Abstract
Erythropoiesis stimulating agents (ESAs) have been reported to activate erythropoietin receptors (EpoR) on cell types, including endothelial, neuronal, renal tubule, and cardiac cells. ESAs have also been reported to promote angiogenesis. However, those findings are controversial and confounded by methodologic issues. We show that EpoR mRNA was detected in essentially all cell types examined, including primary human endothelial, renal, cardiac, and neuronal cells but 10- to 100-fold lower than Epo-responsive cells using quantitative reverse-transcribed polymerase chain reaction. Total endothelial EpoR protein examined using a new monoclonal antibody was low to undetectable. Surface EpoR on endothelial cells was not detected using [125I]-rHuEpo surface-binding studies. There was no evidence of ESA-induced intracellular signaling in endothelial cells. There was a similar lack of EpoR expression and signaling in other cell types examined. Experiments were performed examining ESA function on these cells. An in vivo rat corneal angiogenesis assay demonstrated neo-vessel formation in response to recombinant human vascular endothelial growth factor (rHuVEGF). However, recombinant mouse Epo did not induce vessel formation. Similarly, ESAs did not reproducibly provide cytoprotection to neuronal, renal, or cardiac cells. Taken together, our data challenge the notion of presence or function of EpoR on nonhematopoietic cells, and call into question the preclinical basis for clinical studies exploring direct, “pleiotropic” actions of ESAs.
Introduction
Erythropoietin receptor (EpoR) and its cognate ligand erythropoietin (Epo) function to prevent apoptosis of erythroid progenitors, allow for erythrocyte maturation, and are essential for definitive erythropoiesis. However, expression of functional EpoR was also reported in endothelial cells (reviewed by Arcasoy1 ). rHuEpo and other erythropoiesis-stimulating agents (ESAs) were reported to stimulate nitric oxide synthase expression, induce proliferation in endothelial cell preparations, and stimulate angiogenesis in chick embryo chorioallantoic membrane, mouse uterine, and rodent tumor models through direct stimulation of endothelial EpoR.
Some data also suggested that EpoR may be functionally expressed in other nonhematopoietic cells, such as cardiac myocytes, kidney, and neuronal cells, and ESAs have been reported to be cytoprotective for these cells.1 Antiapoptotic signaling pathways downstream of EpoR were reportedly activated by ESAs to inhibit cell death associated with cytotoxic insult (eg, ischemia, reperfusion injury, and exposure to cytotoxins) both in vitro and in vivo. It has also been hypothesized that alternative ESA-binding receptor complexes, such as a heteroreceptor composed of the granulocyte-macrophage colony-stimulating factor/interleukin-3 (IL-3)/IL-5 receptor β-common chain and EpoR, may mediate the cytoprotective activities of ESAs.2 These reports have formed the basis for a number of clinical studies examining the “direct” action of ESAs in diseases, such as stroke and myocardial infarction.
However, the data surrounding the expression of functional EpoR or alternative receptors in endothelial and other nonhematopoietic cells are conflicting and confounded for a number of reasons. First, reports describing EpoR protein expression used nonspecific antibodies, which produce false positive results.3,4 Second, when surface EpoR was examined on nonerythroid cells using rHuEpo-binding studies, the reported receptor characteristics were very different from that known for erythroid EpoR: that is, receptor affinity was extremely low and receptor number unusually high compared with erythroid cells.5–7 Although alternative ESA receptor complexes2 could theoretically explain differences in the affinity and receptor number, other studies have found no evidence for alternative ESA receptor complexes.8,9
In addition, there are conflicting data surrounding the presence of functional endothelial EpoR. ESAs were unable to stimulate the expression of vasoactive factors in vitro,10 did not induce endothelial nitric oxide synthase expression or response in rats,11 did not stimulate vasoconstriction of arterioles in humans,12 and did not influence vascular density in rodent tumor models.13 Other studies were confounded by cross-species inactivity of rHuEpo: rHuEpo had no effect on chicken erythroid cells14 yet reportedly stimulated angiogenesis in a chick embryo chorioallantoic membrane assay.15 Similarly, in some nonhematopoietic tissue protection in vivo models, ESAs were unable to preserve renal function after ischemia-reperfusion injury in vivo,16 did not alter lipopolysaccaharide-induced myocardial depression or expression of proapoptotic or antiapoptotic genes in the heart,17 and did not provide neuroprotection in several different models.18,19 Given the methodologic issues, conflicting data regarding the expression and function of EpoR, and other hypothetical ESA-binding receptor complexes in endothelial and other nonhematopoietic cells, we investigated this further.
Methods
Cell source and culture
Cell lines.
EpoR+ control UT-7/Epo cells (human megakaryoblastic leukemia line) and OCIM-1 cells (erythroleukemia cell line) were generous gifts from Dr N. Komatsu (Jichi Medical School, Tochigi, Japan) and Dr V. Broudy (University of Washington, Seattle). Cells were grown in Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum (FBS), 1x penicillin/streptomycin/glutamine (PSG; Invitrogen), and UT-7/Epo were supplemented with 1 U/mL rHuEpo (Amgen). EpoR− control cell line 769-P20 (human renal adenocarcinoma) from ATCC was cultured in Dulbecco modified Eagle medium high glucose (4.5 g/L), 10% FBS, and PSG. Immunoblot-positive and -negative control lysates from COS-7 cells transfected with a FLAG-full-length human EpoR or empty vector (pcDNA3.1a) were generated as described.3 Human neuroblastoma SH-SY5Y cell line was cultured in F12 media with 10% FBS; rat pheochromocytoma PC-12 cell line was cultured in RPMI 1640 with 10% FBS and 5% horse serum.
Primary human cells.
All primary human nonhematopoietic cells and growth media were obtained from Lonza and cultured according to the manufacturer's instructions. Human endothelial cells were from umbilical vein (HUVECs), coronary artery (HCAEC), pulmonary microvascular endothelium (HMVEC-LBl), and dermal microvascular endothelium (HMVEC-dBl). HUVECs were grown in EGM-2 medium with EGM-2 BulletKit growth supplements. Other endothelial cell preparations were grown in EGM-2MV medium with EGM-2MV BulletKit growth supplements. Passages were minimized to less than 5 and not cultured for longer than 2 to 3 weeks. Primary human renal proximal tubule epithelial cells (RPTECs) were cultured at 37°C at 5% CO2 in REGM BulletKit media. Cells were cultured for 1 or 2 passages. Human cardiac myocytes were supplied in culture and not passaged. Human tissue samples for Western immunoblots were derived from Zoion and the National Resource Center.
Rat neonatal cardiac myocytes.
All animal studies were approved by the Institutional Animal Care and Use Committee of Amgen. Rat neonatal cardiac myocytes were purchased from ScienCell Research Laboratories or isolated directly. Isolated myocytes were generated using a method similar to that used in other studies reporting the in vitro cardiac effects of ESAs.21 Hearts from 1- to 3-day-old rats were dissected, finely macerated, and washed with ice-cold phosphate-buffered saline (PBS; 2 or 3 times), dissociated further with 1 mg/mL collagenase (Worthington Biochemical) in PBS prewarmed to 37°C with gentle agitation and repeated pipetting for 25 to 30 minutes. Cells were transferred to culture media (Dulbecco modified Eagle medium, 10% FBS, and PSG; Invitrogen), plated in flasks for 30 to 45 minutes at 37°C and 5% CO2 to allow clumped cells and nonmyocytes to adhere, and then nonadherent cells were transferred to fresh flasks and cultured overnight. Only cultures containing contractile cells (> 20%) were used, generally within 1 week.
mRNA generation and quantitative RT-PCR
Human tissue mRNA was obtained from commercial sources: brain (Ambion), kidney (Clonetech), heart, lung, and liver (Stratagene). Other mRNAs were isolated using Trizol (Invitrogen) per the manufacturer's instructions, and cDNA was synthesized using OmniScript RT (QIAGEN). Quantitative polymerase chain reaction (PCR) was performed as previously described20 except that 24 ng cDNA was used in a Lightcycler 1.5 machine (Roche Applied Science). EPOR PCR primers were located in exons 5 and 820 or both in EPOR exon 8 (forward primer, 5′-CTACCCCACCCCACCTAAAG-3′; reverse primer, 5′-GCCTCGCCATCCCTGTT-3). Human cyclophilin A levels determined with PCR primers located in exons 1 and 4 were used to normalize the EPOR copy number.20 EPOR expression profiles in human tissues/cells were obtained from microarray hybridizations in the Gene Expression Omnibus (www.ncbi.nih.gov/geo).
