .05, **P < .01, ***P < .001 (unpaired Student t test).
PKCs involved in platelet secretion require JNK1
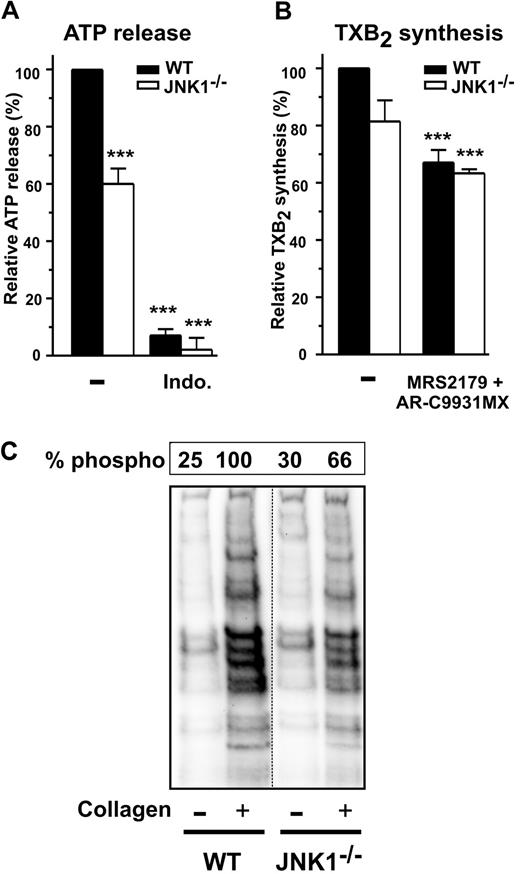
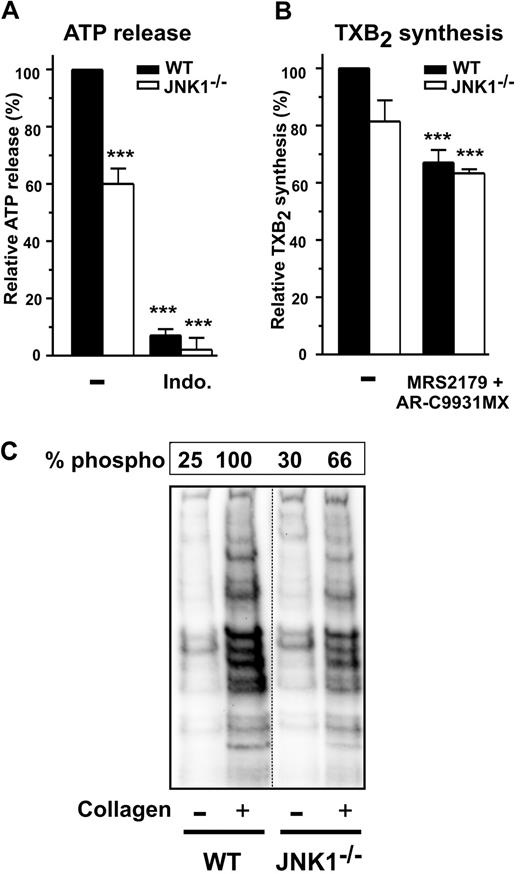
We characterized the role of JNK1 in the signaling pathways leading to platelet secretion, by investigating whether JNK1 acted upstream or downstream from TXA2 synthesis or PKCs, both of which contribute to collagen-induced platelet activation and dense granule secretion.26 As expected, secretion by WT and JNK1−/− platelets, as determined by measuring ATP release after activation with a low dose of collagen (1 μg/mL), was almost completely inhibited by the addition of indomethacin, a cyclooxygenase inhibitor (Figure 5A). In these conditions, levels of TXA2 synthesis, as assessed by determining levels of its stable metabolite, TXB2, were lower (∼ 20%) in JNK1−/− than in WT platelets (Figure 5B), whereas addition of the ADP receptor antagonists AR-C69931MX and MRS2179 inhibited TXB2 synthesis by 33% plus or minus 4% (P < .001) and by 37% plus or minus 1% (P < .001) in WT and JNK1−/− platelets, respectively.
