Tissue factor (TF) on cell surfaces resides mostly in a cryptic state. It is not entirely clear how cryptic TF differs from procoagulantly active TF and how deencryption occurs. Here, we critically evaluated the importance of cystine 186–cystine 209 (Cys186-Cys209) bond formation for TF procoagulant activity and its de-encryption. Chinese hamster ovary cells transfected with TFC186S, TFC209S, or TFC186S/C209S expressed little procoagulant activity at the cell surface. TF monoclonal antibody and activated factor VII (FVIIa) binding studies showed that little TF protein was present at the cell surface in cells expressing mutant TF. Similar data were obtained in human umbilical vein endothelial cells (HUVECs) transduced to express TFC186S, TFC209S, or TFC186S/C209S. Analysis of TF activity in HUVECs expressing similar levels of wild-type TF and TFC186S/C209S showed that TF mutant in the presence of saturating concentrations of FVIIa exhibited similar coagulant activity as that of wild-type TF. More importantly, treatment of HUVECs expressing TFC186S/C209S with HgCl2 or ionomycin increased the cell-surface TF activity to the same extent as that of the wild-type TF. Our data provide clear evidence that TF lacking the Cys186-Cys209 bond is coagulantly active once it is complexed with FVIIa, and TF de-encryption does not require Cys186-Cys209 disulfide bond formation.
Introduction
Tissue factor (TF), a plasma membrane glycoprotein, plays a key role in the initiation of blood coagulation by allosterically activating coagulation factor VIIa (FVIIa). TF is essential for hemostasis, but the aberrant expression of it leads to thrombosis and contributes to inflammation and cancer.1,,,,–6 Thus, the proper regulation of TF expression is critical not only for maintenance of the hemostatic balance but also for health in general. It is well known that TF on cell surfaces exists in 2 different populations: a minor population of coagulant-active TF, which binds FVIIa and the resultant TF-FVIIa complexes cleave macromolecular substrates, factor X (FX) and factor IX, and a major population of cryptic TF, which also binds FVIIa but the resultant TF-FVIIa complexes are incapable of activating macromolecular substrates.7,,–10 Although FVIIa appears to bind preferentially to active TF, the differences between FVIIa binding to active and cryptic TF are not readily distinguishable, and the binding studies often showed a single class of high-affinity binding sites for FVII or FVIIa, suggesting that FVII and FVIIa form stable high-affinity associations with both decrypted and encrypted TF.7,11,–13 It is unclear at present how the coagulantly active TF differs from the cryptic form and how the cryptic TF is converted to the active form. Studies from our laboratory14,–16 and others8,9 demonstrated that exposure of cells to calcium ionophore or other stimuli, which increase the negatively charged phospholipids at the outer leaflet of cell-surface membrane, enhanced the TF coagulant activity at the cell surface, suggesting that availability of negatively charged phospholipids within the vicinity of TF converts the cryptic TF to active TF. In addition to negatively charged phospholipids, dimerization of TF17 and association of TF with cholesterol and lipid rafts18,–20 were also shown to modulate TF procoagulant activity (see review in Egorina et al21 ).
Recent studies suggest that cryptic and active TF exist in different conformations because the cryptic form of TF contains unpaired cysteine thiols at cystine 186 and cystine 209 in the membrane-proximal domain, whereas the active form of TF is thought to have an oxidized cystine 186–cystine 209 (Cys186-Cys209) disulfide bond.22,23 It was further suggested that protein disulfide isomerase (PDI) regulates TF activity by targeting this disulfide bond.23 The validity of the proposal that TF encryption/de-encryption involves PDI-mediated disulfide isomerization has been questioned.15 It has recently been suggested that differences in cell-model systems might have contributed to opposing conclusions on the importance of disulfide isomerization in TF encryption/de-encryption.24 However, this suggestion has been repudiated.25 Despite this unresolved controversy, it has recently been reported that PDI plays a critical role in thrombus formation in vivo.26,27 Although these studies provide no direct evidence that TF actually exists in the reduced form in vivo and PDI de-encrypts TF by forming the Cys186-Cys209 disulfide bond or that the de-encrypted TF is responsible for thrombus formation, it was strongly implied that this appears to be the mechanism responsible for thrombus formation.