EpoR immunoblot analysis
Cells were trypsinized (Invitrogen), washed with sterile PBS, and then resuspended in lysis buffer containing a protease inhibitor cocktail (50mM Tris-HCl, pH 7.2, 150mM NaCl, 1% Triton X, 0.1% Na deoxycholate, one complete protease inhibitor tablet per 50 mL, 0.1 mg/mL 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride [Pefabloc-SC], and 10 μg/mL pepstatin) at a concentration equal to or less than 3 × 107 cells/mL and processed at 4°C. In control experiments with low EpoR expressing adherent cell line A2780, there was no difference in the amount or pattern of EpoR expression detected with A82 on a Western blot when cells were detached with chelating agent ethylenediaminetetraacetic acid (Versene, Invitrogen) versus trypsin treatment (data not shown). To extract protein from tissues, tissue sections were washed with ice-cold PBS to remove embedding media optimal cutting temperature compound and processed as described for cell lysates.
Cell lysates were subjected to polyacrylamide gel electrophoresis (NuPAGE; Invitrogen) and transferred to Invitrolon polyvinylidene fluoride membranes (Invitrogen) and immunoblotted with anti-EpoR mAb A82 as described.22 After transfer, membranes were washed with Tris-buffered saline–Tween 20 (TBS-T; 25mM Tris-HCl, pH 7.5, 150mM NaCl, 0.15% Tween-20), then blocked at room temperature for 1 hour with 5% (wt/vol) nonfat dry milk in TBS-T. Blocked membranes were incubated with A82 (0.1 μg/mL) in 2.5% (wt/vol) milk plus TBS-T buffer for 2 hours at room temperature. Blots were washed in TBS-T, incubated in 0.01 μg/mL anti–rabbit IgG horseradish peroxidase-linked whole antibody (Jackson ImmunoResearch Laboratories) at room temperature for 1 hour, and then washed 3 times in TBS-T. For detection, membranes were incubated for 5 minutes with ECL Plus reagent (Invitrogen) and then exposed to Hyperfilm ECL x-ray film.
Semiquantitative measurements of EpoR protein
Soluble EpoR was purified from conditioned medium from CHO cells expressing a cDNA encoding the EpoR signal peptide and the first 225 amino acids of the human EpoR (Met1-Pro249) by affinity and conventional chromatography (EpoR-ECD; amino acids 1-225) with absorbance (A280) protein concentration determination.
A 2-fold serial dilution of HUVEC cell lysates or a 2-fold serial dilution of EpoR-ECD mixed into EpoR− 769-P cells was generated and subjected to immunoblot analysis and EpoR was detected with A82. Band intensities (25 kDa EpoR-ECD or 59 kDa full-length EpoR in cell lysates) were compared on the same immunoblot. The functional form of EpoR is a homodimer; thus, EpoR levels were divided by 2 and were reported as homodimers per cell (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
[125I]-rHuEpo cell surface binding
[125I]-rHuEpo binding studies were performed as previously described with some modification.20 Cells were dissociated with Versene (Invitrogen), washed in binding buffer, passed through a 40-μm nylon cell strainer, and pelleted by centrifugation. The supernatant was discarded, and cells were resuspended at 1.25 × 107 cells/mL in binding buffer. [125I]-rHuEpo was obtained from GE Healthcare. The total binding arm used 0.083nM [125I]-rHuEpo, and nonspecific binding was determined by adding 50nM unlabeled rHuEpo (∼ 500-fold molar excess). Cells were incubated at 37°C for 2.5 hours, reactions were stopped by addition of 700 μL of ice-cold PBS/0.5% BSA and centrifuged. Cell pellets were washed again and bound [125I]-rHuEpo was counted in a Packard Cobra II auto γ-counter (Perkin-Elmer). Specific binding was calculated by subtracting nonspecific binding from total binding, and results are expressed as the mean plus or minus SEM. Student t test using Statview software, Version 3.0 (Adept Scientific) assessed statistical significance. In addition, a Dunnett test assessing multiple comparisons was also performed using SAS 9.1.3, Windows XP Professional Edition. In some experiments, binding characteristics were determined by Scatchard analysis as described previously.23
rHuEpo signaling studies
Endothelial cells.
Primary endothelial cell cultures and control cell lines (769-P, UT-7/Epo) were cultured overnight in 0.1% FBS. Replicate cultures of each were exposed to vehicle control (0.25% human serum albumin [HSA]), growth factor cocktail GFC (100 ng/mL rHuEGF, Roche Diagnostics; 500 ng/mL rHuIGF-1, R&D Systems; and 500 ng/mL rHuHGF, R&D Systems), or 300 U/mL rHuEpo for 5 and 30 minutes. Cells were lysed in BioSource cell-extraction buffer (Invitrogen) containing phenylmethanesulphonylfluoride and protease inhibitors (Sigma-Aldrich) for 30 minutes on ice with periodic vortexing. Lysates were centrifuged at 4°C, protein (5 μg) was electrophoresed using NuPAGE 4% to 12% Bis-Tris gels and transferred onto polyvinylidene fluoride membranes in NuPAGE transfer buffer, 20% methanol (Invitrogen). Membranes were blocked in TBS-T (0.1% Tween 20) and 5% (wt/vol) nonfat dry milk (Bio-Rad) for 2 hours at room temperature and then incubated in TBS-T/5% BSA (Sigma-Aldrich) containing a cocktail of pretitrated antibodies specific to phospho-p90RSK (Ser380), phospho-AKT (Ser473), phospho-ERK1/2 (Thr202/Tyr204), phospho-S6 (Ser235/236), and eIF4E as a loading control (Pathscan Multiplex Western Cocktail I; Cell Signaling Technologies). Anti–rabbit secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technologies) was used to detect primary antibody binding. Chemiluminescent substrate SuperSignal West Dura Extended Duration Substrate (Pierce Chemical) was used for detection. Signals were detected using an Alpha Innotech FluorChem HD Imager.
SH-SY5Y, cardiac myocytes, and RPTECs.
Cells were seeded at 2 to 3 × 106 cells in a 10-cm dish, grown for 24 to 48 hours, and then starved overnight in low serum containing basal media (0.5% FBS for SH-SY5Y and cardiac myocytes, 0.1% for RPTECs) before treatment with 10 U/mL rHuEpo, a volume equivalent of vehicle (volume equivalent of rHuEpo formulation buffer: 20mM sodium citrate, 100mM sodium chloride, pH 6.9, in media-negative control), or 1 or more growth factors, including rHuIFN-γ (100 ng/mL), rHuIGF (500 ng/mL), rHuHGF (500 ng/mL), rMsIFN-γ (100 μg/mL; R&D Systems), and rHuEFG (100 ng/mL; Roche Diagnostics) for up to 30 minutes. UT-7/Epo cells were starved of rHuEpo overnight before stimulation with rHuEpo and used as a positive control. After stimulation, cells were lysed in protein lysis buffer (Meso Scale Discovery), subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis immunoblotting, and probed with antibodies against phospho ERK1/2, STAT5, and AKT at 1:1000 dilution (Cell Signaling Technologies) and binding detected with a 1:2000 dilution of anti–rabbit horseradish peroxidase conjugate and the ECL detection system (Pierce Chemical). Blots were stripped and reprobed with antibodies to total ERK1/2, STAT5, and AKT, respectively, at 1:1000 dilution (Cell Signaling Technologies). Anti–β-tubulin antibody used at 1:1000 dilution (Sigma-Aldrich) confirmed protein-loading equivalence.