The PKCs involved in platelet secretion require JNK1. Dense granule secretion (measured by ATP release, A) and TXA2 synthesis (measured by production of TXB2, B) were determined for WT and JNK1−/− washed platelets (108) after stimulation with collagen (1 μg/mL), with stirring, over 3 minutes. When indicated, platelets were first incubated for 10 minutes at 37°C with the cyclooxygenase inhibitor indomethacin (5μM) or the antagonists of ADP receptors, MRS2179 (100μM) and AR-C69931MX (10μM). Data are expressed as the relative percentage of ATP released (A) or of TXB2 produced (B). Each bar represents the mean plus or minus SEM of 3 experiments. ***P < .001 (unpaired Student t test). (C) The PKC activity of WT and JNK1−/− platelets (108) induced by collagen (1 μg/mL), with stirring, over a period of 3 minutes. Stimulated platelets were lysed and samples analyzed by Western blotting with an antibody against phosphorylated PKC substrates. The levels of phosphorylation of PKC substrates were evaluated as described in “Methods,” for WT and JNK1−/− platelets. The Western blot shown is representative of at least 3 independent experiments.
The PKCs involved in platelet secretion require JNK1. Dense granule secretion (measured by ATP release, A) and TXA2 synthesis (measured by production of TXB2, B) were determined for WT and JNK1−/− washed platelets (108) after stimulation with collagen (1 μg/mL), with stirring, over 3 minutes. When indicated, platelets were first incubated for 10 minutes at 37°C with the cyclooxygenase inhibitor indomethacin (5μM) or the antagonists of ADP receptors, MRS2179 (100μM) and AR-C69931MX (10μM). Data are expressed as the relative percentage of ATP released (A) or of TXB2 produced (B). Each bar represents the mean plus or minus SEM of 3 experiments. ***P < .001 (unpaired Student t test). (C) The PKC activity of WT and JNK1−/− platelets (108) induced by collagen (1 μg/mL), with stirring, over a period of 3 minutes. Stimulated platelets were lysed and samples analyzed by Western blotting with an antibody against phosphorylated PKC substrates. The levels of phosphorylation of PKC substrates were evaluated as described in “Methods,” for WT and JNK1−/− platelets. The Western blot shown is representative of at least 3 independent experiments.
We investigated the role of JNK1 in PKC activity, by Western blotting with an antibody against phosphorylated PKC substrates. The phosphorylated bands observed when WT platelet aggregation was induced by collagen were not observed in the presence of an inhibitor of PKC activity (Ro-31-8220; results not shown). Smaller amounts of these phosphorylated proteins (52%) were observed in JNK1−/− platelets (Figure 5C), suggesting the possibility that JNK1 acts upstream from PKCs.
We then investigated the functional relationship between JNK1 and other JNK isoforms in TXA2 synthesis, PKC activity, and the aggregation of WT and JNK1−/− platelets. Indeed, JNK2 (54 kDa) was detected in similar amounts in WT and JNK1−/− platelets by Western blots (Figure 6A), whereas JNK3 was not detected (results not shown). In addition, JNK2 underwent phosphorylation on platelet activation with low doses of collagen; however, this phosphorylation was impaired in JNK1−/− platelets (63% reduction; Figure 6B). Interestingly, SP600125, an inhibitor of all JNK isoforms (JNK1/2/3), totally inhibited platelet aggregation induced by low doses of collagen not only in WT but also in JNK1−/− platelets (Figure 6C). This strongly suggests that JNK kinases other than JNK1 contribute to platelet aggregation and that JNK2 activation is partially dependent on JNK1, although a nonspecific effect of SP600125 cannot be excluded.