Despite enthusiasm for this new model, other investigators in the field, in addition to us, have raised questions about the validity of this model.28,29 The very idea that the Cys186-Cys209 disulfide bond is critical for TF coagulant activity and the cryptic TF contains unpaired cysteine thiols at this position comes from the earlier observation that ablation of the Cys186-Cys209 disulfide bond by mutating both cysteine residues severely impaired the procoagulant activity of TF.30 However, there was no direct evidence in this or other reports22,23 that TF lacking the Cys186-Cys209 disulfide bond actually behaves like cryptic TF, that is, binds to FVIIa, but the resultant TF-FVIIa complexes fail to activate FX. Furthermore, there are no published studies that examined whether a TF mutant in which Cys186-Cys209 disulfide bond formation is precluded is resistant to de-encryption.
In the present study, we provide clear evidence that diminished procoagulant activity of TF in cells expressing TF mutants lacking the ability to form a Cys186-Cys209 disulfide bond results from diminished antigen expression at the cell surface and the mutant's lower affinity for FVIIa. Our data show that TF mutants lacking the Cys186-Cys209 disulfide bond exhibit the same specific coagulant activity as the wild-type TF in the presence of saturating concentrations of FVIIa. More importantly, the TF mutant could be de-encrypted to the same extent as the wild-type TF when treated with an oxidizing agent. These data suggest that Cys186-Cys209 disulfide bond formation is not critical for TF de-encryption.
Methods
Reagents
Recombinant human FVIIa was from Novo Nordisk. Purified human FX and factor Xa were purchased from either Enzyme Research Laboratories or Haematologic Technologies Inc. Preparation and characterization of monospecific polyclonal antibodies against human TF was described earlier.31 TF monoclonal antibodies (TF910H10, TF85G9, and TF96B4) were kindly provided by Wolfram Ruf (The Scripps Research Institute). Alexa Fluor 488–conjugated annexin V and secondary antibodies conjugated to Oregon Green or Rhodamine Red were obtained from Invitrogen. Annexin V was kindly provided by Jonathan F. Tait (University of Washington). FuGENE HD transfection reagent was from Roche Diagnostics Corp; hygromycin B was from A.G. Scientific. HgCl2, ionomycin, trypan blue, cephalin, and other reagents were from Sigma-Aldrich.
Cell culture
Primary human umbilical vein endothelial cells (HUVECs), EBM-2 basal medium and growth supplements were purchased from Lonza. Endothelial cells were cultured in EBM-2 basal medium supplemented with the growth supplements and 5% fetal bovine serum. Endothelial cell passages between 3 and 7 were used in the present studies. Wild-type Chinese hamster ovary (CHO)–K1 cells were obtained from ATCC and cultured in F12K media containing 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). HL60 cells were purchased from ATCC and cultured in Iscove-modified Dulbecco medium (ATCC) and 20% FBS. The HEK293AD cell line was obtained from Cell Biolabs and cultured in DMEM medium with 10% FBS and 1% penicillin/streptomycin. All cell types were cultured at 37°C and 5% CO2 in a humidified incubator.
Generation of TF disulfide mutant constructs by site-directed mutagenesis
Site-directed mutagenesis was performed with the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene) following the manufacturer's protocol. The wild-type TF gene cloned in the expression plasmid pcDNA3.1 hygro vector (Invitrogen) was taken as the template for site-specific mutagenesis. To generate TFC186S or TFC186A and TFC209S or TFC209A disulfide mutants, the following synthetic oligonucleotides were designed: 5′-GATAAAGGAGAAAACTACTCTTTCAGTGTTCAAG-CAGTG-3′ or 5′-GGATAAAGGAGAAAACTACGCTTTCAGTGTTCAA-GCAGTG-3′ and 5′-CAGACAGCCCGGTAGAGTCTATGGGCCA GGAGAAAG-3′ or 5′-CAGACAGCCCGGTAGAGGCTATGGGCCAGGAGAAAG-3′, respectively, where the carboxyl terminal cysteines (at amino acid positions 186 and 209) were substituted by serine (bold and underlined) or alanine (bold and italics). The double-mutant clones were generated by subsequential mutation of the other cysteine. The mutants were verified by DNA sequencing of the plasmids (DNA sequencing service provided by Eurofins MWG/Operon).
Transient and stable expression of TF disulfide mutants
CHO-K1 cells (cultured in 24-well plates) were transfected transiently with equal amounts of plasmid constructs (1 μg/well) encoding wild-type TF or TF disulfide mutants (TFC186A, TFC209A, TFC186S, TFC209S, or TFC186SC209S) with the use of FuGENE HD transfection reagent. After 48 hours, the transfected cells were analyzed for cell-surface TF activity and antigen expression. For the generation of the stable cell lines, the transfected CHO-K1 cells were grown in the presence of hygromycin B (1 mg/mL) containing medium and several stable transfectant clones from wild type, and the mutants were selected for further analysis. Cell sorting and single- cell cloning were used to establish the homogenous, stable cell lines. Stable cell lines were maintained under selection pressure with hygromycin B.