Cytoprotection
Cardiac myocytes.
Methods used to investigate the ESA-mediated cardiac myocyte protection were similar to those described by others.2,21 Cells were detached with 0.025% trypsin/0.01% ethylenediaminetetraacetic acid (Cambrex) for 3 to 5 minutes at room temperature and then plated into 96-well plates at 7500 cells/well and allowed to recover overnight. Overnight recovery was sufficient for reexpression of surface receptors potentially affected by trypsin as evidenced by responses to the positive control rMsIFNγ (Figure 3A) and rHuEpo-induced signaling in UT-7/Epo cells (data not shown). Cells were treated with rHuEpo (0.2-20 U/mL) or darbepoetin alfa (DA, 1-2500 ng/mL) for 1 to 24 hours before exposure (6-24 hours) to cytotoxins: H2O2 (3-300μM; Sigma-Aldrich), staurosporine (50-300nM; Sigma-Aldrich), or hypoxia (1% O2) in the presence or absence of FBS and glucose. Negative controls were treated with media diluent only. Cell viability was assessed using WST-1 viability reagent (Roche Applied Science), and apoptosis was measured using CellProbe HT Caspase 3/7 whole cell assay reagent (Beckman Coulter). Caspase inhibitor Z-VAD-FMK (25μM; Calbiochem) was used as a positive control. An inactive (I) rHuEpo analog (I-rHuEpo [NM385]; Lys45Asp and Ser100Glu substitutions) was used as a negative control in some studies.24
Neuronal cell lines.
Using methods similar to those reported to induce cell death in neuronal cell lines,25 SH-SY5Y and PC-12 cells were plated into 96-well plates at 1.4 × 104 to 2.0 × 104 cells/well, pretreated with rHuEpo (0.2-200 000 U/mL) for up to 24 hours, and exposed to hypoxia (1% O2) for 5 to 24 hours. After hypoxia, some cultures were exposed to normoxia for 20 hours to mimic reperfusion injury. In addition to hypoxia, starvation in medium lacking glucose was also used in some experiments. Cell viability was determined with CellTitre-Glo Luminescent assay (Promega).
RPTECs.
The methods used to investigate ESA-mediated RPTEC protection were similar to those described by others.26 Cells were plated into 96-well plates at 1 × 104 cells per well and pretreated with DA (1.5, 15, or 150 ng/mL) for 24 hours before treatment with cisplatin (Platinol AQ; Bristol-Myers Squibb) at 10μM or 20μM. Growth medium without DA was used as the control. Apoptosis was measured after 48 hours of cisplatin treatment using a Cell Probe HT Caspase 3/7 kit (Beckman Coulter).
Rat corneal angiogenesis
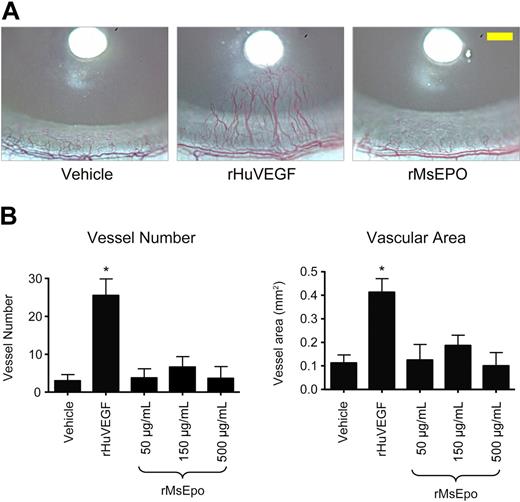
Experiments were performed with CD rats as described.27 Nylon disks soaked in 0.1% BSA, 420 ng/μL rHuVEGF (R&D Systems), or 50, 150, or 500 μg/mL recombinant mouse Epo (rMsEpo; Amgen) were surgically implanted into the rat cornea (n = 8 per group). After 7 days, corneas were photographed as described.27 Briefly, corneas were photographed at 25× using a Nikon SV-3 Ophthalmic Slit Lamp (Nikon Ophthalmic Instruments) with a Nikon D-1 digital camera. Images were optimized, reformatted, and resized for image analysis, and transferred to a Metamorph IA system and analyzed using the Metamorph image analysis system (Universal Imaging Corporation). For each corneal image, the number of blood vessels intersecting the midpoint between the implanted disk and the limbus was counted, and blood vessel area was determined by digital thresholding and automated pixel counting. All evaluations were performed in a blinded manner; results are expressed as the mean plus or minus SEM, and the experiment was repeated. Statistical analysis was performed with 1-way analysis of variance using StatView software, Version 5.0.1 (SAS Institute). Fisher post hoc test was used to determine any statistical significance between groups. To confirm bioactivity of rMsEpo, 3H-thymidine incorporation assays were performed as described24 using Baf3 cells expressing human EpoR (Baf3-HuEpoR) or mouse EpoR (Baf3-MsEpoR). Maximum proliferative responses were observed with a supra-maximal concentration approximately 50 ng/mL (∼ 10 U/mL equivalent) on the plateau phase of the dose-response curve; there was no evidence of a bell-shaped dose-response.
Results
EpoR mRNA and protein in endothelial and other nonhematopoietic cells
EPOR mRNA expression profiling was performed in various hematopoietic and nonhematopoietic cell/tissue types. EPOR transcript levels were examined using a publicly available microarray database and demonstrated that levels were highest in cells/tissue types enriched for erythroid progenitors (eg, fetal liver and bone marrow–derived CD71+ cells) and substantially lower in nonhematopoietic tissues (eg, brain, heart, kidney) and nonerythroid hematopoietic cells (Figure 1A). Transcript levels were also measured in nonhematopoietic cells, including endothelial cell preparations using quantitative reverse-transcribed (RT) PCR (Figure 1B). Endothelial cells derived from the lung microvasculature (HMVEC-LBl), dermal microvasculature (HMVEC-dBl), umbilical vein (HUVEC), and coronary artery (HCAEC) expressed low levels of EPOR transcripts, approximately 50-fold lower than those in positive control UT-7/Epo cells. Endothelial EPOR levels were similar to/lower than those found in other human nonhematopoietic tissues analyzed (Figure 1B).
Nonerythroid cells express low to undetectable levels of EpoR. (A) EPOR mRNA was quantified in hematopoietic and nonhematopoietic cells using a publicly available microarray from the Gene Expression Omnibus (www.ncbi.nih.gov/geo) with mean fluorescence units plus or minus SEM for probe 215054 presented. Data were normalized using global mean scaling. Similar findings were also observed with other EPOR probes (data not shown). PB indicates peripheral blood; BM, bone marrow. (B) Levels of EPOR transcripts were quantified by quantitative RT-PCR in positive control UT-7/Epo cells (+), negative/low control 769-P cells (−), primary human endothelial cells, and normal human nonhematopoietic tissues using primers located in exons 5 and 8. Results were normalized to levels of cyclophilin A mRNA. Data are mean ± SEM (n = 3 per set) and are representative of 3 separate experiments. These findings were reproduced with a different EPOR primers in exon 8 (data not shown). (C) EpoR protein expression analysis in endothelial cells using immunoblot (IB) analysis with anti-EpoR monoclonal antibody A82 and cyclophilin B as a loading control (experiment performed twice). Positive controls FLAG-EpoR transfected into COS-7 cells, UT-7/Epo, and OCIM-1 cells were used to determine the position of EpoR on the blot. FL indicates full-length EpoR at 59 kDa. The smaller proteins detected in erythroid samples were confirmed to contain EpoR sequences and probably reflect degradation products.3,22 (D) Surface EpoR was determined using [125I]-rHuEpo binding. Data are mean ± SEM (n = 5 per cell type) and are representative of 3 experiments. Inset with expanded axis represents absence of specific binding of rHuEpo to endothelial cells.