Complementary roles of JNK1 and other JNK isoforms in platelet aggregation, TXA2 synthesis, and PKC activity. The levels of JNK2 expression (A) and phosphorylation (B) were evaluated by Western blotting with appropriate antibodies directed against total JNK2 or phosphorylated JNK2 for WT and JNK1−/− platelets (108) stimulated with collagen (1 μg/mL) for 3 minutes, with stirring. Tubulin was used as a loading control. The Western blot shown is representative of at least 3 independent experiments. The complementary roles of JNK1 and the other JNK isoforms were evaluated for platelet aggregation (C), TXA2 synthesis (measured by evaluating production of TXB2 (D), and PKC activity (by Western blotting with an antibody directed against phosphorylated PKC substrates; E), in WT or JNK1−/− platelets (108), incubated for 10 minutes at 37°C with DMSO (0.1%, vol/vol), as a control, or with the pan-JNK inhibitor SP600125 (10μM), before stimulation with collagen (1 μg/mL) for 3 minutes, with stirring. The traces and Western blot shown are representative of at least 3 independent experiments. Thromboxane production was assessed by analysis of the supernatants of aggregated platelets, as described in “Methods.” Each bar represents the mean plus or minus SEM of 3 experiments. ***P < .001 (unpaired Student t test).
Complementary roles of JNK1 and other JNK isoforms in platelet aggregation, TXA2 synthesis, and PKC activity. The levels of JNK2 expression (A) and phosphorylation (B) were evaluated by Western blotting with appropriate antibodies directed against total JNK2 or phosphorylated JNK2 for WT and JNK1−/− platelets (108) stimulated with collagen (1 μg/mL) for 3 minutes, with stirring. Tubulin was used as a loading control. The Western blot shown is representative of at least 3 independent experiments. The complementary roles of JNK1 and the other JNK isoforms were evaluated for platelet aggregation (C), TXA2 synthesis (measured by evaluating production of TXB2 (D), and PKC activity (by Western blotting with an antibody directed against phosphorylated PKC substrates; E), in WT or JNK1−/− platelets (108), incubated for 10 minutes at 37°C with DMSO (0.1%, vol/vol), as a control, or with the pan-JNK inhibitor SP600125 (10μM), before stimulation with collagen (1 μg/mL) for 3 minutes, with stirring. The traces and Western blot shown are representative of at least 3 independent experiments. Thromboxane production was assessed by analysis of the supernatants of aggregated platelets, as described in “Methods.” Each bar represents the mean plus or minus SEM of 3 experiments. ***P < .001 (unpaired Student t test).
We next investigated the role of the other JNK isoform(s) on TXA2 synthesis and PKC activity. SP600125 inhibited TXA2 synthesis in WT and JNK1−/− platelets by 78% plus or minus 3% (P < .001) and 86% plus or minus 5% (P < .001), respectively (Figure 6D), and PKC activity in WT and JNK1−/− platelets by 51% and 84%, respectively (Figure 6E). Altogether, these results suggest that JNK1 and other JNK isoforms play complementary roles.
