Phagocytosis in macrophages is receptor mediated and relies on actin polymerization coordinated with the focal delivery of intracellular membranes that is necessary for optimal phagocytosis of large particles. Here we show that phagocytosis by various receptors was inhibited in primary human macrophages infected with wild-type HIV-1 but not with a nef-deleted virus. We observed no major perturbation of F-actin accumulation, but adaptor protein 1 (AP1)–positive endosome recruitment was inhibited in HIV-1–infected cells. Expression of negative factor (Nef) was sufficient to inhibit phagocytosis, and myristoylation as well as the LL and DD motifs involved in association of Nef with AP complexes were important for this inhibition. We observed that Nef interferes with AP1 in association with membranes and/or with a cleaved regulatory form of AP1. Finally, an alteration of the recruitment of vesicle-associated membrane protein (VAMP3)– and tumor necrosis factor-α (TNFα)–positive recycling endosomes regulated by AP1, but not of VAMP7-positive late endosomes, was observed in phagocytic cups of HIV-1–infected macrophages. We conclude that HIV-1 impairs optimal phagosome formation through Nef-dependent perturbation of the endosomal remodeling relying on AP1. We therefore identified a mechanism of macrophage function down-regulation in infected cells.
Introduction
Macrophages play crucial functions at the interface between innate and adaptive immunity. They recognize, take up, and degrade microorganisms and are responsible for clearance of cell debris during developmental processes, elimination of dead red blood cells in the liver, as well as clearance of pathogenic microorganisms. They also participate in the generation of specific adaptive immune responses by presenting microbial-derived peptides associated with major histocompatibility complex class II (MHCII) to T lymphocytes and by secreting proinflammatory cytokines.1 Macrophages possess a wide variety of receptors that sense and bind microorganisms, including receptors for surface determinants such as the Toll-like receptors, and receptors for mannose or beta glucans (Dectin-1). Other receptors recognize opsonins, molecules of the immune system covering the surface of microorganisms. Some of these receptors, including receptors for the Fc portion of immunoglobulins (crystallizable fragment receptor [FcR]) and complement receptor 3 (CR3) receptors have strong phagocytic capacities; their stimulation induces the efficient uptake of the bound microorganism. Phagocytosis induced by FcRs is the best characterized pathway. It activates a signaling cascade that involves small guanosine-5′-triphosphate–binding proteins of the Rho and adenosine diphosphate–ribosylation factor families and eventually leads to actin polymerization, plasma membrane remodeling, and extension of pseudopods around the particle.2,,–5
Extension of the plasma membrane around large particles is supported by a process of internal membrane delivery that involves the fusion machinery relying on vesicle-associated, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (v-SNAREs).6,–8 Although the major part of the membrane forming a phagosome is of plasmalemmal origin,9 the recycling endosomes bearing the SNARE protein vesicle-associated membrane protein (VAMP3)–Cellubrevin,10,11 a subpopulation of late endosomes bearing the SNARE protein VAMP7/TI-VAMP,12 and the endoplasmic reticulum with the SNARE protein ERS24/Sec22b13,14 have been shown to be recruited and undergo focal exocytosis at the site of phagocytosis. These internal membranes are therefore required for efficient phagocytosis of large particles. The adaptor protein 1 (AP1) clathrin-associated adaptor complex plays an important role in the regulation of recycling endosome dynamics during phagocytosis under the control of the adenosine diphosphate–ribosylation factor 1.15
HIV-1 infects predominantly CD4+ T lymphocytes but also macrophages and dendritic cells. Macrophages are thought to represent a major reservoir for HIV persistence because infectious virions can be retained in an infectious state for prolonged periods of time inside macrophages and may be released in a delayed manner and in different locations. Therefore, macrophages were proposed to be important for pathogenesis and dissemination.16,–18 In addition, HIV-1 infection has been reported to impair the functions of macrophages both in vivo and in vitro. Whereas macrophages from HIV-1–infected patients are deficient for phagocytosis of apoptotic neutrophils,19 infected monocyte-derived macrophages are impaired in vitro in phagocytosis of Candida albicans and Toxoplasma gondii,20,21 as well as phagocytosis mediated by FcR and CR3.22,–24 The molecular mechanisms responsible for these alterations of macrophage functions observed during HIV-1 infection are still elusive and have not been investigated with regard to recent data on early steps of phagosome formation.
In addition to gag, pol, and env, which encode the essential structural and enzymatic viral proteins, HIV-1 contains 6 additional genes: tat, rev, vif, vpr, vpu, and nef. Negative factor (Nef) is a major virulence factor that is present in high abundance during the early stages of the viral life cycle.25 It is a multifunctional myristoylated protein of 27 to 34 kDa that has no apparent enzymatic activity but interacts with many host cell proteins. Nef perturbs and/or uses distinct cellular machineries, induces cell surface down-regulation of many receptors, interferes with signal transduction pathways, and enhances viral infectivity. Indeed, the best documented effect of Nef during the course of viral infection is related to its ability to disturb the clathrin-dependent trafficking machinery involved in the transport of transmembrane proteins through endosomal compartments. This leads to the modulation of the level of cell surface expression for some receptors, including CD4, the main HIV-1 receptor.
In this study, we analyzed the phagocytic capacities of HIV-1–infected primary human macrophages. We observed a defect in phagocytosis when the cells were infected with wild-type HIV-1 but not a nef-deleted strain (HIV-1ΔNef). Expression of Nef was sufficient to impair phagocytosis. In addition, HIV-1 wild-type (HIV-1WT), but not HIV-1ΔNef infection, perturbed early events of membrane remodeling necessary for optimal phagosome formation, but not F-actin recruitment. Taken together, these results provide evidence for a role of Nef in interfering with endosomal dynamics that relies on AP1 and that is important for phagosome formation.
Methods
Reagents
Phorbol 12-myristate 13-acetate (used at 150 ng/mL from a 1-mg/mL stock in dimethyl sulfoxide), 4′,6-diamidino-2-phenylindole, and complement C5–deficient serum were from Sigma-Aldrich. The following antibodies were used: rabbit anti–sheep red blood cell (SRBC; IGN Biochemicals), mouse monoclonal anti-RBC (clone TIB111, supernatant; made in-house); rabbit anti-RBC immunoglobulin M (IgM; Accurate); mouse monoclonal anti–human CD4 phycoerythrin (PE)/cyanin 5 (Cy5), mouse monoclonal anti–human CD14 peridinin-chlorophyll-protein complex, isotype control mouse IgG1k PE/Cy5, isotype control mouse IgG2b PE/Cy5, mouse monoclonal anti-AP1 gamma adaptin (clone 88), mouse monoclonal anti–human tumor necrosis factor-α (TNFα; Becton Dickinson); rat monoclonal anti–human CCR5 Alexa 647, rat IgG2ak-Alexa647 isotype control (BioLegend); mouse monoclonal anti-HIV p24 (Dako); goat polyclonal anti-HIV p24 (AbD Serotec); anti-HA (Roche); rabbit polyclonal anti–human Rho GDI alpha (Santa Cruz Biotechnology); mouse monoclonal anti–human transferrin receptor (Invitrogen); rabbit polyclonal anti-VAMP3/Cellubrevin (serum TG2) and mouse monoclonal anti-VAMP7/TI-VAMP (clone 158.2; given by Dr Thierry Galli, Institut Jacques Monod); anti-Giantin (clone TA10, ScFv recombinant antibody fused to human Fc portion; given by Dr Franck Perez [Institut Curie] and described in Moutel et al26 ); and Aminomethylcoumarin-, Cy2-, Cy3-, or Cy5-labeled F(ab′)2 anti–goat, –human, –mouse, –rabbit, or –rat IgG, horseradish peroxidase–labeled anti–mouse and anti–rabbit IgG (Jackson ImmunoResearch Laboratories). Alexa350/633-coupled phalloidins were from Molecular Probes (Invitrogen). Zymosan A (Sigma-Aldrich) was coupled to Cy3 (GE Healthcare).
Plasmids
The constructs encoding the HA-tagged Nef, green fluorescent protein (GFP)–tagged Nef, and vectors for expression of WT or mutated HIV-1NL4-3 Nef fused to HA or GFP were as described.27,28 The plasmids encoding GST-tagged Nef mutants were as described.29 All plasmids were transformed in Escherichia coli XL1Blue and sequenced.