Nonerythroid cells express low to undetectable levels of EpoR. (A) EPOR mRNA was quantified in hematopoietic and nonhematopoietic cells using a publicly available microarray from the Gene Expression Omnibus (www.ncbi.nih.gov/geo) with mean fluorescence units plus or minus SEM for probe 215054 presented. Data were normalized using global mean scaling. Similar findings were also observed with other EPOR probes (data not shown). PB indicates peripheral blood; BM, bone marrow. (B) Levels of EPOR transcripts were quantified by quantitative RT-PCR in positive control UT-7/Epo cells (+), negative/low control 769-P cells (−), primary human endothelial cells, and normal human nonhematopoietic tissues using primers located in exons 5 and 8. Results were normalized to levels of cyclophilin A mRNA. Data are mean ± SEM (n = 3 per set) and are representative of 3 separate experiments. These findings were reproduced with a different EPOR primers in exon 8 (data not shown). (C) EpoR protein expression analysis in endothelial cells using immunoblot (IB) analysis with anti-EpoR monoclonal antibody A82 and cyclophilin B as a loading control (experiment performed twice). Positive controls FLAG-EpoR transfected into COS-7 cells, UT-7/Epo, and OCIM-1 cells were used to determine the position of EpoR on the blot. FL indicates full-length EpoR at 59 kDa. The smaller proteins detected in erythroid samples were confirmed to contain EpoR sequences and probably reflect degradation products.3,22 (D) Surface EpoR was determined using [125I]-rHuEpo binding. Data are mean ± SEM (n = 5 per cell type) and are representative of 3 experiments. Inset with expanded axis represents absence of specific binding of rHuEpo to endothelial cells.
Analysis of endothelial EpoR protein expression was performed using a recently discovered rabbit anti–human EpoR monoclonal antibody (A82) that binds the N-terminus of EpoR and was shown to be suitable for the sensitive and specific detection of human EpoR by immunoblot.22 A 59-kDa protein was detected in total cell lysates from positive control cell lines (COS-7 cells transfected with FLAG-EpoR, UT-7/Epo, and OCIM-1) cells, whereas no protein was detected in negative controls (COS-7 cells transfected with an empty vector and 769-P cells; Figure 1C). An anti-FLAG antibody was shown previously to position full-length FLAG-EpoR at 59 kDa with these controls.3 Additional EpoR derived proteins smaller than 59 kDa were also detected in positive controls with A82 and are probably degradation products.22 In endothelial cells, a faint EpoR band at 59 kDa was detected in HUVEC, HCAEC, and HMVEC-LBI cells, but no EpoR protein was detected in HMVEC-dBI cells (Figure 1C). Levels of EpoR protein were estimated to be approximately 80 to 160 homodimers/cell according to a semiquantitative A82 immunoblot analysis that compared HUVEC cell lysates with titrations of known quantities of EpoR-ECD (supplemental Figure 1). In contrast, UT-7/Epo cells expressed 300- to 1000-fold higher levels of EpoR protein than HUVEC cells (Figure 1C).22
Because of the detection of low levels of total endothelial EpoR protein by Western blot analysis, experiments were performed to assess cell-surface EpoR using competitive binding of radiolabeled [125I]-rHuEpo on intact cells (Figure 1D). Although EpoR+ UT-7/Epo cells showed specific surface binding of [125I]-rHuEpo, specific binding to preparations of intact endothelial cells was not detected in multiple repeat experiments.
Lack of rHuEpo-mediated signaling in primary human endothelial cells
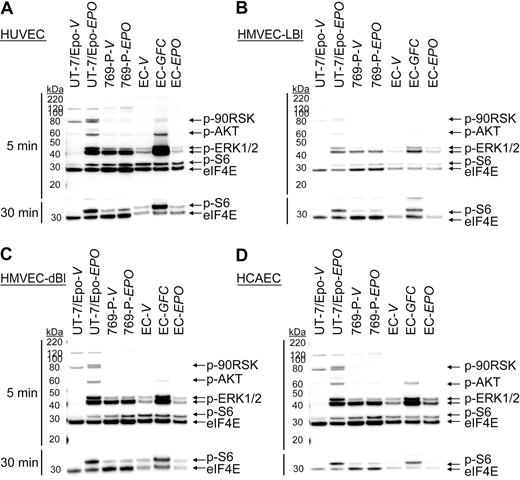
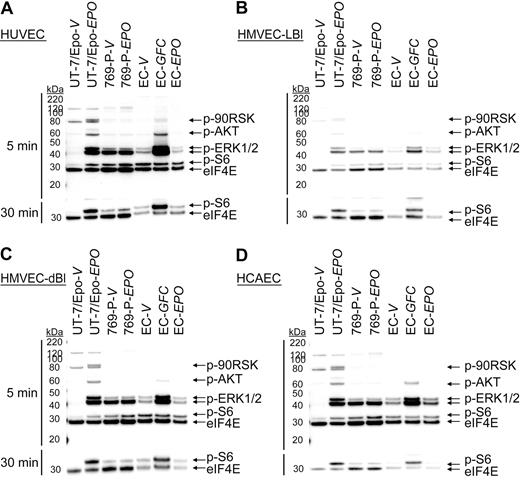
Experiments were performed to determine whether low levels of EpoR protein expression were sufficient to mediate a functional response to rHuEpo. Endothelial cells were stimulated with vehicle or 300 U/mL of rHuEpo for 5 and 30 minutes after overnight starvation (0.1% FBS), and immunoblots were prepared using specific antibodies for the phosphorylated forms of signaling proteins known to be activated downstream of EpoR (p90RSK, AKT, ERK1/2, and S6). As a positive control, the same endothelial cell cultures were stimulated in parallel with a GFC (includes rHuEGF, rHuHGF, and rHuIGF-1) known to induce phosphorylation of some of the same signaling proteins as rHuEpo. UT-7/Epo cells (EpoR+ control) and 769-P cells (EpoR− control) were treated in parallel. As shown in Figure 2, significant induction of p90RSK, AKT, ERK1/2, and S6 phosphorylation was observed in UT-7/Epo cells treated with rHuEpo relative to vehicle but not in 769-P cells. When HUVECs were stimulated with the GFC, increased AKT, ERK1/2, and S6 phosphorylation was observed relative to vehicle (Figure 2A) with induction of S6 phosphorylation apparent after 30 minutes (Figure 2A bottom panel). This demonstrated that growth factor surface receptors and signaling pathways were intact and endothelial cell cultures were responsive to extracellular stimuli under the culture conditions used. In contrast, no significant induction of protein phosphorylation was detected in HUVECs treated with rHuEpo (Figure 2A; supplemental Figure 2). Similarly, no rHuEpo-induced signaling was observed in parallel experiments using HMVEC-LBl (Figure 2B), HMVEC-dBl (Figure 2C), and HCAEC (Figure 2D). These studies suggested an absence of functional EpoR expression in these endothelial cells.
rHuEpo does not activate EpoR signaling pathways in endothelial cells. Endothelial cells (EC) were exposed to vehicle (V), GFC, or rHuEpo (EPO) for 5 (top panel) or 30 minutes (bottom panel). Cell lysates were analyzed by immunoblotting using antibodies specific to phospho-p90RSK (p-90RSK), phospho-AKT (p-AKT), phospho-ERK1/2 (p-ERK1/2), phospho-S6 (p-S6), and eIF4E (loading control). Because of p-S6 being further downstream in the signaling cascade, an extended treatment time of 30 minutes was needed to evaluate phospho-S6. Note lack of signaling in: (A) HUVEC cells, (B) HMVEC-LBI cells, (C) HMVEC-dBI cells, and (D) HCAEC cells when treated with rHuEpo.
rHuEpo does not activate EpoR signaling pathways in endothelial cells. Endothelial cells (EC) were exposed to vehicle (V), GFC, or rHuEpo (EPO) for 5 (top panel) or 30 minutes (bottom panel). Cell lysates were analyzed by immunoblotting using antibodies specific to phospho-p90RSK (p-90RSK), phospho-AKT (p-AKT), phospho-ERK1/2 (p-ERK1/2), phospho-S6 (p-S6), and eIF4E (loading control). Because of p-S6 being further downstream in the signaling cascade, an extended treatment time of 30 minutes was needed to evaluate phospho-S6. Note lack of signaling in: (A) HUVEC cells, (B) HMVEC-LBI cells, (C) HMVEC-dBI cells, and (D) HCAEC cells when treated with rHuEpo.