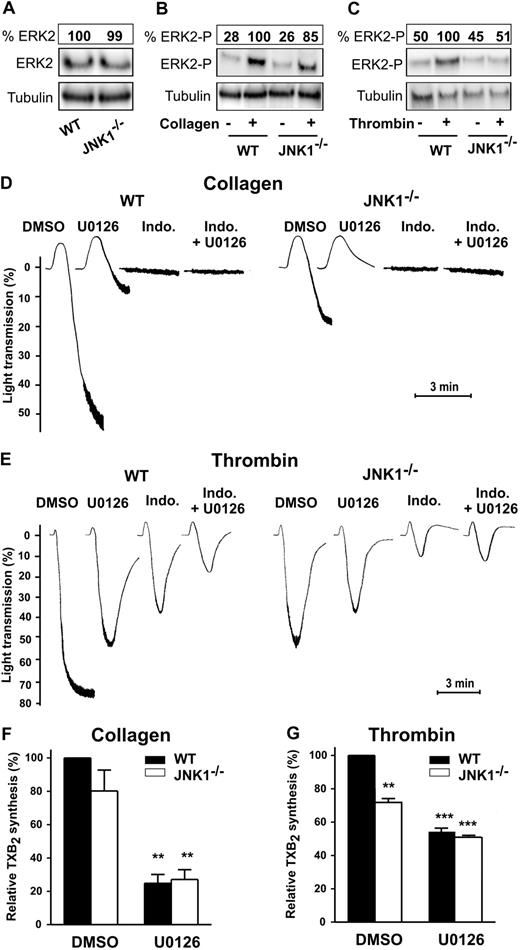
Complementary roles of JNK1 and ERK2
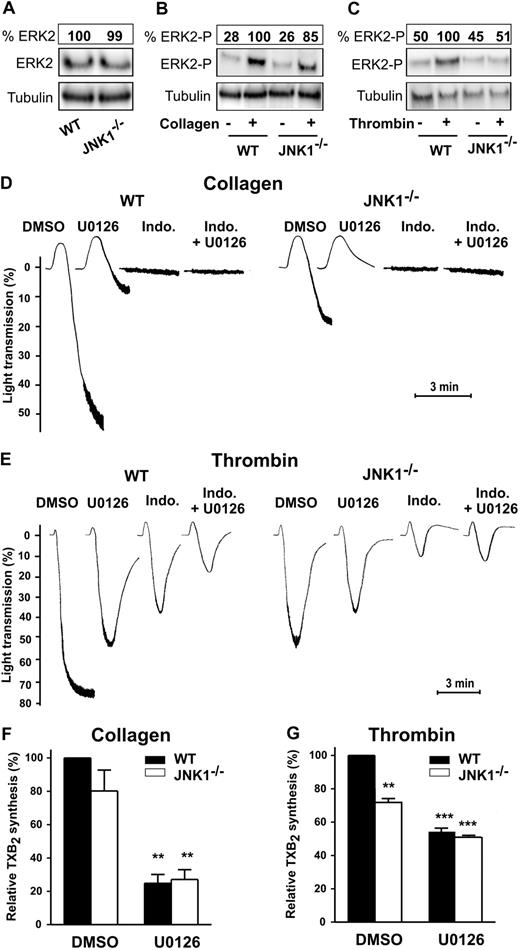
ERK2 modulates aggregation after platelet activation by threshold concentrations of agonists.19 We investigated the potential redundancy or complementary roles of JNK1 and ERK2 by assessing both its activation status and its role in JNK1−/− platelets.
Whereas total ERK2 level (both nonphosphorylated and phosphorylated) was similar in WT and JNK1−/− platelets (Figure 7A), after stimulation by collagen (1 μg/mL; Figure 7B), or thrombin (65 mU/mL; Figure 7C) ERK2 underwent either low or undetectable phosphorylation, respectively, in JNK1−/− platelets. We next compared the levels of platelet aggregation and TXA2 synthesis in WT and JNK1−/− platelets in the presence of U0126, an inhibitor of MEK (therefore leading to the inhibition of the MEK downstream substrate, ERK2). Platelet aggregation induced by collagen, in the presence of U0126, was inhibited almost totally in WT platelets, and totally in JNK1−/− platelets. Aggregation to collagen was also completely inhibited after addition of indomethacin with or without U0126 in JNK1−/− and WT platelets (Figure 7D). This suggests that ERK2 is a major actor in collagen-induced aggregation, acting upstream of JNK1.