Generation of recombinant adenoviral constructs of wild-type TF and cysteine mutants
Wild-type TF or TF mutants in pcDNA 3.1 were excised with HindIII and BamHI and subcloned into pacAd5 CMVK-NpA Shuttle vector (Cell Biolabs) at HindIII and BamHI restriction sites. The individual TF shuttle plasmids and pacAd5 9.2-100 adenovirus backbone DNA were linearized with PacI for cotransfection into HEK293AD cells. The recombinant viruses were produced according to the specifications provided with the kit. The high-titer adenoviral particles were purified with the ViraBind adenovirus purification kit (Cell Biolabs), and the viral titer was determined by the QuickTiter adenovirus immunoassay kit (Cell Biolabs).
Adenoviral transduction of endothelial cells
HUVECs grown to 50% to 60% confluence were transduced with wild-type or mutant TF (25 multiplicities of infection [MOI]/cell) for 4 hours in a minimal volume of complete growth medium, and then more fresh growth medium was added to the cells. The cells were grown for 48 hours and washed twice with buffer A [10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.15M NaCl, 4mM KCl, and 11mM glucose, pH 7.5)] before they were used in experiments.
TF activity assay
The procoagulant activity of wild-type TF and TF mutants was measured in a FX activation assay as described earlier.15 Briefly, confluent cell monolayers expressing wild-type or mutant TF were incubated with FVIIa (100nM) for 10 minutes at 37°C in buffer B (buffer A containing 1 mg/mL bovine serum albumin and 5mM CaCl2). Then FX (1μM) was added to the cells, and the amount of FXa generated at 2 minutes was measured in a chromogenic assay with the use of the substrate Chromogenix S-2765. Any variation in the concentrations of FVIIa and FX used for measuring TF activity is listed in figure legends.
Clotting assays
The procoagulant activity of HL60 cells and HUVECs was also measured by a 1-stage clotting assay with the use of a STArt coagulometer (Diagnostica Stago). For HL60 cells, the experimental conditions were essentially the same as those described previously.19 Clot formation was initiated by the addition of 100 μL of pooled normal human plasma to 200 μL of cell suspension in buffer B supplemented to contain 12.5mM CaCl2 (2 × 105 cells/200 μL; both plasma and cell suspension were prewarmed to 37°C). For adenoviral-infected HUVECs, cells were detached from the dish with 0.53mM EDTA (ethylenediaminetetraacetic acid; in buffer A) treatment for 2 to 3 minutes. The cells were washed twice with buffer A followed by brief centrifugation to remove EDTA and resuspended (1 × 106 cells/mL) in buffer B/12.5mM CaCl2. The clotting assay was performed as described for HL60 cells. The clotting times were converted to arbitrary units of TF procoagulant activity from a standard curve constructed with relipidated recombinant TF (Innovin; Dade Behring) diluted in rabbit brain cephalin (cephalin was reconstituted as suggested in the product information) or diluted with uninfected control HUVECs. TF activity present in 1000-fold diluted Innovin was taken as 1 U/mL.
Determination of 125I-FVIIa and 125I-TF monoclonal antibody binding to cells
FVIIa and TF monoclonal antibody (mAb), TF85G9, TF910H10, and TF96B4, were labeled with 125I with the use of polypropylene tubes coated with IODO-GEN (Thermo Scientific Pierce Research Products) and Na 125I (PerkinElmer Life Sciences) according to the manufacturer's instructions and as described previously.32 The cell monolayers were chilled on ice, and 125I-FVIIa (100nM) or 125I-TF mAb (100nM) was added to cells in ice-cold buffer B. After 2 hours of incubation at 4°C, the supernatant was removed, and the cells were washed 4 times with ice-cold buffer B to remove the unbound radiolabeled ligand. The surface-bound radiolabeled ligand was eluted by treating the cells with 0.1M glycine (pH 2.3) for 3 minutes, and the radioactivity counts of the eluate were measured in a γ-counter. To determine TF-specific FVIIa binding, parallel experiments were performed in which the cells were treated with polyclonal anti-TF immunoglobulin G (IgG; 100 μg/mL) for 45 minutes at room temperature before the radiolabeled ligand was added. 125I-FVIIa associated with cells pretreated with anti-TF IgG was deducted from 125I-FVIIa associated with cells treated with control vehicle to obtain TF-specific binding. Because preliminary studies showed little nonspecific binding of 125I-TF mAbs to cells, no further attempts were made to use excess unlabeled TF mAbs to block nonspecific binding of 125I-TF mAbs to determine the specific TF mAbs binding to the cells. Dose-dependent binding studies confirmed that a concentration of 100nM TF mAb or FVIIa was sufficient to saturate TF binding sites on cells expressing either wild-type TF or TF mutants.