Lack of rHuEpo-induced signaling in other nonhematopoietic cells
As shown in Figure 1A and B, low levels of EPOR mRNA were detected in nonhematopoietic tissues. Consistent with this finding, EpoR protein was detected with A82 in RPTECs and the neuronal SH-SY5Y cell line at levels comparable with protein detected in samples from human heart, kidney, liver, or brain tissue samples (supplemental Figure 3).
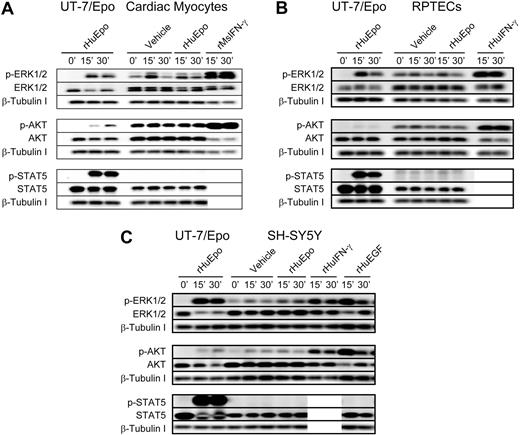
Given the low-level EpoR protein detected in RPTEC and SH-SY5Y cells, rHuEpo-induced signaling was examined in these cells and in rat neonatal cardiac myocytes (Figure 3). Although rHuEpo induced phosphorylation of intracellular proteins in the positive control cell line UT-7/Epo, rHuEpo (10 U/mL) was unable to induce phosphorylation of ERK1/2, AKT, or STAT5 above that seen with the vehicle control in cardiac myocytes (Figure 3A). Signaling was, however, induced in myocytes with positive control cytokine rMsIFN-γ. rHuEpo was similarly unable to induce detectable signaling in RPTECs (Figure 3B) or SH-SH5Y cells (Figure 3C), whereas signaling was detected in positive controls. Interestingly, addition of vehicle control alone modestly increased phosphorylation of ERK1/2 and AKT in some of these nonhematopoietic cells (Figure 3; supplemental Figure 2). This increase was less than in the positive growth factor controls, indicating that cell manipulation alone can induce low-level signaling.
Lack of rHuEpo-induced signaling in cardiac, neuronal, and renal cell types. Signaling analyses in (A) rat neonatal cardiac myocytes, (B) primary human RPTECs, and (C) human neuronal cell line SH-SY5Y. Cells were serum deprived overnight and stimulated with 10 U/mL rHuEpo or a volume equivalent of vehicle for up to 30 minutes, with UT-7/Epo cells serving as a control for rHuEpo activity. Positive control cytokines to confirm receptors and pathways were intact for signal transduction included: (A) rMsIFN-γ; (B) rHuIFN-γ in addition to a GFC of rHuEGF, rHuIGF and rHuHGF; and (C) rHuIFN-γ and rHuEGF. Phosphorylation of EpoR downstream signaling proteins ERK1/2, AKT, and STAT5 were evaluated by immunoblot analysis in addition to total amounts of signaling protein and β-tubulin as a loading controls. Experiments were repeated a minimum of 3 times with independent preparations of each cell type with similar results. Note induction of phosphoproteins in response to positive control cytokines and the absence of signaling in response to rHuEpo.
Lack of rHuEpo-induced signaling in cardiac, neuronal, and renal cell types. Signaling analyses in (A) rat neonatal cardiac myocytes, (B) primary human RPTECs, and (C) human neuronal cell line SH-SY5Y. Cells were serum deprived overnight and stimulated with 10 U/mL rHuEpo or a volume equivalent of vehicle for up to 30 minutes, with UT-7/Epo cells serving as a control for rHuEpo activity. Positive control cytokines to confirm receptors and pathways were intact for signal transduction included: (A) rMsIFN-γ; (B) rHuIFN-γ in addition to a GFC of rHuEGF, rHuIGF and rHuHGF; and (C) rHuIFN-γ and rHuEGF. Phosphorylation of EpoR downstream signaling proteins ERK1/2, AKT, and STAT5 were evaluated by immunoblot analysis in addition to total amounts of signaling protein and β-tubulin as a loading controls. Experiments were repeated a minimum of 3 times with independent preparations of each cell type with similar results. Note induction of phosphoproteins in response to positive control cytokines and the absence of signaling in response to rHuEpo.
Lack of ESA activity in nonhematopoietic functional assays
Although ESAs were unable to induce intracellular signaling in endothelial and other nonhematopoietic cells, we sought to determine whether there were any phenotypic effects with ESAs on these cell types.
Lack of rMsEpo activity in a rat corneal angiogenesis model.
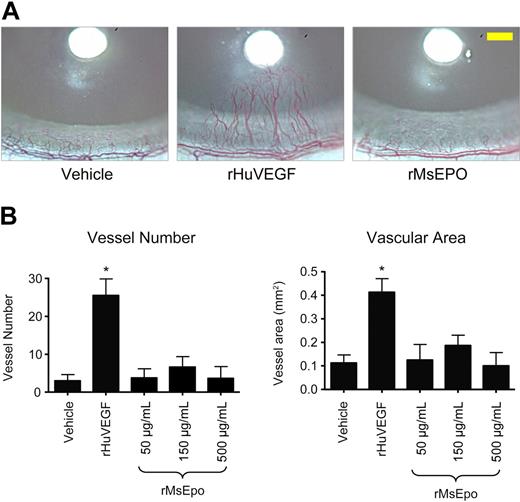
To determine whether ESAs could induce angiogenesis in vivo, rMsEpo was tested in a rat corneal angiogenesis model, a well-established model of the angiogenic potential of agents.27 Bioactivity of the rMsEpo used in these experiments was confirmed in proliferation assays using murine and human EpoR-expressing Baf3 cells (EC50 = 1.7 ng/mL and 1.91 ng/mL, respectively). Growth-factor-soaked nylon disks were placed into the avascular rat cornea at a fixed distance from the surrounding limbal vessels (Figure 4A), and 2 vascular endpoints were evaluated: (1) the number of blood vessels that intersect the midpoint between the disk and the limbus and (2) the blood vessel area (Figure 4B). Implantation of disks soaked in vehicle induced a background angiogenic response, whereas treatment with positive control rHuVEGF (420 ng/μL) induced the sprouting of neovessels from the limbal vessels toward the developing rHuVEGF-diffusion gradient (Figure 4A) and significantly increased (P < .001) vessel number and vascular area (Figure 4B). In contrast, nylon disks soaked with 3 different concentrations of rMsEpo (500, 150, or 50 μg/mL, peptide mass equivalent to 100 000, 30 000, or 10 000 U/mL rHuEpo) did not induce a significant angiogenic response at any of the concentrations tested above vehicle control (Figure 4).
Mouse Epo does not stimulate angiogenesis in the rat cornea. Nylon disks coated with rMsEpo at 3 different concentrations (500, 150, or 50 μg/mL; peptide mass equivalent to 100 000, 30 000, or 10 000 U/mL rHuEpo) were implanted into the corneal stroma of rats (n = 8/group). Disks coated with vehicle (PBS + 0.1% BSA) or 420 ng/μL of rHuVEGF were used as negative and positive controls, respectively. Seven days later, the angiogenic response was evaluated from digital images of each cornea. (A) Representative images from each treatment group are shown. Scale bar in the right panel (yellow) represents 0.5 mm. (B) Quantitative measures of angiogenesis. Two vascular endpoints were evaluated: the number of blood vessels that intersect the midpoint between the disk and the limbus (left panel), and the blood vessel area (right panel). Data represent mean ± SEM. *P < .001 vs vehicle control (analysis of variance with Fisher post hoc test). Experiment was repeated with similar results.