Complementary roles of JNK1 and ERK2 in platelet aggregation and TXA2 synthesis. The levels of ERK2 expression (A) and phosphorylation were evaluated in WT and JNK1−/− platelets after activation by collagen (1 μg/mL; B) or thrombin (65 mU/mL; C) for 3 minutes, with stirring. Stimulated platelets were lysed and samples analyzed by Western blotting with appropriate antibodies directed against total ERK2 (A) or phosphorylated ERK2 (B-C). Tubulin was used as a loading control. The Western blot shown is representative of at least 3 independent experiments. The complementary roles of JNK1 and ERK2 were evaluated for platelet aggregation (D-E) and for TXA2 synthesis (measured by evaluating production of TXB2; F-G), in WT or JNK1−/− platelets (108) stimulated with collagen (1 μg/mL) or thrombin (65 mU/mL) for 3 minutes, with stirring. Platelets were first incubated for 10 minutes at 37°C with DMSO (0.1%, vol/vol), as a control, or the MEK inhibitor U0126 (10μM) plus or minus indomethacin (5μM). Thromboxane synthesis was determined in the supernatants of aggregated platelets, as described in “Methods.” Each bar represents the mean plus or minus SEM of 3 experiments. **P < .01, ***P < .001 (unpaired Student t test). Traces are representative of at least 3 independent experiments.
Complementary roles of JNK1 and ERK2 in platelet aggregation and TXA2 synthesis. The levels of ERK2 expression (A) and phosphorylation were evaluated in WT and JNK1−/− platelets after activation by collagen (1 μg/mL; B) or thrombin (65 mU/mL; C) for 3 minutes, with stirring. Stimulated platelets were lysed and samples analyzed by Western blotting with appropriate antibodies directed against total ERK2 (A) or phosphorylated ERK2 (B-C). Tubulin was used as a loading control. The Western blot shown is representative of at least 3 independent experiments. The complementary roles of JNK1 and ERK2 were evaluated for platelet aggregation (D-E) and for TXA2 synthesis (measured by evaluating production of TXB2; F-G), in WT or JNK1−/− platelets (108) stimulated with collagen (1 μg/mL) or thrombin (65 mU/mL) for 3 minutes, with stirring. Platelets were first incubated for 10 minutes at 37°C with DMSO (0.1%, vol/vol), as a control, or the MEK inhibitor U0126 (10μM) plus or minus indomethacin (5μM). Thromboxane synthesis was determined in the supernatants of aggregated platelets, as described in “Methods.” Each bar represents the mean plus or minus SEM of 3 experiments. **P < .01, ***P < .001 (unpaired Student t test). Traces are representative of at least 3 independent experiments.
In thrombin aggregation of WT platelets (Figure 7E), U0126 and indomethacin exhibited an additive inhibition (from 27% and 47%, separately, to 74% together), consistent with distinct pathways for ERK2 and cyclooxygenase/TXA2. In JNK1−/− platelets, the slightly lower extent of thrombin aggregation compared with WT (27%) suggests a minor engagement of JNK1. U0126 induced an additional inhibition (27%), suggesting a JNK1-independent partial engagement of ERK2.
In addition, the levels of TXA2 synthesis were moderately lower in JNK1−/− platelets than in WT platelets stimulated with collagen (∼ 20%) or with thrombin (28% ± 3%, P < .01), whereas U0126 inhibited TXA2 synthesis to a similar extent in WT and JNK1−/− platelets activated by collagen (75% ± 5% and 73% ± 6%, respectively; P < .01; Figure 7F) or thrombin (46% ± 2% and 49% ± 1%, respectively; P < .001; Figure 7G). Thus, JNK1 involvement in TXA2 synthesis is only minor. Moreover, altogether, our findings are consistent with ERK2 exerting its effects on platelet aggregation via 2 distinct pathways: one independent of JNK1 and the other common to JNK1 and ERK2.
Discussion
Our data, based on the study of JNK1−/− mice, provide evidence for a key role of JNK1 in platelet function and thrombosis. JNK1−/− mice displayed features of unstable hemostasis, with long bleeding times and rebleeding, despite normal platelet counts, normal expression of key adhesive receptors (including GPIbα, GPVI, and integrin αIIbβ3), and normal ultrastructure. JNK1−/− mice also displayed delayed arterial and venular thrombosis in vivo and defective thrombus formation in an in vitro whole-blood perfusion assay carried out with a fibrillar collagen matrix and arterial shear conditions. JNK1−/− platelets displayed impaired secretion activities, decreased levels of integrin αIIbβ3 activation, and platelet aggregation. These effects were dependent on PKC activity. Other MAP kinases, such as ERK2 and other JNK isoforms, involved in collagen-induced platelet aggregation, affect TXA2 synthesis and PKC activity, suggesting functional complementarity between the various MAP kinases.