Cell culture and transfection
RAW264.7 macrophages were grown and transfected as described.11
Human monocytes were isolated from blood of healthy donors (Etablissement Français du Sang Ile-de-France) by density gradient sedimentation in Ficoll (GE Healthcare), followed by a negative selection with magnetic beads (Monocyte negative kit; Dynal). Monocytes were derived into macrophages for 7 days in complete culture medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 100 μg/mL streptomycin/penicillin, and 2mM l-glutamine [Invitrogen/Gibco]) containing 10 ng/mL recombinant human macrophage colony-stimulating factor (rhM-CSF; R&D systems). Cells were transfected with the Nucleofector II device (Amaxa GmbH Europe/World) and the Human Macrophage Nucleofector Kit. Briefly, 6 × 105 macrophages were nucleofected with 4 μg of plasmid and then cultured in complete culture medium for 6 hours.
Flow cytometric analysis
To determine the purity of CD14+ monocytes, cells were fixed in 4% paraformaldehyde for 15 minutes at 23°C then stained with peridinin-chlorophyll-protein complex anti-CD14 antibodies for 30 minutes at 23°C. Cells were washed once with phosphate-buffered saline 1×/2% FCS and once with phosphate-buffered saline 1× before analysis by flow cytometry (FACSCalibur and CellQuest Software; Becton Dickinson).
Virus production and human macrophages infection
Proviral infectious clones of the macrophage-tropic virus isolate ADA (HIV-1ADAWT) and the same clone disrupted for the Nef open reading frame (HIV-1ADAΔNef) were kindly provided by Luciana da Costa (Federal University). Virions were produced by transient transfection of 293T cells with proviral plasmids as described.30 Virus concentration in cell culture supernatants was measured by reverse-transcriptase assay as described.30 Human monocytes were isolated on Ficoll gradient as described in “Cell culture and transfection,” then allowed to adhere to dishes for 2 hours in FCS-free medium and differentiated into macrophages in complete culture medium supplemented with 10 ng/mL rhM-CSF. After 8 days, monocyte-derived macrophages were seeded in 24-well plates at a density of 3 × 105 cells/well and cultivated in complete culture medium. After 3 days of incubation, HIV-1ADAWT and HIV-1ADAΔNef viruses (4000 cpm/mL) were added. Viruses were washed after 2 days of incubation; then, at 4, 6, 8, 10, and 12 days after infection, half of the cell supernatants was collected and virus lysed with 0.5% Nonidet P40 before determination of p24 levels by enzyme-linked immunosorbent assay (Innotest).
Phagocytosis assays and quantitation
Phagocytosis of RBCs was performed as described.12 To quantitate phagocytosis, the number of internalized particles (RBCs or zymosans) was counted at 60 minutes in 50 cells randomly chosen on the coverslips, and the phagocytic index (ie, the mean number of phagocytosed particles per cell) was calculated. The index obtained for transfected or infected cells was divided by the index obtained for control cells and expressed as a percentage of control cells. We also counted the number of initial cell-associated particles (at 3 minutes), calculated the association index (mean number of associated particles per cell), and expressed it as a percentage of control cells.
Immunofluorescence and wide-field imaging analysis
Immunofluorescence and image acquisition were performed as described,12 except that the samples were examined under an inverted wide-field microscope (Zeiss Axiovert 200M or Leica DMB) equipped with an oil- immersion objective (100× PL APO HCX, 1.3 or 1.4 NA, respectively) and a cooled CCD camera (ORCA ER Hamamatsu or MicroMAX Princeton Instruments, respectively). Z-series of images were taken at 0.2-μm increments. Three-dimensional reconstructions were obtained using the IsoSurface function of Imaris 5.7 software (Bitplane AG).
Fluorescence quantitation
Quantitation of fluorescence was performed using ImageJ Color Profiler software (National Institutes of Health) on selected linear regions in a merge z projection of maximum intensities of 8-bit stacks with an inverted wide-field microscope (Leica DMB) equipped with an oil immersion objective (100× PL APO HCX, 1.4 NA) and a cooled CCD camera (MicroMAX Princeton Instruments). Two areas of the cell were quantified in a linear region: the phagocytic cup and the cell body. The maximum of fluorescence intensities of each area was background corrected by subtracting the maximum value from a cell-free region. Ratio values (index) were obtained by dividing the maximum of fluorescence intensities in the phagocytic cups by the maximum of fluorescence intensities in the cell body or the bulk plasma membrane.
Protein purification
The glutathione-S-transferase (GST)–tagged Nef constructs were expressed in E coli BL21 and checked for protein induction by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Coomassie blue staining. Protein purification was performed after induction using 0.5mM Isopropyl β-D-1-thiogalactopyranoside (IPTG), overnight at 28°C. Bacteria were lysed in 50mM Tris (tris(hydroxymethyl)aminomethane), pH 7.5, 500mM NaCl, 2mM MgCl2, 1 mM dithiothreitol, 1% Triton X-100, protease inhibitors (Complete Protease Inhibitor Cocktail; Roche), and 0.5 mg/mL lysozyme during 30 minutes at 4°C. The suspension was then sonicated. After clearing the bacterial lysate by centrifugation at 42 500g for 30 minutes at 4°C, GST fusion proteins were bound to glutathione sepharose beads (GE Healthcare) and then eluted with 20mM Tris, pH 8, 100mM NaCl, 2mM β-mercaptoethanol, and 50mM glutathione. Purified proteins were dialyzed in 20mM Tris, pH 8, 100mM NaCl, and 2mM β-mercaptoethanol. Purified proteins were stored at −80°C.
GST pull-down assays, subcellular fractionation, and coimmunoprecipitation
GST pull-downs were performed with 6 to 8 million RAW264.7 cells grown to subconfluence per point. Cells were lysed in lysis buffer 1 (20mM Tris, pH 7.5, 150mM NaCl, and 0.5% Nonidet P40) supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail; Roche), 1 mM sodium orthovanadate, and 50mM NaF. An aliquot of the supernatant was kept to assess the amount of protein in the total lysate. Pull-down was performed by incubating the cell lysate with 20 μg of GST or 40 μg of GST-Nef fusion proteins and glutathione sepharose beads for 1 hour at 4°C with rotation. After 3 washes in lysis buffer 1, bound material was stored or analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting.
Immunoprecipitation was performed with 12 to 16 million RAW264.7 cells grown to subconfluence per point. Cells were transfected with a vector encoding GFP- or HA-tagged Nef. After 15 hours, cells were scraped and resuspended in culture medium without serum.
For simple coimmunoprecipitation, cells were centrifuged at 13 000g for 15 seconds, the supernatant was removed, and cells were lysed on ice in complete lysis buffer 1. The lysate was centrifuged at 13 000g for 10 minutes at 4°C. An aliquot of the supernatant was kept to assess the amount of protein in the total lysate. After a preclearing step with protein G sepharose (GE Healthcare), the supernatant was incubated rotating with 1 μg of antibody and protein G Sepharose at 4°C for 3 hours. The beads were then washed 3 times at 4°C in lysis buffer 1. The 7 samples were stored or analyzed by Western blotting.
For subcellular fractionation, cells were centrifuged at 13 000g for 15 seconds, the supernatant was removed, and cells were homogenized on ice in buffer 2 (250mM sucrose, 3mM imidazole, pH 7.5) supplemented with protease inhibitors (complete ethylenediaminetetraacetic acid–free; Roche) by repeated passages through a 25-gauge needle. The lysate was centrifuged at 600g for 10 minutes at 4°C. Supernatant was the cytosolic fraction, whereas the membrane pellet was solubilized by rotating for 30 minutes in lysis buffer 3 (0.5% Triton X-100, 10mM 1,4-piperazinediethanesulfonic acid (PIPES), pH 7.5, 300mM sucrose, 100mM NaCl, 3mM MgCl2, 3mM ethylenediaminetetraacetic acid) supplemented with protease inhibitors. Suspension was centrifuged at 5000g for 10 minutes at 4°C to remove insoluble debris. The supernatant was the membrane fraction. An aliquot of each supernatant was kept to assess the amount of protein and check for fraction purity. Immunoprecipitation and Western blotting were then performed as described in the previous paragraph, except that beads were washed in lysis buffers 2 and 3, respectively.