Immunofluorescence confocal microscopy
HUVECs grown on glass coverslips were transduced with wild-type or 1 of the 3 Cys mutant TF adenoviruses (25 MOI) and cultured for 48 hours. The cells were then fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, and processed for immunofluorescence staining of TF as described earlier.33 Nuclei were stained with DAPI (4,6 diamidion-2-phenylindole). The immunostained cells were viewed with an Axio ObserverZ1 microscope with the use of an 63× (oil) plan-apochromate lens. Images were acquired from a field of view at 0.4-μm z-axis increments with the use of the LSM 510 Meta confocal system (Carl Zeiss). The laser setting wavelengths were 369 plus or minus 10 nm excitation and 450 plus or minus 30 nm emission for DAPI, 488 plus or minus 10 nm excitation and 525 plus or minus 10 nm emission for Oregon Green, and 543 plus or minus 10 nm excitation and 575 plus or minus 10 nm emission for Rhodamine Red. For accurate comparison of TF expression levels between wild-type and mutant TF, all images were obtained with the use of identical gain and off-set settings for the detector. The images were processed with LSM Zen 2007 (Zeiss) software and imported to Adobe Photoshop (Version 7.0; Adobe) for compilation of figures.
Fluorescence-activated cell-sorting analysis
Fluorescence-activated cell-sorting analysis was performed essentially as described previously12 with the use of TF polyclonal antibodies (3 μg/mL).
TF antigen enzyme-linked immunoabsorbent assay
For determining total TF antigen in cell lysates, adenoviral-infected HUVECs were solubilized in Tris-buffered saline containing 2% Triton X-100 and 10mM EDTA, and the cell lysates were freeze-thawed thrice before they were used. To measure total TF antigen levels, a 96-well plate was coated with rabbit anti-TF IgG (10 μg/mL) overnight at 4°C. After blocking the wells with 0.1% gelatin, cell lysates of adenoviral-infected HUVECs or recombinant TF apoprotein diluted in 2% Triton X-100 (to generate standard curve) was added to the wells. TF antigen captured by the primary antibody was measured with biotinylated rabbit anti–human TF antibody followed by alkaline phosphatase–conjugated streptavidin and color development with BluePhos Microwell Phosphatase Substrate System (KPL). For cell-surface enzyme-linked immunoabsorbent assay (ELISA), HUVECs cultured in a 96-well culture plate were infected with adenovirus encoding wild-type or mutant TF. After 48 hours, the monolayers were fixed with 4% paraformaldehyde for 30 minutes at 4°C and then blocked with 1% bovine serum albumin. The amount of TF antigen on the cell surface was determined in an ELISA using rabbit anti-TF polyclonal antibody or TF mAb (TF96B4), followed by anti–rabbit IgG or anti–mouse IgG conjugated with streptavidin horseradish peroxidase and color development with SureBlue TMB Microwell peroxidase substrate (KPL).
Cell-surface biotinylation and immunoprecipitation
Cell-surface biotinylation was performed essentially as described previously.12 For immunoprecipitation, biotin-labeled cells (from 60-mm culture dish) were solubilized in buffer A containing 1% Triton X-100 (1.5 mL) supplemented with cocktail of protease inhibitors, and the cell lysates were incubated overnight at 4°C with high-capacity neutravidin-agarose resin (50 μL; Pierce Chemical). After the removal of the unbound material, the beads were washed 3 times with the washing buffer, and the bound material was eluted with 100 μL of 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Eluted samples were subjected to SDS-PAGE, followed by immunoblot analysis with anti-TF polyclonal antibodies.
Data collection and statistical analysis
All experiments were repeated 3 to 6 times. Data shown in the figures represent mean plus or minus SEM or a representative experiment.