In a previous study, using a pan-JNK inhibitor, we showed that JNKs played a role in thrombus formation but did not identify the precise isoform involved.21 Here, we clearly identify JNK1 as one of the JNK isoforms involved in hemostasis and thrombosis. Occlusion times for the arterioles of JNK1−/− and WT mice differed to a greater extent in this study (4.3 times greater in JNK1−/− mice) than in our previous study, based on the use of a pan-JNK inhibitor in WT mice (1.9 times greater with the inhibitor). This difference between the 2 studies may be the result of differences in genetic backgrounds (C57BL/6 mice in this study and FVB mice in the previous study) or incomplete inhibition of JNK activity by the pan-JNK inhibitor in vivo. Regardless of the explanation, our results demonstrate the existence of a clear platelet defect. However, in the absence of studies of radiation chimeras, we cannot rule out the contribution of a vasculature defect to the phenotype. However, this is unlikely because our previous in vivo findings based on the use of JNK inhibitors21 and the markedly lower levels of thrombus formation by isolated JNK1−/− platelets in our in vitro blood flow studies strongly suggest a significant and direct effect of JNK1 on platelet function.
Our observations of unstable hemostasis and thrombus formation in JNK1−/− mice led us to address the role of JNK1 in platelet function. We found that the platelet aggregation induced by low concentrations of agonists, such as collagen, thrombin, or PAR4-AP, was dependent on JNK1, whereas ADP-induced aggregation was independent of this MAP kinase. Moreover, granule secretion and integrin αIIbβ3 activation were impaired in JNK1−/− platelets stimulated with thrombin or PAR4-AP, but not in platelets activated by ADP. These findings suggest that JNK1 activation plays an indirect role in integrin αIIbβ3 activation, probably through a mechanism involving secretion. We investigated the mechanism underlying the effect of JNK1 on platelet secretion, by evaluating the involvement of JNK1 in TXA2 synthesis and PKC activity, which has been shown to be involved in collagen-induced platelet secretion. We confirmed that TXA2 synthesis was required for full secretion and that TXA2 synthesis was partially dependent on integrin αIIbβ3 as previously described27 (and results not shown) in WT and JNK1−/− platelets. JNK1−/− platelets displayed moderate impairment of TXA2 synthesis and partial impairment of PKC activity. The mechanism by which JNK1 affects PKC activities is unknown. JNK1 could act directly on PKC activities or indirectly by inhibiting platelet aggregation. Further studies are required to identify potential substrates regulated by collagen-induced JNK1 activity and acting on platelet secretion. PKCs have also been reported to regulate the JNK signaling pathway in proliferative cells.28 Thus, we cannot exclude the possibility that JNK1 acts downstream from PKCs in platelets. Indeed, JNK1 plays a minor role in the platelet secretion induced by phorbol-12-myristate-13-acetate, with no effect on PKC substrates (results not shown).