Statistics
The statistical significance of the data was tested with an unpaired Student t test, and calculated goodness-of-fit value (P value) is indicated in the figures. Differences were considered significant if P value was less than .05 (*) and .005 (**).
Results
Inhibition of phagocytic activity of primary human macrophages infected with HIV-1 wild-type relies on Nef
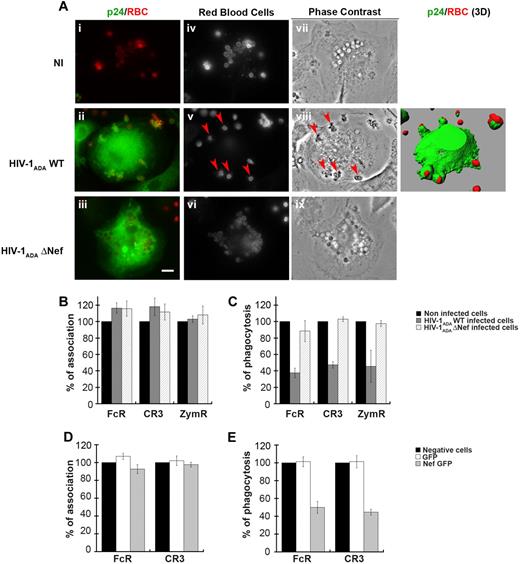
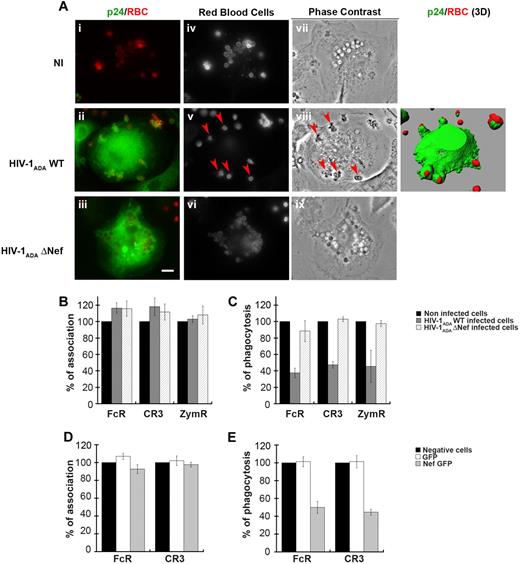
To examine the effects of HIV-1 infection on phagocytosis, we derived primary human macrophages from blood monocytes isolated from healthy donors. Monocytes were cultivated for 11 days in the presence of rhM-CSF. Expression of CD4 and CCR5 at the surface of differentiated macrophages was checked by flow cytometry (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Macrophages were then infected with different doses of HIV-1ADAWT or nef-deleted HIV-1ADA (HIV-1ADAΔNef) for 4 to 12 days, and the culture supernatant was analyzed every 2 days by enzyme-linked immunosorbent assay for p24 production. As described previously,31,32 HIV-1 wild-type and nef-deleted variants replicate with similar kinetics in macrophages with a peak of viral production 8 days after infection (supplemental Figure 1B). We therefore infected macrophages for 8 days with HIV-1ADAWT or HIV-1ADAΔNef before submitting the cells to phagocytic assays. For this, primary macrophages were allowed to interact with IgG- or complement-opsonized sheep red blood cells (IgG-RBCs or C-RBCs, respectively), or with Cy3-labeled zymosan (heat-inactivated yeast) for 60 minutes at 37°C. Macrophages were then fixed and stained to detect external RBCs, then permeabilized and labeled with anti-p24 antibodies to detect HIV-1–infected cells. As shown in Figure 1A for phagocytosis of IgG-RBCs, several IgG-RBC particles were internalized in noninfected cells, as observed by phase contrast. In contrast, infection of primary macrophages with HIV-1ADAWT often led to a defect in phagocytosis, with particles blocked outside the cells in abortive phagosomes as shown on the 3-dimensional reconstruction, whereas macrophages infected with HIV-1ADAΔNef had ingested many particles. This suggested that infection of primary human macrophages with HIV-1 induced a Nef-dependent inhibition of FcR-mediated phagocytosis. The efficiency of phagocytosis was calculated for p24-positive HIV-1–infected macrophages and compared with noninfected cells in phagocytic assays with IgG-RBCs, C-RBCs, and zymosan (Figure 1B-C). Although association of the particles to cells was not affected (Figure 1B), we observed that phagocytosis by FcR, CR3, and ZymR was inhibited by 62% plus or minus 6%, 53% plus or minus 4%, and 54% plus or minus 19%, respectively, in cells infected with HIV-1ADAWT virus (Figure 1C). Importantly, phagocytosis was not simply delayed; the same inhibition of phagocytosis was observed 3 hours after the initiation of phagocytosis (data not shown). Of note, no inhibition was observed in the p24-negative cells of the same coverslips in infected conditions, which indicates that there was no diffusive factor inhibiting phagocytosis in neighboring p24-negative cells in these experimental conditions (supplemental Figure 1C). Finally, phagocytosis was not impaired in macrophages infected with HIV-1ADAΔNef, highlighting a crucial role for the Nef accessory protein in the mechanism of inhibition of phagocytosis by HIV-1 (Figure 1C).
Inhibition of phagocytosis in HIV-1–infected human macrophages. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or were noninfected for 8 days. The cells were incubated for 60 minutes with IgG-RBCs at 37°C, then fixed and stained with Cy2-anti–rabbit IgG to reveal external RBCs (iv-vi). Particles internalized in closed phagosomes are also detected by phase contrast (vii-ix). The cells were then permeabilized and labeled with anti-p24, followed by Cy3-anti–mouse IgG. Merged images show p24 in green and RBCs in red (i-iii). Arrowheads indicate RBCs in phagocytic cups. Z stacks of wide-field fluorescent images were acquired using a piezo, and 1 medial optical section is shown. The stacks of images are presented as 3-dimensional reconstructions (right panel). Bar represents 10 μm. (B-C) HIV-1ADAWT or HIV-1ADAΔNef–infected cells were incubated for 60 minutes at 37°C with IgG-RBCs, C3bi-RBCs, or Cy3-zymosans. The cells were then treated as in panel A except that they were labeled with anti-p24, followed by Cy2-anti–mouse IgG when Cy3-zymosan was used. The efficiencies of association (B) and phagocytosis (C) were calculated for 50 infected and 50 noninfected cells (control). Results are expressed as a percentage of control cells. The means ± SEM of 3 independent experiments are plotted. (D-E) Primary human macrophages transiently expressing GFP or Nef-GFP were incubated with IgG-RBCs or C3bi-RBCs for 60 minutes at 37°C. The cells were then fixed and stained as in panel A. The efficiencies of association (D) and phagocytosis (E) were calculated and expressed as in panels B-C. The means ± SEM of 3 independent experiments are plotted.
Inhibition of phagocytosis in HIV-1–infected human macrophages. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or were noninfected for 8 days. The cells were incubated for 60 minutes with IgG-RBCs at 37°C, then fixed and stained with Cy2-anti–rabbit IgG to reveal external RBCs (iv-vi). Particles internalized in closed phagosomes are also detected by phase contrast (vii-ix). The cells were then permeabilized and labeled with anti-p24, followed by Cy3-anti–mouse IgG. Merged images show p24 in green and RBCs in red (i-iii). Arrowheads indicate RBCs in phagocytic cups. Z stacks of wide-field fluorescent images were acquired using a piezo, and 1 medial optical section is shown. The stacks of images are presented as 3-dimensional reconstructions (right panel). Bar represents 10 μm. (B-C) HIV-1ADAWT or HIV-1ADAΔNef–infected cells were incubated for 60 minutes at 37°C with IgG-RBCs, C3bi-RBCs, or Cy3-zymosans. The cells were then treated as in panel A except that they were labeled with anti-p24, followed by Cy2-anti–mouse IgG when Cy3-zymosan was used. The efficiencies of association (B) and phagocytosis (C) were calculated for 50 infected and 50 noninfected cells (control). Results are expressed as a percentage of control cells. The means ± SEM of 3 independent experiments are plotted. (D-E) Primary human macrophages transiently expressing GFP or Nef-GFP were incubated with IgG-RBCs or C3bi-RBCs for 60 minutes at 37°C. The cells were then fixed and stained as in panel A. The efficiencies of association (D) and phagocytosis (E) were calculated and expressed as in panels B-C. The means ± SEM of 3 independent experiments are plotted.