Results
HgCl2-mediated increase in TF coagulant activity in HL60 cells depends on PS exposure
Controversy exists on potential mechanisms by which thiol-oxidizing agent HgCl2 increases TF activity on cell surfaces of various cell types. On the basis of data obtained with HL60 cells, Chen et al22 proposed that HgCl2-mediated TF de-encryption is independent of phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane and involves the formation of Cys186-Cys209 disulfide bond in the membrane-proximal domain of TF. In contrast, our studies with breast carcinoma cells (MDA-MB-231) suggested that increased PS exposure at the cell surface in response to HgCl2 treatment was responsible for the increased TF activity.15 Recently, it had been suggested that MDA-MB-231 cells are not suitable for the study of TF de-encryption.24 Although several loopholes exist in this argument,25 it necessitated us to reexamine the role of increased PS exposure in HgCl2-mediated decryption of TF in the HL60 cell-model system. Consistent with the published findings,22 HgCl2 (100μM) treatment markedly increased TF procoagulant activity in phorbol myristate acetate (PMA)–stimulated HL60 cells. A brief exposure of HL60 cells to HgCl2 (30 seconds) increased TF procoagulant activity measured in FX activation assay by approximately 10-fold, and a 3-minute treatment with HgCl2 led to a 20-fold increase in TF activity. However, treatment of HL60 cells with annexin V markedly reduced HgCl2-mediated increased TF activity in HL60 cells (Figure 1A). Similar data were obtained when TF procoagulant activity was measured in a clotting assay (Figure 1B). Examination of PS exposure at the cell surface by confocal microscopy with Alexa Fluor 488–conjugated annexin V showed limited PS at the cell surface of PMA-stimulated HL60 cells, and HgCl2 treatment markedly increased PS levels at the cell surface (Figure 1C). Cell viability measurement by trypan blue exclusion showed no significant cell death in HL60 cells treated with HgCl2 for 30 seconds or 3 minutes (control, 2.1% ± 0.91%; HgCl2 for 30 seconds, 2.6% ± 0.72%; HgCl2 for 3 minutes, 3.5% ± 1.7%, mean ± SD; n = 4).
Increased TF procoagulant activity associated with HgCl2 treatment in HL60 cells depended on PS exposure at the cell surface. (A) HL60 cells (1 × 106/mL) were stimulated with PMA (1μM) in serum-free medium for 6 hours at 37°C to induce TF expression. PMA-stimulated HL60 cells (2 × 105 cells in 200 μL) were treated with HgCl2 (100μM) for 30 seconds and 3 minutes at 37°C in the presence or absence of annexin V (400nM). At the end of HgCl2 treatment, FVIIa (10nM) and FX (175nM) were added to the cells, and the amount of FXa generated at the end of the 2-minute activation period was measured in a chromogenic assay (n = 3; mean ± SEM). *P < .05; §P < .001. (B) HL60 cells were stimulated with PMA and treated with HgCl2 as described above, and TF activity was measured in a clotting assay. TF activity is shown in arbitrary units. (C) HL60 cells stimulated with PMA and treated with HgCl2 as described in panel A were stained for cell-surface expression of PS (Alexa Fluor 488 [AF488]–annexin V staining) and TF. Control and treated cells were washed with buffer A and then incubated with AF488–annexin V in the binding buffer at room temperature for 15 minutes. The cells were then washed, fixed, and immunostained with anti-TF antibodies followed by Rhodamine Red–conjugated secondary antibodies. The immunofluorescence was analyzed by confocal microscopy (Axio Observer Z1 microscope, Plan-APOCHROMAT 63×/1.4 NA oil objective lens, Carl Zeiss LSM 510 Meta confocal system). (D) PMA-stimulated HL60 cells were incubated with 400nM annexin V for 30 minutes at 37°C and then the unbound annexin V was removed, and the cells were washed once with buffer B before they were treated with either control buffer or HgCl2 (100μM) for 3 minutes. In one set, annexin V (400nM) was added to cells again at the time of the HgCl2 treatment. TF activity was measured in FX activation assay as described for panel A.
Increased TF procoagulant activity associated with HgCl2 treatment in HL60 cells depended on PS exposure at the cell surface. (A) HL60 cells (1 × 106/mL) were stimulated with PMA (1μM) in serum-free medium for 6 hours at 37°C to induce TF expression. PMA-stimulated HL60 cells (2 × 105 cells in 200 μL) were treated with HgCl2 (100μM) for 30 seconds and 3 minutes at 37°C in the presence or absence of annexin V (400nM). At the end of HgCl2 treatment, FVIIa (10nM) and FX (175nM) were added to the cells, and the amount of FXa generated at the end of the 2-minute activation period was measured in a chromogenic assay (n = 3; mean ± SEM). *P < .05; §P < .001. (B) HL60 cells were stimulated with PMA and treated with HgCl2 as described above, and TF activity was measured in a clotting assay. TF activity is shown in arbitrary units. (C) HL60 cells stimulated with PMA and treated with HgCl2 as described in panel A were stained for cell-surface expression of PS (Alexa Fluor 488 [AF488]–annexin V staining) and TF. Control and treated cells were washed with buffer A and then incubated with AF488–annexin V in the binding buffer at room temperature for 15 minutes. The cells were then washed, fixed, and immunostained with anti-TF antibodies followed by Rhodamine Red–conjugated secondary antibodies. The immunofluorescence was analyzed by confocal microscopy (Axio Observer Z1 microscope, Plan-APOCHROMAT 63×/1.4 NA oil objective lens, Carl Zeiss LSM 510 Meta confocal system). (D) PMA-stimulated HL60 cells were incubated with 400nM annexin V for 30 minutes at 37°C and then the unbound annexin V was removed, and the cells were washed once with buffer B before they were treated with either control buffer or HgCl2 (100μM) for 3 minutes. In one set, annexin V (400nM) was added to cells again at the time of the HgCl2 treatment. TF activity was measured in FX activation assay as described for panel A.