The aggregation of platelets induced by threshold concentrations of agonists is modulated by MAP kinases. We therefore investigated whether JNK1, JNK2, and ERK2 displayed redundant or complementary functions.19 The milder granule secretion phenotype associated with JNK1 deficiency may be accounted for by redundancy with other JNK isoforms. JNK1 and JNK2 are ubiquitously expressed in proliferating cells, whereas JNK3 seems to be restricted essentially to the brain, testis, and heart.29 We detected JNK2, but not JNK3 (results not shown), in platelets. Based on the use of a pharmacologic inhibitor of all isoforms of JNK (SP600125), we demonstrated that another isoform of JNK, not directly identified, was complementary to JNK1 in platelet aggregation, TXA2 synthesis, and PKC activity. However, we cannot exclude a nonspecific inhibition of platelet function by SP600125. Nevertheless, our results indicated that JNK2 phosphorylation after collagen activation is dependent on JNK1 and also independent of JNK1. In the presence of low doses of collagen, approximately 30% of TXA2 synthesis was dependent on ADP secretion, whereas approximately 80% of TXA2 synthesis was dependent on JNKs, as shown by experiments with SP600125. This suggests that JNK1 and another isoform have effects on TXA2 synthesis independently of ADP secretion. This model was confirmed in the presence of high doses of collagen, antagonists of ADP receptors, and the pan-JNK inhibitor. In these conditions, JNK1 and another JNK isoform directly affect TXA2 synthesis (70% reduction; results not shown). These findings suggest that JNK isoforms affect TXA2 synthesis independently of ADP secretion. This hypothesis is supported by recent studies demonstrating the regulation of cytosolic phospholipase A2 phosphorylation and activity by JNKs in cytotoxic T lymphocytes and macrophages.30,31 Further studies are required to determine the respective roles of the MAP kinase JNKs and to exclude the hypothesis of a nonspecific inhibition of platelet function by SP600125. However, in the absence of specific JNK2 inhibitors, such studies will require mice with megakaryocyte-specific JNK2 gene invalidation because mice lacking both JNK1 and JNK2 are not viable.
We confirm that ERK2 plays a critical role in collagen-induced platelet aggregation dependent on TXA2 synthesis. Moreover, the aggregation and the quantification of TXA2 synthesis in WT and JNK1−/− platelets suggest that ERK2 is involved in platelet aggregation via a TXA2 pathway, consistent with published data.26 The other possibility is that the decrease in TXA2 synthesis observed in the presence of the MEK inhibitor is a consequence of the inhibition of platelet aggregation. Moreover, ERK2 and JNK1 act on collagen-induced platelet aggregation both by a common pathway as well as by independent pathways. The major part of ERK2 activation occurs upstream of JNK1 or on another pathway, whereas the minor part of ERK2 activation is dependent on JNK1. ERK2 activation is probably dependent on Rap1 as suggested by others,26 but other candidates, such as ADP release32,33 or the P2X1 pathway, could also be involved.18 Further studies are required to investigate the role of JNK1 along the ERK2 pathway.
ERK2 also plays a role in thrombin-induced platelet aggregation and TXA2 synthesis. The aggregation in WT and JNK1−/− platelets suggests that ERK2 acts through a pathway both dependent and independent of JNK1, despite undetectable ERK2 phosphorylation in JNK1−/− platelets. Moreover, JNK1 acts upstream of ERK2, suggesting that the complementary roles of JNK1 and ERK2 are distinct whether platelet aggregation is induced by collagen or thrombin.
Our study thus provides direct evidence for a central role of JNK1 in platelet secretion and thrombus formation, as well as for other complementary MAP kinases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr H. Enslen for his help with JNK1-deficient mice, Dr C. Gachet for advice, Dr P. Foubert for technical assistance, and K. Jambou from CDTA-Orléans/CNRS/Transgenesis, Archiving and Animal Models TAAM-UPS44.
This work was supported by Inserm and the Agence Nationale de la Recherche. A.K. held a PhD fellowship from the Ministère de l'Enseignement et de la Recherche. F.A. was supported by the Agence Nationale de la Recherche and a postdoctoral fellowship from the Fondation Lefoulon-Delalande.
Authorship
Contribution: F.A. and A.K. designed and performed research, analyzed data, and wrote the paper; P.N. performed and analyzed electron microscopy imaging; E.S. analyzed flow cytometry experiments; M.F.H. provided vital reagents; R.J.D. constructed JNK1 knockout mice; J.-P.R. discussed results and wrote the manuscript; and M.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marijke Bryckaert, Inserm U770, Hôpital Bicêtre, 80 rue du Général Leclerc, 94276 Le Kremlin Bicêtre Cedex, France; e-mail: marijke.bryckaert@inserm.fr.
References
Author notes
F.A. and A.K. contributed equally to this study.