To further analyze the role of Nef in the inhibition of phagocytosis, we transiently expressed Nef in primary human macrophages by nucleofection (Figure 1D-E). Macrophages were allowed to phagocytose IgG-RBCs and C-RBCs as described previously. We observed that phagocytosis by FcR and CR3 was inhibited by 44% and 53%, respectively, in primary human macrophages transiently expressing Nef-GFP, whereas the association of particles was not affected. Therefore, expression of Nef alone was sufficient to inhibit phagocytosis in human macrophages.
These results show that infection of primary human macrophages with HIV-1ADAWT leads to a reduction in the efficiency of phagocytosis by various receptors and that the Nef accessory protein is important for this inhibition.
HIV-1 infection of macrophages does not perturb F-actin cup formation
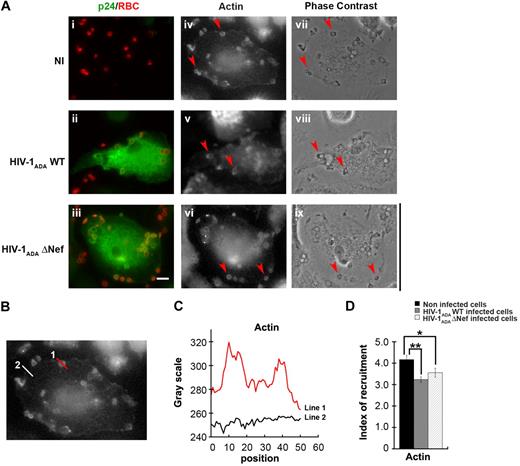
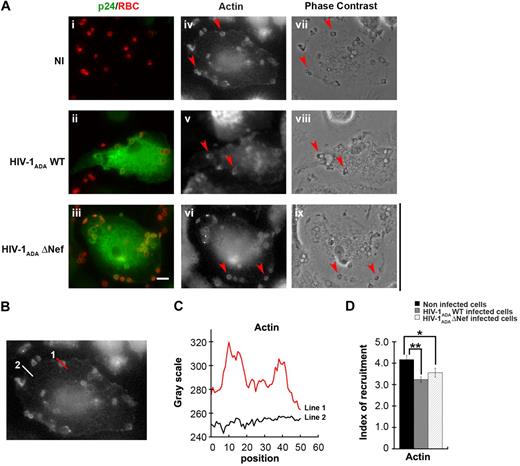
HIV-1 and Nef in particular were reported to perturb actin polymerization as well as membrane trafficking in different cell types,25 both events necessary for optimal phagosome formation in macrophages.4,,,–8 Because actin is strictly required for phagocytosis, we first set out to analyze early steps of actin reorganization in phagocytosing cells. For this, primary human macrophages were infected as in Figure 1 and then allowed to phagocytose IgG-opsonized particles for 5 minutes prior to fixation (Figure 2). Cells were then stained with anti-p24 antibodies to detect infected cells and Alexa633-phalloidin for F-actin detection (Figure 2A). The presence of F-actin at sites of particle attachment defines a phagocytic cup. In macrophages infected with HIV-1ADAWT, as well as in cells infected with HIV-1ADAΔNef, F-actin recruitment was observed at sites of particle attachment. To better define the recruitments observed, we quantified the fluorescence associated with F-actin at sites of particles attachment and compared the signal obtained with the signal measured in other sites of the cell (Figure 2B-D). We observed that F-actin was enriched at phagocytic sites by a factor of 4.2 plus or minus 0.2 in noninfected cells. Comparison of actin enrichment measured for infected and noninfected cells showed that the recruitment of F-actin at sites of phagocytosis was slightly but significantly affected in cells infected with HIV-1ADAWT (77% ± 3.2% of noninfected cells, P < .005) or cells infected with HIV-1ADAΔNef (85% ± 4.9% of control cells, P < .05; Figure 2E). This result shows that HIV-1 infection does not cause profound defects in F-actin cup formation and that the effect observed on actin is not correlated with the expression of Nef but may depend on other viral factor(s).
Presence of F-actin at phagocytic sites in HIV-1–infected macrophages. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or noninfected for 8 days. The cells were incubated for 5 minutes with IgG-RBCs, then fixed and stained with AMCA-anti–rabbit IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-p24, followed by Cy2-anti–goat IgG and Alexa633-phalloidin to stain F-actin (iv-vi). Merged images show p24 in green and RBCs in red (i-iii). Arrowheads indicate examples of F-actin cups. Cells were analyzed by wide-field fluorescence microscopy and phase contrast (vii-ix). Bar represents 10 μm. (B) Noninfected cells were treated as described in panel A. (C) The profile of F-actin fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (D) Noninfected cells and infected cells were treated as described in panel A. The fluorescence intensities measured in the phagocytic cups were background subtracted and divided by the fluorescence intensities measured for cortical actin in the cell body after background substration. This ratio defined the index of recruitment. The means ± SEM of 3 independent experiments are plotted (n = 78 actin cups per condition).
Presence of F-actin at phagocytic sites in HIV-1–infected macrophages. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or noninfected for 8 days. The cells were incubated for 5 minutes with IgG-RBCs, then fixed and stained with AMCA-anti–rabbit IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-p24, followed by Cy2-anti–goat IgG and Alexa633-phalloidin to stain F-actin (iv-vi). Merged images show p24 in green and RBCs in red (i-iii). Arrowheads indicate examples of F-actin cups. Cells were analyzed by wide-field fluorescence microscopy and phase contrast (vii-ix). Bar represents 10 μm. (B) Noninfected cells were treated as described in panel A. (C) The profile of F-actin fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (D) Noninfected cells and infected cells were treated as described in panel A. The fluorescence intensities measured in the phagocytic cups were background subtracted and divided by the fluorescence intensities measured for cortical actin in the cell body after background substration. This ratio defined the index of recruitment. The means ± SEM of 3 independent experiments are plotted (n = 78 actin cups per condition).
HIV-1 infection of macrophages impairs AP1 recruitment to the site of phagocytosis
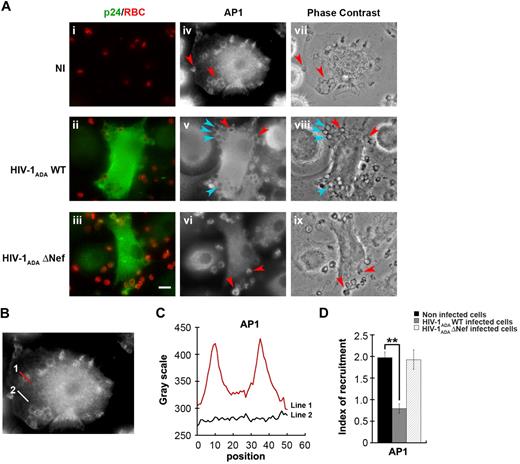
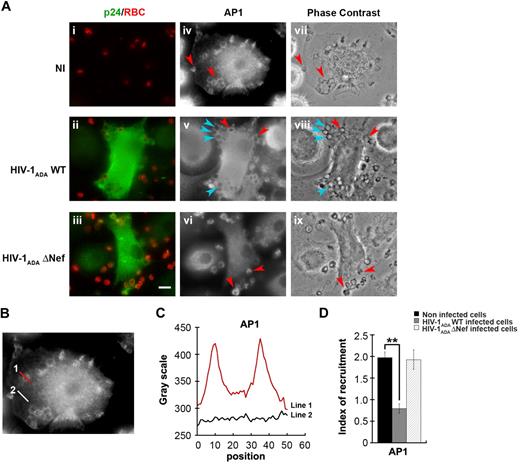
We next investigated the impact of HIV-1 infection on the endosomal membrane remodeling processes that are important for efficient phagocytosis. Because AP1 is recruited in phagocytic cups and is required for optimal FcR-mediated phagocytosis15,33 and because Nef was reported to interfere with adaptor complexes,34,–36 we analyzed the recruitment of AP1 in macrophages infected with HIV-1 (Figure 3). As observed previously in RAW264.7 murine cells,15 AP1 is recruited and enriched in phagocytic cups of primary human macrophages by a factor of 2 plus or minus 0.1 in noninfected cells (Figure 3A-B). Interestingly, the recruitment of AP1 at sites of phagocytosis was inhibited in cells infected with HIV-1ADAWT (60% ± 3% of control cells), but there was no inhibition in macrophages infected with HIV-1ADAΔNef (97.3% ± 9.4% of control cells). These results indicate that HIV-1 infection of human macrophages severely impairs the recruitment of AP1 complexes at phagocytic sites and that Nef is important for this effect.