Although the aforementioned data suggest that HgCl2-mediated increase in PS at the cell surface is responsible for the increased TF activity after HgCl2 treatment, one cannot exclude the possibility that basal PS levels may be sufficient to support HgCl2-mediated increased TF activity because annexin V also inhibits basal TF activity. To address this, we have examined whether the concentration of annexin V that is sufficient to inhibit the basal TF activity could also inhibit the increased TF activity associated with HgCl2 treatment. Annexin V dose-dependent studies showed that approximately 5nM annexin V was sufficient to inhibit half-maximal TF activity of control PMA-stimulated cells, whereas approximately 125nM annexin V was required to inhibit the same in HgCl2-treated cells (data not shown). In a further experiment, the basal PS in PMA-stimulated HL60 cells was blocked by incubating the cells with annexin V and then washed to remove unbound annexin V before they were treated with HgCl2. The data of this experiment showed that the cell-bound annexin V significantly inhibited TF activity of control PMA-stimulated cells but not in HgCl2-treated cells. The addition of annexin V again to HgCl2-treated cells markedly inhibited the TF activity (Figure 1D). Overall these data are consistent with the hypothesis that increased anionic phospholipids are primarily responsible for increased TF activity at the cell surface in HL60 cells exposed to the thiol-oxidizing agent HgCl2.
Impaired TF protein expression in cells transfected with TF mutants lacking Cys186-Cys209 disulfide bond
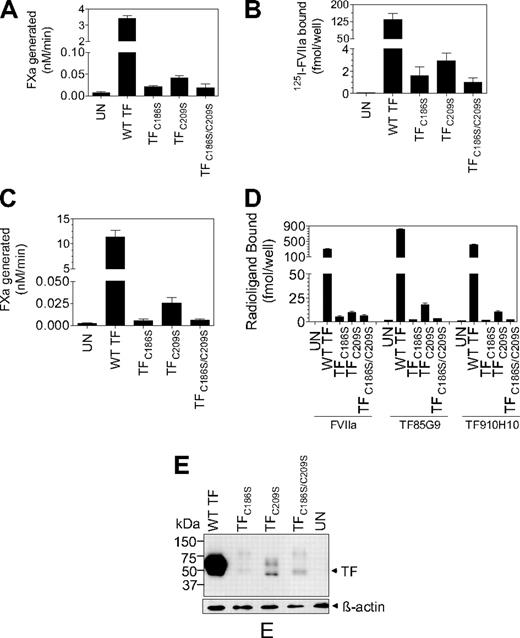
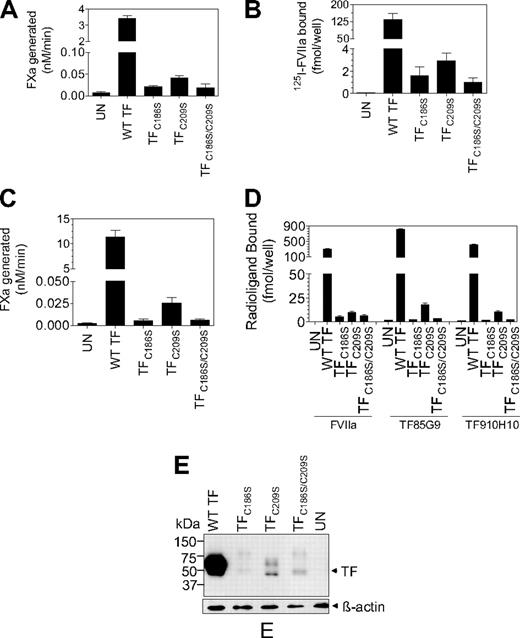
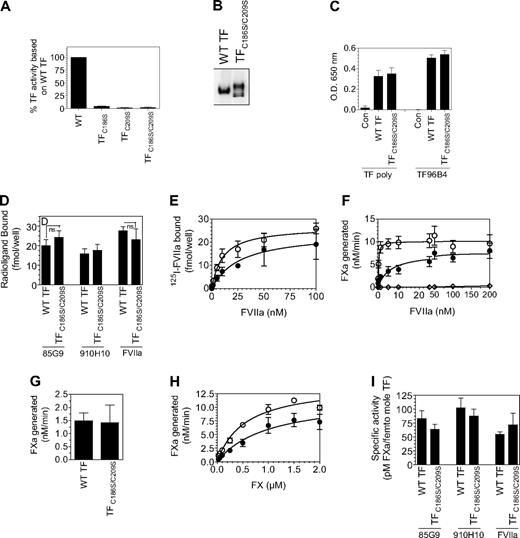
The recent hypothesis implicates that cryptic TF contains unpaired cysteine thiols at Cys186 and Cys209 and that de-encryption of TF involves the formation of disulfide bond between Cys186 and Cys209. This hypothesis is primarily based on the observation that cells transfected with TF mutants, in which the formation of the Cys186-Cys209 bond was precluded, exhibit no or reduced TF procoagulant activity on their cell surfaces compared with the cells transfected with wild-type TF.22,23 However, there was no unequivocal data in the literature showing that these TF mutants mimic cryptic TF and are resistant to de-encryption because they cannot form the disulfide bond. Therefore, in the present study we systematically investigated underlying mechanism(s) responsible for the reduced TF procoagulant activity in cells expressing TF mutants incapable of forming the Cys186-Cys209 disulfide bond. TF mutants (TFC186S, TFC209S, or TFC186S/C209S) were constructed by site-directed mutagenesis, and CHO cells were transfected transiently with wild-type TF, TFC186S, TFC209S, or TFC186S/C209S. As noted in earlier studies,23,30 cells transfected with TFC186S, TFC209S, or TFC186S/C209S exhibited low TF procoagulant activity, approximately 