Mouse Epo does not stimulate angiogenesis in the rat cornea. Nylon disks coated with rMsEpo at 3 different concentrations (500, 150, or 50 μg/mL; peptide mass equivalent to 100 000, 30 000, or 10 000 U/mL rHuEpo) were implanted into the corneal stroma of rats (n = 8/group). Disks coated with vehicle (PBS + 0.1% BSA) or 420 ng/μL of rHuVEGF were used as negative and positive controls, respectively. Seven days later, the angiogenic response was evaluated from digital images of each cornea. (A) Representative images from each treatment group are shown. Scale bar in the right panel (yellow) represents 0.5 mm. (B) Quantitative measures of angiogenesis. Two vascular endpoints were evaluated: the number of blood vessels that intersect the midpoint between the disk and the limbus (left panel), and the blood vessel area (right panel). Data represent mean ± SEM. *P < .001 vs vehicle control (analysis of variance with Fisher post hoc test). Experiment was repeated with similar results.
Lack of ESA activity in nonhematopoietic cytoprotection assays.
As studies have reported that ESAs are cytoprotective in a number of nonhematopoietic cell types,8,21,26 we investigated this further. Rat neonatal cardiac myocytes were exposed to cytotoxic environments in vitro (staurosporine, H2O2, hypoxia) in the presence or absence of ESAs. The effect on apoptosis and/or viability was examined (Table 1). Figure 5A is a representative study examining potential dose-dependent effects of ESAs (rHuEpo and DA) on staurosporine-induced apoptosis as measured by caspase-3/caspase-7 activity. The positive control was caspase inhibitor Z-VAD-FMK and negative control inactive (I) rHuEpo. Although Z-VAD-FMK significantly reduced caspase-3/caspase-7 activity (P < .001) at all doses examined, ESAs were unable to significantly reduce caspase-3/caspase-7 activity (Figure 5A). Although trends for reduced caspase-3/caspase-7 activity were observed at high rHuEpo concentrations more than 30 ng/mL (> 6 U/mL), a similar trend was observed with inactive I-rHuEpo but not with DA. Multiple independent experiments examining the effect of ESAs on staurosporine-induced apoptosis produced inconsistent, irreproducible, and, at best, modest (10%-20%) effects (Table 1). Other cytotoxic insults (H2O2 and hypoxia) were investigated in rat cardiac myocytes. Again, ESAs did not in a dose-dependent or reproducible manner reduce apoptosis or cell death (Table 1). ESAs did not provide cytoprotection in other nonhematopoietic cell types reported to respond to ESAs.8,26 DA did not in a dose-dependent manner reduce caspase-3/caspase-7 activity in RPTECs exposed to cisplatin (Figure 5B; Table 1). In neuronal cell lines, pretreatment of SH-SY5Y cells with rHuEpo, even at supra-pharmacologic doses (> 200 U/mL equivalent to > 1 μg/mL) did not improve cell viability during hypoxia in the presence or absence of glucose starvation (Figure 5C; Table 1). Similar findings were observed in a rat neuronal cell line PC12 (Table 1). Taken together, our studies suggest that functional EpoR or other hypothesized receptor complexes are not detectable on the nonhematopoietic cell types examined.
ESAs did not demonstrate cytoprotective activities in nonhematopoietic cells. (A) A representative experiment in rat neonatal cardiac myocytes evaluating caspase-3/caspase-7 activity after treatment with increasing doses of rHuEpo and DA, positive control caspase inhibitor Z-VAD-FMK, and negative control inactive I-rHuEpo. Cells were pretreated for 2 hours with test article, and then apoptosis was induced with 150nM staurosporine in the presence of test article for 22 hours. Data are mean ± SEM and shown with Student t test for significance (n = 12 per group) with caspase-3/caspase-7 activity measured as relative fluorescence units (RFU) and relative to the vehicle control at 0. There was significant reduction of caspase-3/caspase-7 activity at all doses of Z-VAD-FMK (P < .001) but no significant reduction with any other treatment. (B) A representative experiment using primary human RPTECs, which were pretreated with DA at concentrations from 1.5 to 150 ng/mL or with media alone for 24 hours before the addition of cisplatin at a final concentration of 20μM. Control groups included pretreatment with DA without cisplatin treatment (white bars). Apoptosis was measured after 48 hours of cisplatin treatment using a caspase-3/caspase-7 assay. Data are mean ± SEM (n = 6 per group). Note the inability of DA at any concentration to inhibit cisplatin-induced caspase-3/caspase-7 activity. (C) Human neuronal cell line SH-SY5Y was pretreated with 200 U/mL rHuEpo for 0, 2, or 24 hours before induction of hypoxia (1.4%-1.6% O2 for 24 hours). Some treatment groups were also starved of glucose for 24 hours during hypoxia and compared with normoxia. Cell viability was determined with a Cell Titer Glo luminescent assay (relative light units [RLU]) with mean ± SEM shown (n = 6 per group). Student t test was used to determine statistical significance. No significant increase in cell viability was observed with rHuEpo treatment.
ESAs did not demonstrate cytoprotective activities in nonhematopoietic cells. (A) A representative experiment in rat neonatal cardiac myocytes evaluating caspase-3/caspase-7 activity after treatment with increasing doses of rHuEpo and DA, positive control caspase inhibitor Z-VAD-FMK, and negative control inactive I-rHuEpo. Cells were pretreated for 2 hours with test article, and then apoptosis was induced with 150nM staurosporine in the presence of test article for 22 hours. Data are mean ± SEM and shown with Student t test for significance (n = 12 per group) with caspase-3/caspase-7 activity measured as relative fluorescence units (RFU) and relative to the vehicle control at 0. There was significant reduction of caspase-3/caspase-7 activity at all doses of Z-VAD-FMK (P < .001) but no significant reduction with any other treatment. (B) A representative experiment using primary human RPTECs, which were pretreated with DA at concentrations from 1.5 to 150 ng/mL or with media alone for 24 hours before the addition of cisplatin at a final concentration of 20μM. Control groups included pretreatment with DA without cisplatin treatment (white bars). Apoptosis was measured after 48 hours of cisplatin treatment using a caspase-3/caspase-7 assay. Data are mean ± SEM (n = 6 per group). Note the inability of DA at any concentration to inhibit cisplatin-induced caspase-3/caspase-7 activity. (C) Human neuronal cell line SH-SY5Y was pretreated with 200 U/mL rHuEpo for 0, 2, or 24 hours before induction of hypoxia (1.4%-1.6% O2 for 24 hours). Some treatment groups were also starved of glucose for 24 hours during hypoxia and compared with normoxia. Cell viability was determined with a Cell Titer Glo luminescent assay (relative light units [RLU]) with mean ± SEM shown (n = 6 per group). Student t test was used to determine statistical significance. No significant increase in cell viability was observed with rHuEpo treatment.
Discussion
Here, we describe further investigations on the expression and function of EpoR on endothelial and other nonhematopoietic cells. Using quantitative RT-PCR, EPOR mRNA levels in endothelial and other nonhematopoietic cells/tissues were 10- to 100-fold lower than EPOR+ erythroid lineage cells, respectively. These data contrast with studies using less quantitative methods.6,28,29 Low levels of nonhematopoietic EPOR transcription are most probable because of the basal promoter, which does not contain a TATA-box,30 a characteristic of ubiquitously expressed genes. However, high-level erythroid EPOR transcription is regulated by erythroid transcription factor GATA-1,30 providing 1 level of control of EPOR. Thus, it would not be surprising to find low levels of EPOR transcripts in nonhematopoietic tissues that do not contain GATA-1.
Transcription is essential for protein expression, but mRNA levels do not necessarily correlate with protein levels. Although EpoR protein has been described as widely and highly expressed in endothelial cells and other nonhematopoietic cell types,1,21,28,31 accurate determination of EpoR protein expression has been confounded with the use of nonspecific antibodies that provide false-positive results.3,4 We have recently generated an anti-EpoR monoclonal antibody (A82) that is suitable for the specific and sensitive detection of EpoR protein using immunoblot assays but is not suitable for specific detection with IHC.22 EpoR protein was expressed at low levels in some (∼ 80-160 total EpoR homodimers/cell), but not all, primary endothelial preparations. Other nonhematopoietic tissues had low to undetectable levels of total EpoR protein. In hematopoietic cells, less than 10% of total EpoR protein is trafficked to the cell surface.32 Thus, approximately less than 8 to 16 homodimers/cell may be on the endothelial surface, substantially lower than rHuEpo-responsive human cell lines (200-12 000 surface EpoR)20,33 and primary human erythroid cells (300-1100 surface EpoR).5 Although rHuEpo was reported to signal with low surface levels of EpoR,8 we saw no such effect in endothelial cells. Rather, surface EpoR was undetectable using rHuEpo binding; there was no evidence of signaling and no effect on endothelial cell function.