Inhibition of AP1 recruitment at phagocytic sites in HIV-1 infected cells. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or noninfected for 8 days. The cells were incubated for 5 minutes with IgG-RBCs, fixed, and stained with AMCA-anti–rabbit IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-p24 followed by Cy2-anti–goat IgG and with anti-AP1 (γ-adaptin) followed by Cy3-anti–mouse IgG (iv-vi). Merged images show p24 in green and RBCs in red (i-iii) and phase-contrast images are shown (vii-ix). Red arrowheads indicate AP1 enrichment in some forming phagosomes, whereas cyan arrowheads show no enrichment. Bar represents 10 μm. (B) Noninfected cells were treated as described in panel A. (C) The profile of AP1 (γ-adaptin) fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (D) The AP1 fluorescence intensity measured in the phagocytic cups were background subtracted and divided by the AP1 fluorescence intensity measured in the cell body after background substraction. This ratio defines the index of recruitment. The means ± SEM of 3 independent experiments, each with 6 noninfected and 6 infected cells, are plotted.
Inhibition of AP1 recruitment at phagocytic sites in HIV-1 infected cells. (A) Primary human macrophages were infected with HIV-1ADAWT or HIV-1ADAΔNef or noninfected for 8 days. The cells were incubated for 5 minutes with IgG-RBCs, fixed, and stained with AMCA-anti–rabbit IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-p24 followed by Cy2-anti–goat IgG and with anti-AP1 (γ-adaptin) followed by Cy3-anti–mouse IgG (iv-vi). Merged images show p24 in green and RBCs in red (i-iii) and phase-contrast images are shown (vii-ix). Red arrowheads indicate AP1 enrichment in some forming phagosomes, whereas cyan arrowheads show no enrichment. Bar represents 10 μm. (B) Noninfected cells were treated as described in panel A. (C) The profile of AP1 (γ-adaptin) fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (D) The AP1 fluorescence intensity measured in the phagocytic cups were background subtracted and divided by the AP1 fluorescence intensity measured in the cell body after background substraction. This ratio defines the index of recruitment. The means ± SEM of 3 independent experiments, each with 6 noninfected and 6 infected cells, are plotted.
Nef motifs involved in phagocytosis inhibition in macrophages
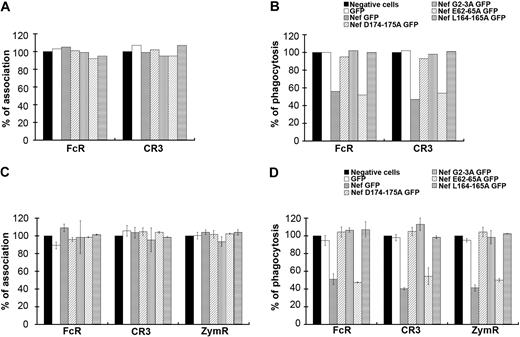
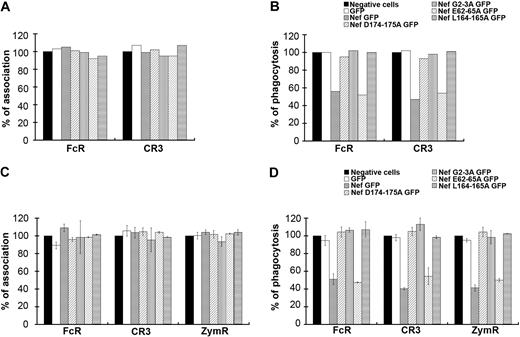
To gain further insight into the role of Nef during phagocytosis, we expressed mutants of the protein with substitutions in the LL (Nef L164-165A) and the DD (Nef D174-175A) motifs, which were both reported to bind to AP complexes34,–36 (Figure 4A-B). The DD motif was also shown to be involved in the interaction of Nef with the V1H subunit of the endosomal vacuolar adenosine triphosphatase.37,38 We also expressed a mutant in the myristoylation site (Nef G2A) and a mutant in the acidic cluster (Nef E62-65A), which was reported to be important for MHCI trafficking regulation.39,40 All these constructs were expressed as GFP fusion proteins in primary human macrophages after nucleofection. The efficiencies of particle binding and phagocytosis were monitored in cells expressing the fusion proteins and compared with GFP-negative cells on the same coverslips. Inhibition of phagocytosis was observed in Nef wild-type– and Nef E62-65A–expressing cells, but not in Nef L164-165A–, Nef D174-175A–, and Nef G2A–expressing cells (Figure 4B). The association of particles was not affected by the expression of any of the Nef mutants (Figure 4A). Because it is difficult to obtain enough primary macrophages transiently expressing the various constructs to perform this phagocytic assay, we also used the RAW264.7 murine macrophage cell line, which is readily transfectable. Transient expression of Nef in RAW264.7 macrophages leads to inhibitory effects similar to that observed in primary human macrophages (Figure 4D). Expression of Nef wild-type and Nef E62-65A led to inhibition of phagocytosis by FcR and CR3 as observed for primary human macrophages from one donor, and also to inhibition of uptake of zymosan. In contrast, expression of Nef L164-165A, Nef D174-175A, and Nef G2A did not affect phagocytosis. Association was not impaired by the expression of Nef wild-type or mutants (Figure 4C).
Motifs of Nef involved in phagocytosis inhibition. (A-B) Primary human macrophages transiently expressing GFP or Nef-GFP or the indicated Nef mutants: Nef D174-175A GFP, Nef G2A GFP, Nef E62-65A GFP, and Nef L164-165A GFP were allowed to phagocytose IgG-RBCs or C3bi-RBCs for 60 minutes at 37°C. The samples were processed as in Figure 1. The efficiencies of association (A) and phagocytosis (B) were calculated for 50 GFP-positive and 50 GFP-negative cells (control). Results are expressed as a percentage of control GFP-negative cells. One experiment is shown. (C-D) RAW264.7 macrophages transiently expressing GFP or Nef-GFP or the indicated mutants were allowed to phagocytose IgG-RBCs, C3bi-RBCs, or Cy3-zymosans for 60 minutes at 37°C. The samples were processed as in Figure 1. The efficiencies of association (C) and phagocytosis (D) were calculated for 50 GFP-positive and 50 GFP-negative cells (control). Results are expressed as a percentage of control cells. The means ± SEM of at least 3 independent experiments are plotted.
Motifs of Nef involved in phagocytosis inhibition. (A-B) Primary human macrophages transiently expressing GFP or Nef-GFP or the indicated Nef mutants: Nef D174-175A GFP, Nef G2A GFP, Nef E62-65A GFP, and Nef L164-165A GFP were allowed to phagocytose IgG-RBCs or C3bi-RBCs for 60 minutes at 37°C. The samples were processed as in Figure 1. The efficiencies of association (A) and phagocytosis (B) were calculated for 50 GFP-positive and 50 GFP-negative cells (control). Results are expressed as a percentage of control GFP-negative cells. One experiment is shown. (C-D) RAW264.7 macrophages transiently expressing GFP or Nef-GFP or the indicated mutants were allowed to phagocytose IgG-RBCs, C3bi-RBCs, or Cy3-zymosans for 60 minutes at 37°C. The samples were processed as in Figure 1. The efficiencies of association (C) and phagocytosis (D) were calculated for 50 GFP-positive and 50 GFP-negative cells (control). Results are expressed as a percentage of control cells. The means ± SEM of at least 3 independent experiments are plotted.
Together, these results indicate that the LL and DD motifs, required for interactions of Nef with components of the endocytic machinery, including AP complexes, as well as the myristoylation site important for membrane anchoring are important for the inhibition of phagocytosis in macrophages.