1% or less compared with cells expressing wild-type TF (Figure 2A). Radiolabeled FVIIa binding studies showed that only traces of FVIIa associated with cells expressing TFC186S, TFC209S, or TFC186S/C209S compared with cells expressing wild-type TF (Figure 2B). This indicates that either mutant TF is unable to support FVIIa binding or that cells transfected with TF mutant constructs express little TF protein. Immunoblot analysis of cell lysates with anti-TF antibodies, which showed a prominent TF band in cells transfected with wild-type TF but not TF mutant constructs, suggested that the later assumption was true (data not shown). Although transient transfections were carried out with equal amounts of wild-type or TF mutant plasmid DNA in parallel, one cannot rule out the possibility of heterogeneity in the transfection efficiency between the wild-type and TF mutants. Therefore, stable cell lines that expressed wild-type TF, TFC186S, TFC209S, or TFC186S/C209S were generated. Similar to the data noted with transiently transfected cells, stable cell lines expressing TFC186S, TFC209S, or TFC186S/C209S also exhibited low levels of TF procoagulant activity, ie, less than 1% of the activity of cells expressing wild-type TF (Figure 2C). Quantification of TF antigen levels at the cell surface with 2 different TF mAbs showed that TF antigen levels in cells expressing TFC186S, TFC209S, or TFC186S/C209S were 2% or lower of TF antigen levels found in cells expressing wild-type TF. Consistent with these data, 125I-FVIIa binding to cells expressing TFC186S, TFC209S, or TFC186S/C209S was also low compared with cells expressing wild-type TF (Figure 2D). Western blot analysis of cell lysates confirmed that total TF protein levels were also low in cells expressing TFC186S, TFC209S, or TFC186S/C209S compared with wild-type TF (Figure 2E). Although, based on an earlier study,30 we expected somewhat reduced TF protein expression in cells transfected with TFC186S, TFC209S, or TFC186S/C209S, such drastically low TF protein expression in cells expressing the mutant TF was unexpected. To assure that the aforementioned data were not a result of an unknown experimental artifact or limited to the specific set of plasmid constructs we used in the above experiments, we reconstructed TF mutants in which cysteine residues were mutated to alanine (instead of serine) and/or cloned into a different plasmid vector expressing green fluorescent protein (GFP; pEGFP-N1). Evaluation of TF procoagulant activity and TF protein levels in CHO cells transfected with the reconstructed TF mutants essentially gave similar results as described in Figure 2. Confocal microscopy of the GFP-TF chimerics showed faint or no GFP fluorescence at the cell surface and intracellularly in cells expressing the mutant TF, whereas bright GFP fluorescence was observed at the cell surface and in the Golgi in cells expressing wild-type TF (data not shown).