Another explanation for lack of cellular response is that EpoR protein may not be trafficked to the cell surface because of the absence of required cofactors, such as Jak2.34 Similarly, downstream signaling pathways may be repressed or absent. For example, OCIM-1 cells express surface EpoR but do not respond to rHuEpo,23 and overexpression of EpoR in CTLL2 cells did not induce rHuEpo responsiveness.35 This suggest that EpoR protein expression is necessary but not sufficient for cells to respond to ESAs for a number of permissive factors. Some investigators have also reported that EpoR may exist as a heteroreceptor with the granulocyte-macrophage colony-stimulating factor/IL-3/IL-5 β common chain and could also be activated by ESAs.2,36 However, this is controversial as other studies refute this possibility.8,9
Although our data are consistent with other studies that found ESAs did not stimulate responses in endothelial cells,10–13 it is difficult to reconcile these findings with reports that ESAs induced angiogenesis in vivo.7,15,37–41 However, some positive results are difficult to interpret because of cross-species inactivity of ESAs (ie, rHuEpo is not active in chicken erythroid progenitors14 yet is reportedly active)15,38 and the use of low-potency Epo-mimetic peptides (> 65 000-fold less potent than rHuEpo) that induced angiogenesis but not erythroid activity.42,43 Experimental artifacts may also explain conflicting results. For example, ESAs were reported to induce phosphorylation of AKT, ERK1/2, and other downstream signaling in endothelial cells compared with untreated cells.44 However, the magnitude of increased signaling after rHuEpo treatment was very low (2- to 3-fold). In control experiments, addition of vehicle alone induced low-level phosphorylation of ERK and AKT. This vehicle effect may be the result of serum factors contained in the vehicle45 or modulations in temperature or pH.45–47 Therefore, vehicle effects may explain, in part, some of the low-level responses reported with rHuEpo in endothelial cells.
Multiple published studies have reported that ESAs have direct effects on nonhematopoietic cells and tissues and mediate cytoprotection.1 However, ESAs did not induce signaling or provide cytoprotective effects on cardiac myocytes, renal proximal tubule epithelial cells, or neuronal cell lines. Although we did observe minor effects in some groups, in some experiments (eg, Figure 5A), we attributed those results to experimental variation given the lack of consistent, reproducible, dose-related effects and that similar changes were seen with the inactive ESA. The lack of cytoprotective effects is consistent with a number of reports. For example, whereas rHuEpo reportedly reduced ischemia reperfusion-induced renal injury and preserved renal function in some studies,48 it did not in others.16 DA did not alter lipopolysaccharide-evoked myocardial depression or the expression of proapoptotic or antiapoptotic genes in the heart.17 The reported cytoprotective activity of rHuEpo in rat spinal cord compression and contusion injury models49 was not reproduced by others.19 Conflicting cytoprotective findings have been reported with ESAs in murine models of amyotrophic lateral sclerosis,50,51 and rHuEpo did not provide neuroprotection in a rabbit bacterial meningitis model.18 A recent study found no effect of rHuEpo on hepatocytes in vitro and concluded reports of in vivo liver cytoprotective effects were via indirect mechanisms.52 Indeed, it is theoretically possible that some reported effects in vivo may be mediated through ESA-induced changes in iron metabolism or by increasing hemoglobin levels, thus increasing oxygen delivery to tissues. However, our data with the findings of others suggest that ESAs do not have broad, reproducible, robust, direct angiogenic or cytoprotective activities. Based on these results, we do not think that clinical studies examining an alleged “direct” effect of ESAs on heart or brain function or repair are well founded.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Norma Rogers for binding analyses, Ivonne Archibeque for quantitative RT-PCR analysis, Bee Sun for signaling analyses, Juan Estrada for the rat cornea angiogenesis model, Amy Lafayette for generating cardiac myocytes, Wen Gu for statistical analysis, and Kathryn Boorer for editorial assistance.
Authorship
Contribution: A.M.S. generated myocyte cytoprotection data and designed and supervised nonhematopoietic signaling, mRNA studies, and lead writing of the manuscript and editing; A.C. designed, interpreted, and supervised rat angiogenesis study and cowrote the manuscript; I.M. supervised and interpreted endothelial signaling studies; S.K. generated and analyzed rat angiogenesis data; K.P. generated endothelial signaling data; L.L. designed, supervised, and interpreted neuronal cell line cytoprotection studies; L.B. generated and interpreted Western immunoblot data; S.S. generated and interpreted RPTEC cytoprotection and binding data; S.E. supervised studies and cowrote and edited the manuscript; and C.G.B. revised and edited the manuscript.
Conflict-of-interest disclosure: All authors are employees of and/or own stock in Amgen Inc, a manufacturer and marketer of ESAs.
Correspondence: Angus M. Sinclair, MS 15-2-A, 1 Amgen Center Dr, Thousand Oaks, CA 91320; e-mail: anguss@amgen.com.
References
Author notes
A.M.S. and A.C. contributed equally to this study.

![Figure 1. Nonerythroid cells express low to undetectable levels of EpoR. (A) EPOR mRNA was quantified in hematopoietic and nonhematopoietic cells using a publicly available microarray from the Gene Expression Omnibus (www.ncbi.nih.gov/geo) with mean fluorescence units plus or minus SEM for probe 215054 presented. Data were normalized using global mean scaling. Similar findings were also observed with other EPOR probes (data not shown). PB indicates peripheral blood; BM, bone marrow. (B) Levels of EPOR transcripts were quantified by quantitative RT-PCR in positive control UT-7/Epo cells (+), negative/low control 769-P cells (−), primary human endothelial cells, and normal human nonhematopoietic tissues using primers located in exons 5 and 8. Results were normalized to levels of cyclophilin A mRNA. Data are mean ± SEM (n = 3 per set) and are representative of 3 separate experiments. These findings were reproduced with a different EPOR primers in exon 8 (data not shown). (C) EpoR protein expression analysis in endothelial cells using immunoblot (IB) analysis with anti-EpoR monoclonal antibody A82 and cyclophilin B as a loading control (experiment performed twice). Positive controls FLAG-EpoR transfected into COS-7 cells, UT-7/Epo, and OCIM-1 cells were used to determine the position of EpoR on the blot. FL indicates full-length EpoR at 59 kDa. The smaller proteins detected in erythroid samples were confirmed to contain EpoR sequences and probably reflect degradation products.3,22 (D) Surface EpoR was determined using [125I]-rHuEpo binding. Data are mean ± SEM (n = 5 per cell type) and are representative of 3 experiments. Inset with expanded axis represents absence of specific binding of rHuEpo to endothelial cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-10-248666/5/m_zh89991050720001.jpeg?Expires=1768235117&Signature=jQoyhgXETJ8UW6GlHFZxYiijjQlabOmX4rOYWGkbOZyo-awoU8avlz-UnDWMjOqcXgP01K72OKaWExrVzMHnipNKtasXefk2BwYnVrrfi66ttCpPwRhmArnaP1AY9stfxhFaCQqMuQfBFI1sXCS8SoPNkYUk3XtZ8LzeNd1RISTX1aY2SrWryccmXc4uPxEGIMg7vdmtD~a9HdkfIVQEHxOnRL-VvxfwKOuiM52KoGo8pJDp4uwgu0F158exuZwvq0M1-NI-6dGmflwqct3KZI6NEIBdl~kszR8ntAZjTldQ291r0p-bI3KObWTPOdwyhEUJCfB7Gc3MM-DYvJ487A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. ESAs did not demonstrate cytoprotective activities in nonhematopoietic cells. (A) A representative experiment in rat neonatal cardiac myocytes evaluating caspase-3/caspase-7 activity after treatment with increasing doses of rHuEpo and DA, positive control caspase inhibitor Z-VAD-FMK, and negative control inactive I-rHuEpo. Cells were pretreated for 2 hours with test article, and then apoptosis was induced with 150nM staurosporine in the presence of test article for 22 hours. Data are mean ± SEM and shown with Student t test for significance (n = 12 per group) with caspase-3/caspase-7 activity measured as relative fluorescence units (RFU) and relative to the vehicle control at 0. There was significant reduction of caspase-3/caspase-7 activity at all doses of Z-VAD-FMK (P < .001) but no significant reduction with any other treatment. (B) A representative experiment using primary human RPTECs, which were pretreated with DA at concentrations from 1.5 to 150 ng/mL or with media alone for 24 hours before the addition of cisplatin at a final concentration of 20μM. Control groups included pretreatment with DA without cisplatin treatment (white bars). Apoptosis was measured after 48 hours of cisplatin treatment using a caspase-3/caspase-7 assay. Data are mean ± SEM (n = 6 per group). Note the inability of DA at any concentration to inhibit cisplatin-induced caspase-3/caspase-7 activity. (C) Human neuronal cell line SH-SY5Y was pretreated with 200 U/mL rHuEpo for 0, 2, or 24 hours before induction of hypoxia (1.4%-1.6% O2 for 24 hours). Some treatment groups were also starved of glucose for 24 hours during hypoxia and compared with normoxia. Cell viability was determined with a Cell Titer Glo luminescent assay (relative light units [RLU]) with mean ± SEM shown (n = 6 per group). Student t test was used to determine statistical significance. No significant increase in cell viability was observed with rHuEpo treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-10-248666/5/m_zh89991050720005.jpeg?Expires=1768235117&Signature=0NCR5pvb~UMqx-7Te94cwWdpFCfpIlYQmwbiWV8SwvizIhPDlF8ARBS~xG-WA~-7rFTMhKaFQb9VDDfQbTFnTRUuiWKwrAjDLWIVNbTbUgNBie5NS1UUvvHC-lSk5DlY56UzaEtsNcSTaJyo6UA7EFRMTW0UmeblUqrsAUUZ0XWjuf6WRVGOf07-swbXpHXHZhn51pAa59sfpird-LOIa1FRtL3Sn4Tps6LDRD2mg2kIWhlHewY7PsuBOMq9UuJTfTCVwcyI9a82gYs-dxcexUYo04gn5TeKjRbSvUe3S5cWZTypM0NU4D0YSkL092R3fJTihXW2KACHfp1OXev1sw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Nonerythroid cells express low to undetectable levels of EpoR. (A) EPOR mRNA was quantified in hematopoietic and nonhematopoietic cells using a publicly available microarray from the Gene Expression Omnibus (www.ncbi.nih.gov/geo) with mean fluorescence units plus or minus SEM for probe 215054 presented. Data were normalized using global mean scaling. Similar findings were also observed with other EPOR probes (data not shown). PB indicates peripheral blood; BM, bone marrow. (B) Levels of EPOR transcripts were quantified by quantitative RT-PCR in positive control UT-7/Epo cells (+), negative/low control 769-P cells (−), primary human endothelial cells, and normal human nonhematopoietic tissues using primers located in exons 5 and 8. Results were normalized to levels of cyclophilin A mRNA. Data are mean ± SEM (n = 3 per set) and are representative of 3 separate experiments. These findings were reproduced with a different EPOR primers in exon 8 (data not shown). (C) EpoR protein expression analysis in endothelial cells using immunoblot (IB) analysis with anti-EpoR monoclonal antibody A82 and cyclophilin B as a loading control (experiment performed twice). Positive controls FLAG-EpoR transfected into COS-7 cells, UT-7/Epo, and OCIM-1 cells were used to determine the position of EpoR on the blot. FL indicates full-length EpoR at 59 kDa. The smaller proteins detected in erythroid samples were confirmed to contain EpoR sequences and probably reflect degradation products.3,22 (D) Surface EpoR was determined using [125I]-rHuEpo binding. Data are mean ± SEM (n = 5 per cell type) and are representative of 3 experiments. Inset with expanded axis represents absence of specific binding of rHuEpo to endothelial cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-10-248666/5/m_zh89991050720001.jpeg?Expires=1768340018&Signature=Et1lQSR9wdv1MiWOdxabevu14XRIjycQpGAgNHB0liEHAaoGtZDsyPY82YONm5bLc-vLjygl1Ke8pCBWKVxkUoZXqcdqQdHukMGQpB3kKb2J93sPWAxVaFTT0jD5KrqTg1VedCY7hqjJGQMbV15wtmZ3b-HiOdYYtZxMRu93wk3HbfII7lu0ugaThCX5pHhPCL-slcB1mow1qb75w5Sqmewx8j0kT0viFVoY4KrbdyBm9MWUz5uBilfPtJQ1suxyRbpPbWhhD5Q7BU4PzB8e-n2ugi7AZWEQq2A2GQhCdZdgg-mHKmHJa~Z8a9I90aEbnoRJmM4w2DsWlrEguxb7Rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. ESAs did not demonstrate cytoprotective activities in nonhematopoietic cells. (A) A representative experiment in rat neonatal cardiac myocytes evaluating caspase-3/caspase-7 activity after treatment with increasing doses of rHuEpo and DA, positive control caspase inhibitor Z-VAD-FMK, and negative control inactive I-rHuEpo. Cells were pretreated for 2 hours with test article, and then apoptosis was induced with 150nM staurosporine in the presence of test article for 22 hours. Data are mean ± SEM and shown with Student t test for significance (n = 12 per group) with caspase-3/caspase-7 activity measured as relative fluorescence units (RFU) and relative to the vehicle control at 0. There was significant reduction of caspase-3/caspase-7 activity at all doses of Z-VAD-FMK (P < .001) but no significant reduction with any other treatment. (B) A representative experiment using primary human RPTECs, which were pretreated with DA at concentrations from 1.5 to 150 ng/mL or with media alone for 24 hours before the addition of cisplatin at a final concentration of 20μM. Control groups included pretreatment with DA without cisplatin treatment (white bars). Apoptosis was measured after 48 hours of cisplatin treatment using a caspase-3/caspase-7 assay. Data are mean ± SEM (n = 6 per group). Note the inability of DA at any concentration to inhibit cisplatin-induced caspase-3/caspase-7 activity. (C) Human neuronal cell line SH-SY5Y was pretreated with 200 U/mL rHuEpo for 0, 2, or 24 hours before induction of hypoxia (1.4%-1.6% O2 for 24 hours). Some treatment groups were also starved of glucose for 24 hours during hypoxia and compared with normoxia. Cell viability was determined with a Cell Titer Glo luminescent assay (relative light units [RLU]) with mean ± SEM shown (n = 6 per group). Student t test was used to determine statistical significance. No significant increase in cell viability was observed with rHuEpo treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-10-248666/5/m_zh89991050720005.jpeg?Expires=1768340018&Signature=48XtVVSUXsZ00QK7aZZ~VL0UppNu8wBH9zDIxC~QT~Nsaq0jKJL3vNOcNWu2Vq3f~F9irrC4eM0JA1QmIotTc7G7khD6uoVBpC3O7fJlqm428XauYsGtee5NIpmGsC5ve-vMeiyX9ZIZ1bib3vyKYVL2bHf3~5ECt1ywbLcgM0w~cWEjJll5Z-4VyK1YwdGCEQ0klCxrYJTcMVnkoSydECYAhP~KN8~Y2ik---h40n-EH0LDujnLqwzBQTr8Y8LP4g6~K1JXj0in-YvpAP-VhNxKaKXbIAJTTVhFgUdgjGHz5tOAA0f8DKRfN74f7V9RckTwOtDJdBYN9bZQ-evD0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)