Interaction between Nef and AP1 in cytosol and membrane fractions of macrophages
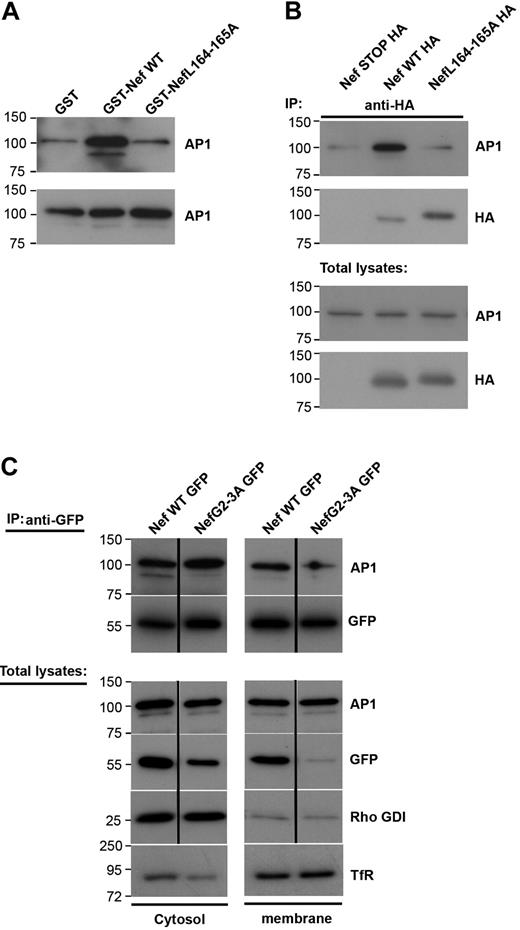
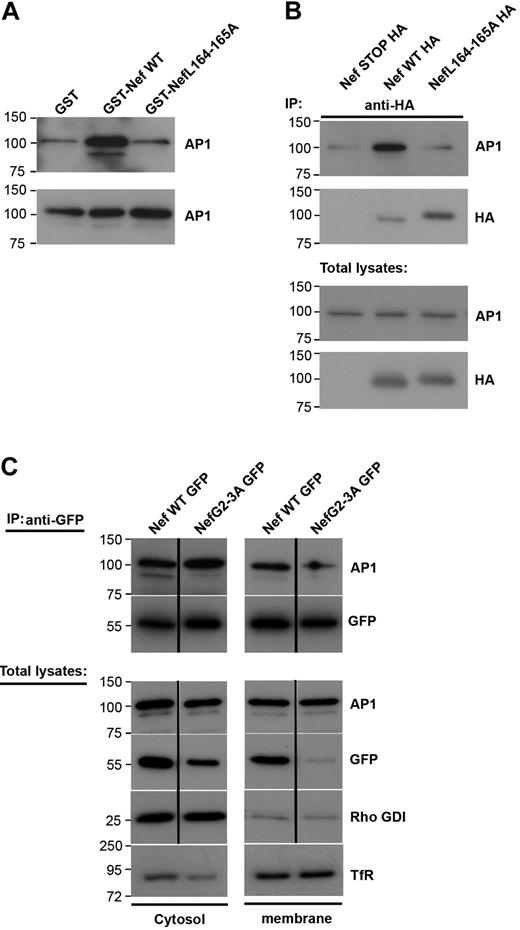
Because Nef inhibits the recruitment of AP1 to the phagocytic cup during phagocytosis (Figure 3), we set out to analyze further the link between Nef and AP1 in macrophages. For this, we performed GST pull-down assays with purified recombinant GST-Nef wild-type or GST-Nef-L164-165A proteins and cell lysates from the RAW264.7 macrophages (Figure 5A). As reported previously in fibroblastic cell lines,29,35,41 Nef interacts with AP1 in cell extracts and this interaction depends on the dileucine motif of Nef (Figure 5A). As reported earlier,15 AP1 was present in 2 forms in macrophages (ie, a lower and a higher molecular weight product) and Nef was found to associate with both forms. The lower molecular weight band of γ-adaptin represents a cleaved form of the protein that plays a role as a regulator of endosome to plasma membrane recycling. We further tested whether the Nef and AP1 proteins could be found in association in macrophages. We transiently expressed Nef-HA in RAW264.7 macrophages and immunoprecipitated the protein with anti-HA antibodies (Figure 5B). We observed that AP1 was coimmunoprecipitated with Nef but not with a control construct (Nef-STOP-HA). As in pull-down assays, the LL motif of Nef was important for Nef to associate with AP1 (Figure 5B). To better define the interaction between Nef and AP1, we fractionated macrophage extracts and performed coimmunoprecipitation of the cytosolic and membrane fractions (Figure 5C). As expected, AP1 was found in both fractions. Nef was also present in the cytosolic as well as the membrane fraction, as reported previously.42,43 We observed that the coimmunoprecipitation was detectable in both fractions, indicating that Nef is able to interact with AP1 in the cytosol as well as in association with membranous compartments. Interestingly, the nonmyristoylated G2A mutant that was found predominantly in the cytosol, and was enriched during immunoprecipitation, was unable to interact with the lower molecular weight form of γ-adaptin, but was consistently associated with the higher form of AP1 γ-adaptin in the cytosol.
Nef L164-165L motif is required for interaction with AP1 and inhibition of macrophages phagocytosis. (A) GST, GST-Nef WT, or GST-NefL164-165A bound to glutathione sepharose beads were incubated with RAW264.7 macrophages lysates. Bound material (top panel) and total lysates (bottom panel) were subjected to Western blotting with anti-AP1 antibodies. Molecular weight markers are in kilodaltons. (B) RAW264.7 macrophages transiently expressing either Nef STOP-HA or Nef WT-HA or NefL164-165A-HA were lysed and immunoprecipitated with anti-HA antibodies. The precipitates and total lysates were revealed by Western blotting with anti-AP1 antibodies and after stripping with anti-HA antibodies. Molecular weight markers are in kilodaltons. (C) RAW264.7 macrophages transiently expressing either Nef WT-GFP or Nef G2-3A-GFP were fractionated to obtain a cytosolic and a membrane fraction. Then each fraction was immunoprecipitated with anti-GFP antibodies. The precipitates and total lysates were subjected to Western blotting with anti-AP1 antibodies and after stripping with anti-GFP antibodies. Total lysates were also revealed with anti–transferrin receptor antibodies (as marker for the membrane fraction) or with anti-Rho GDI alpha antibodies (as marker for the cytosolic fraction). The cytosolic fraction was contaminated with proteins of membrane origin. Molecular weight markers are in kilodaltons.
Nef L164-165L motif is required for interaction with AP1 and inhibition of macrophages phagocytosis. (A) GST, GST-Nef WT, or GST-NefL164-165A bound to glutathione sepharose beads were incubated with RAW264.7 macrophages lysates. Bound material (top panel) and total lysates (bottom panel) were subjected to Western blotting with anti-AP1 antibodies. Molecular weight markers are in kilodaltons. (B) RAW264.7 macrophages transiently expressing either Nef STOP-HA or Nef WT-HA or NefL164-165A-HA were lysed and immunoprecipitated with anti-HA antibodies. The precipitates and total lysates were revealed by Western blotting with anti-AP1 antibodies and after stripping with anti-HA antibodies. Molecular weight markers are in kilodaltons. (C) RAW264.7 macrophages transiently expressing either Nef WT-GFP or Nef G2-3A-GFP were fractionated to obtain a cytosolic and a membrane fraction. Then each fraction was immunoprecipitated with anti-GFP antibodies. The precipitates and total lysates were subjected to Western blotting with anti-AP1 antibodies and after stripping with anti-GFP antibodies. Total lysates were also revealed with anti–transferrin receptor antibodies (as marker for the membrane fraction) or with anti-Rho GDI alpha antibodies (as marker for the cytosolic fraction). The cytosolic fraction was contaminated with proteins of membrane origin. Molecular weight markers are in kilodaltons.
These results indicate that Nef interferes with AP1 via its dileucine motif by binding to membrane-associated and/or cytosolic AP1.
HIV-1 infection of macrophages perturbs the recruitment of VAMP3-positive but not VAMP7-positive endosomes at the site of phagocytosis
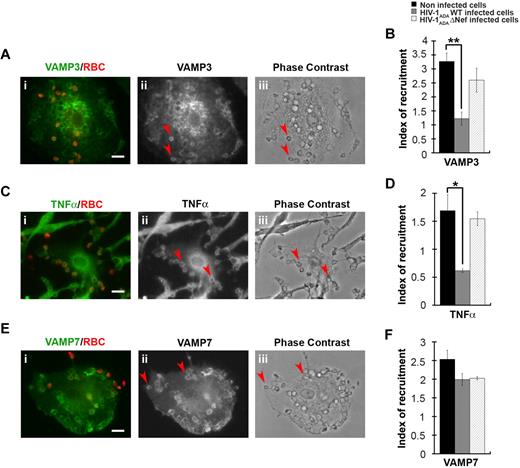
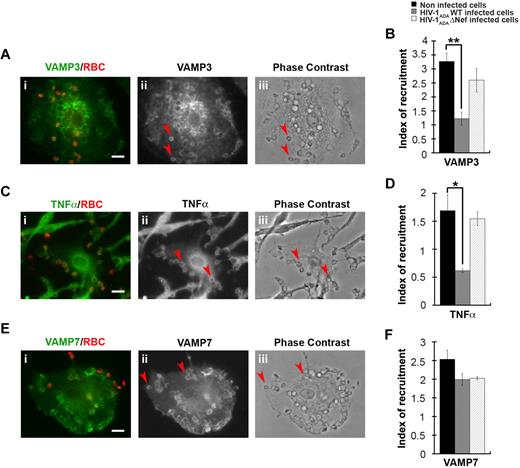
We have previously shown that efficient phagocytosis of large particles relies on focal delivery of endosomal compartments, including recycling endosomes bearing VAMP3 or TNFα under the control of AP1,15 as well as a population of late endosomes expressing VAMP7.12 We therefore analyzed the recruitment of VAMP3, TNFα, and VAMP7, which were enriched in phagocytic cups from noninfected primary human macrophages by a factor of 3 plus or minus 0.3, 1.7 plus or minus 0.3, and 2.5 plus or minus 0.2, respectively (Figure 6). We observed that the recruitment of VAMP3 and TNFα were impaired in HIV-1ADAWT-infected macrophages, whereas the recruitment of VAMP7 was unaffected. No inhibition of recruitment of VAMP3 and TNFα was detected when the cells were infected with HIV-1ADAΔNef (Figure 6B,D,F).
HIV-1 infection perturbs membrane delivery of VAMP3- and TNFα-positive compartments but not VAMP7-positive compartments during phagocytosis. (A) Primary human macrophages were incubated for 5 minutes with IgG-RBCs, fixed, and stained with AMCA-anti–mouse IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-VAMP3, followed by Cy3-anti–rabbit IgG (ii). Merged images show VAMP3 in green and RBCs in red (i). Arrowheads indicate VAMP3 enrichment in phagocytic cup in formation. Cells were analyzed by wide-field fluorescence microscopy and a phase-contrast image is shown (iii). Bar represents 10 μm. (C) Macrophages were treated as in panel A, except that they were labeled with anti-TNFα, followed by Cy3-anti–mouse IgG (ii). Merged images show TNFα in green and RBCs in red (i). Arrowheads indicate TNFα enrichment in phagocytic cup in formation. Bar represents 10 μm. (E) Macrophages were treated as in panel A, except that they were labeled with anti-VAMP7, followed by Cy3-anti–mouse IgG (ii). Merged images show VAMP7 in green and RBCs in red (i). Arrowheads indicate VAMP7 enrichment in phagocytic cup in formation. Bar represents 10 μm. (B,D,F) The VAMP3 (B), TNFα (D), or VAMP7 (F) fluorescence intensities measured in the phagocytic cups were background subtracted, and divided by the respective fluorescence intensities measured in the cell body after background substraction. The ratio defines the index of recruitment. The means ± SEM of 3 independent experiments with 6 noninfected and 6 infected cells are plotted. HIV-1ADAWT infection significantly inhibited VAMP3 and TNFα, but not VAMP7, recruitment at the phagocytic cup compared with control cells.
HIV-1 infection perturbs membrane delivery of VAMP3- and TNFα-positive compartments but not VAMP7-positive compartments during phagocytosis. (A) Primary human macrophages were incubated for 5 minutes with IgG-RBCs, fixed, and stained with AMCA-anti–mouse IgG to reveal external RBCs. The cells were then permeabilized and labeled with anti-VAMP3, followed by Cy3-anti–rabbit IgG (ii). Merged images show VAMP3 in green and RBCs in red (i). Arrowheads indicate VAMP3 enrichment in phagocytic cup in formation. Cells were analyzed by wide-field fluorescence microscopy and a phase-contrast image is shown (iii). Bar represents 10 μm. (C) Macrophages were treated as in panel A, except that they were labeled with anti-TNFα, followed by Cy3-anti–mouse IgG (ii). Merged images show TNFα in green and RBCs in red (i). Arrowheads indicate TNFα enrichment in phagocytic cup in formation. Bar represents 10 μm. (E) Macrophages were treated as in panel A, except that they were labeled with anti-VAMP7, followed by Cy3-anti–mouse IgG (ii). Merged images show VAMP7 in green and RBCs in red (i). Arrowheads indicate VAMP7 enrichment in phagocytic cup in formation. Bar represents 10 μm. (B,D,F) The VAMP3 (B), TNFα (D), or VAMP7 (F) fluorescence intensities measured in the phagocytic cups were background subtracted, and divided by the respective fluorescence intensities measured in the cell body after background substraction. The ratio defines the index of recruitment. The means ± SEM of 3 independent experiments with 6 noninfected and 6 infected cells are plotted. HIV-1ADAWT infection significantly inhibited VAMP3 and TNFα, but not VAMP7, recruitment at the phagocytic cup compared with control cells.
Together, these results show that HIV-1 infection impairs the membrane remodeling processes involving recycling endosomes bearing VAMP3, TNFα, and AP1, but not the late endosomes involving VAMP7. Our results point to a crucial role of Nef in this inhibition.
Discussion
In this study, we revealed a defect in phagocytosis in HIV-1–infected macrophages that can be explained by a default in focal delivery of intracellular membranes. The Nef virulence factor was essential for phagocytosis inhibition, as it interacts with the AP1 complexes that are required for optimal phagosome formation.15,33
We observed that phagocytosis of particles targeting different phagocytic receptors was impaired in macrophages infected with HIV-1, suggesting that the defect observed is related to a mechanism that is common for early phagosome formation, whatever the specificities in signaling that may be associated with specific receptors.2,4 The inhibition of phagocytosis observed is in agreement with previous studies on FcR- and CR3-mediated phagocytosis as well as phagocytosis of Candida albicans.21,–23 We further demonstrated, using Nef-deleted viruses, that Nef is a crucial viral element in perturbing phagocytosis in HIV-1–infected cells in our system, and we observed no inhibitory effects on the neighboring noninfected cells on coverslips containing HIV-1–infected macrophages. This indicates that, in contrast to what was shown for monocyte migration,44 there was no apparent effect of soluble diffusive Nef in this system or of Nef diffusing within nanotubes between macrophages, as reported recently between macrophages and B lymphocytes.45 HIV-1 infection, and Nef expression in particular, is linked with down-regulation of surface expression of receptors, such as CD4, MHCI, MHCII, and CD28 (for review, see Foster and Garcia25 ). Therefore, diminished phagocytic receptor expression could have explained the reduction in phagocytosis efficiency. However, as previously reported,21,–23 we did not observe any defect in particle association to phagocytes, indicating that the binding capacities of Nef-expressing cells are unaffected and the receptor availability is unchanged, for all receptors assayed. Therefore, the defect in phagocytosis is downstream of receptor ligation. Signaling defects associated with phagocytosis by FcR and CR3 have already been described,22,23 and we further investigated the defect observed in infected macrophages, in light of recent findings on actin and membrane reorganization events necessary for optimal phagosome formation.4,,,–8
HIV-1 infection and in particular Nef have been described to perturb signaling to actin polymerization and cell migration.46 Our results clearly show that under our experimental conditions, the presence of F-actin in cups was hardly diminished. Moreover, the slight defect in F-actin recruitment that was measured was not dependent on Nef expression. This is likely linked to differential effects of HIV infection in macrophages versus T lymphocytes. Indeed, migration was shown recently to be inhibited in HIV-1–infected T lymphocytes,47 whereas F-actin–rich protrusions were induced in Nef-expressing macrophages.45 We subsequently analyzed in detail the phagosomes forming after FcR triggering, because these are the best described and are the site of intense and efficient recruitment of diverse membrane compartments. We observed that VAMP3, VAMP7, TNFα, and AP1 were highly enriched in phagocytic cups in human primary macrophages, thus confirming previous data obtained essentially in murine macrophage cell lines.10,–12,15,48 Second, we noticed that VAMP3, AP1, and TNFα recruitment in phagocytic cups, but not that of VAMP7, was altered in macrophages infected with HIV-1 but not in macrophages infected with HIV-1ΔNef. These results provide interesting insight into the mechanism of phagosome formation that is altered in infected macrophages. Nef has been reported to induce the accumulation of multivesicular endosomes and has recently been shown to perturb the multivesicular bodies/autophagic pathway in human macrophages.49 Therefore Nef could have been expected to alter the recruitment in phagocytic cups of the subpopulation of late endosomes bearing VAMP7, but this was not observed. In contrast, our results clearly point to an inhibition by Nef of the focal recycling of endomembranes labeled with AP1, VAMP3, and TNFα. In previous studies, we reported that Nef interferes with transferrin recycling28 and that AP1 was important for the efficient recycling of VAMP3- and TNFα-positive endosomes in macrophages.15 The interaction of Nef with AP1 could therefore explain the defective recruitment of these endosomal compartments to the phagocytic cup.
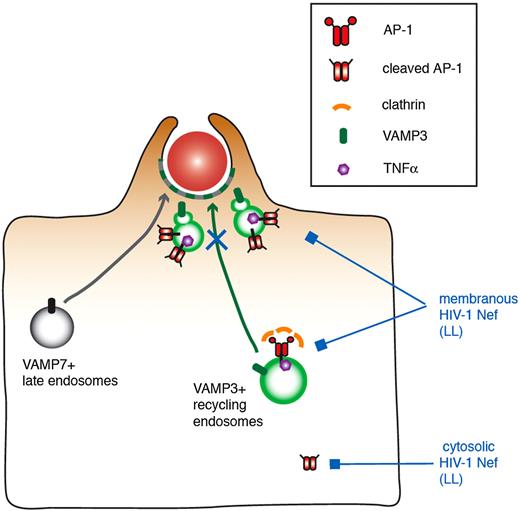
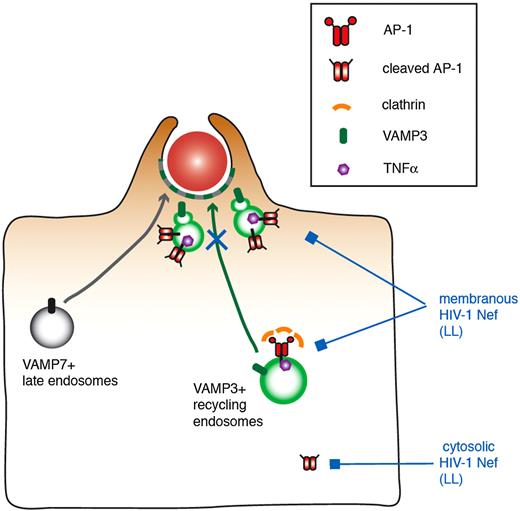
The role of Nef on the AP1 complexes was confirmed by the fact that mutants of Nef impaired in AP1 binding (LL and DD)34,–36 are also impaired in their ability to inhibit phagocytosis. This was not the case of the Nef acidic cluster mutant that was reported to play a role in recruitment of Phosphofurin acidic cluster sorting protein 1 and in MHCI trafficking regulation.39,40 We indeed further showed that Nef interacted with AP1 in macrophages by pull-down and coimmunoprecipitation experiments and that the dileucine motif of Nef was important for this interaction in macrophages, as reported previously in other cells.29,35,41 Nef interacted with both the higher and the lower molecular weight species of γ-adaptin subunit of AP-1. The lower molecular weight protein is of a size compatible with the cleavage of the ear domain, as reported previously in dendritic cells and in monocytes/macrophages.15,50 Based on the lack of clathrin recruitment at sites of particle attachment, we proposed that the complexes associated with the vesicles present under phagocytic cups are the cleaved complexes that could serve as a platform for sorting and retention of some cargos to be delivered at phagocytic sites.15 Here we show that the interaction took place in cytosol and membrane fractions of the cells. Interestingly, we consistently observed that the nonmyristoylated G2A mutant of Nef, which is less associated with membranes than Nef, and inefficient to inhibit phagocytosis, was unable to coimmunoprecipitate the lower molecular weight band form of AP1. Therefore, our data support a speculative model in which Nef interacts via its LL motif with AP1, either membranous, or with the cleaved form of the protein in the cytosol (Figure 7). This in turn would affect the localization of the AP1 complex in HIV-infected macrophages and would impair the plasmalemmal recruitment of endomembranes bearing the VAMP3 and TNFα markers that are known to be essential for optimal phagocytosis.
Speculative model of the role of Nef in the inhibition of membrane remodeling during phagocytosis in HIV-1–infected macrophages. VAMP3-positive recycling endosomes bearing TNFα and VAMP7-positive late endosomes are recruited and fuse with the plasma membrane, thus contributing to pseudopod extension and optimal phagosome formation. AP1, and in particular the cleaved form of the complex that is unable to bind to clathrin, was proposed to regulate the focal delivery of VAMP3- and TNFα-positive membranes. In this study, we show that Nef interacts via its LL motif with AP1, associated either with membranes or with the cleaved form of the protein in the cytosol. This in turn would impair the plasmalemmal recruitment of endomembranes bearing the VAMP3 and TNFα markers that are known to be essential for optimal phagocytosis, without affecting F-actin cup formation or VAMP7-positive compartment delivery.
Speculative model of the role of Nef in the inhibition of membrane remodeling during phagocytosis in HIV-1–infected macrophages. VAMP3-positive recycling endosomes bearing TNFα and VAMP7-positive late endosomes are recruited and fuse with the plasma membrane, thus contributing to pseudopod extension and optimal phagosome formation. AP1, and in particular the cleaved form of the complex that is unable to bind to clathrin, was proposed to regulate the focal delivery of VAMP3- and TNFα-positive membranes. In this study, we show that Nef interacts via its LL motif with AP1, associated either with membranes or with the cleaved form of the protein in the cytosol. This in turn would impair the plasmalemmal recruitment of endomembranes bearing the VAMP3 and TNFα markers that are known to be essential for optimal phagocytosis, without affecting F-actin cup formation or VAMP7-positive compartment delivery.
The inhibition of phagocytosis that we observed was partial, implicating that some phagosomes do form and close in infected cells. Whether these phagosomes mature normally and evolve as phagolysosomes is currently not known, and this will be the focus of a future study. Infection of macrophages by HIV-1 might therefore decrease the efficiency of uptake of newly produced virions, and in association with impaired degradation of virions and increased macrophage survival, this could lead to higher HIV-1 yields. Recent advances in our capacity to manipulate primary human macrophages should allow us to better understand the unique interplay between the virus and macrophages, which may be considered, together with memory T cells, as the last obstacle preventing the eradication of HIV-1 in treated patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Isabelle Maridonneau-Parini (IPBC) and Prof Jean-Louis Mège and Dr Eric Ghigo (Université de la Méditerrannée) for helpful discussions on primary macrophage culture and Prof Roger Garsia (Department of Clinical Immunology, Royal Prince Alfred Hospital) for discussions on HIV-1 infection. We thank Benedicte Capron and Catherine Fabre (EFS Saint Vincent de Paul) for buffy coat supply, Jean-Françcois Alkombre and his team (INRA, Center de Jouy-en-Josas) for collecting samples of sheep blood, Pierre Bourdoncle (Cochin Imaging Facility) for help with image analysis, and Dr Mark Scott for reading the paper.
This work was supported by a collaborative grant from ANRS (Agence Nationale de la Recherche sur le SIDA et les Hépatites C; F.N. and S.B.), and grants from the Fondation pour la Recherche Médicale (INE20041102865), CNRS (ATIP Program), and Ville de Paris (F.N.). J.M. is supported by a doctoral fellowship from ANRS. J.B. is supported by a Bourse de Docteur Ingénieur (BDI) cofunded by CNRS and Inserm.
Authorship
Contribution: J.M., F.H., and J.B. designed and performed research and analyzed data; and A.B., S.B., and F.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Florence Niedergang, Institut Cochin, Phagocytosis and Bacterial Invasion Group, Department of Cell Biology and Host Pathogen Interactions, 22, rue Méchain, F-75014 Paris, France; e-mail: florence.niedergang@inserm.fr.