Analysis of TF procoagulant activity and TF antigen expression in CHO cells transfected with wild-type TF or TF mutants. (A) Monolayers of CHO cells transfected transiently with equal amounts of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S plasmid DNA were incubated with FVIIa (10nM) and FX (175nM), and FXa generation was measured in a chromogenic assay (n = 3; mean ± SEM). (B) CHO cells transfected transiently with equal amounts of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S plasmid DNA were incubated with 125I-FVIIa (10nM) for 2 hours at 4°C in the presence or absence of anti-TF IgG (100 μg/mL), and the amount of 125I-FVIIa associated with cell-surface TF was determined (n = 3; mean ± SEM). (C) Same as in panel A, except CHO cells were stably transfected with wild-type TF, TFC186S, TFC209S, or TFC186S/C209S (n = 6; mean ± SEM). (D) CHO cells stably expressing wild-type TF, TFC186S, TFC209S, or TFC186S/C209S were incubated with saturating concentrations of 125I-FVIIa (± anti-TF IgG) or 125I-TF mAb (10H10 or 5G9; 100nM) for 2 hours at 4°C, and the amount of radioactivity associated with the cell surface was determined. 125I-FVIIa binding shown in the figure was TF specific (n = 3; mean ± SEM). (E) Immunoblot analysis of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S. Cell lysates of cells stably expressing wild-type TF, TFC186S, TFC209S, or TFC186S/C209S were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions followed by immunoblot analysis with polyclonal anti-TF antibodies.
Analysis of TF procoagulant activity and TF antigen expression in CHO cells transfected with wild-type TF or TF mutants. (A) Monolayers of CHO cells transfected transiently with equal amounts of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S plasmid DNA were incubated with FVIIa (10nM) and FX (175nM), and FXa generation was measured in a chromogenic assay (n = 3; mean ± SEM). (B) CHO cells transfected transiently with equal amounts of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S plasmid DNA were incubated with 125I-FVIIa (10nM) for 2 hours at 4°C in the presence or absence of anti-TF IgG (100 μg/mL), and the amount of 125I-FVIIa associated with cell-surface TF was determined (n = 3; mean ± SEM). (C) Same as in panel A, except CHO cells were stably transfected with wild-type TF, TFC186S, TFC209S, or TFC186S/C209S (n = 6; mean ± SEM). (D) CHO cells stably expressing wild-type TF, TFC186S, TFC209S, or TFC186S/C209S were incubated with saturating concentrations of 125I-FVIIa (± anti-TF IgG) or 125I-TF mAb (10H10 or 5G9; 100nM) for 2 hours at 4°C, and the amount of radioactivity associated with the cell surface was determined. 125I-FVIIa binding shown in the figure was TF specific (n = 3; mean ± SEM). (E) Immunoblot analysis of wild-type TF, TFC186S, TFC209S, or TFC186S/C209S. Cell lysates of cells stably expressing wild-type TF, TFC186S, TFC209S, or TFC186S/C209S were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions followed by immunoblot analysis with polyclonal anti-TF antibodies.
TF lacking Cys186-Cys209 disulfide bond retains procoagulant activity
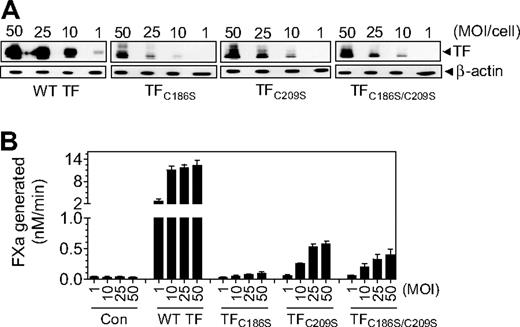
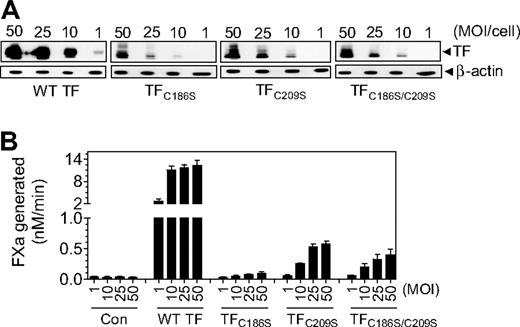
Low levels of TF expression in CHO cells transfected with TFC186S, TFC209S, or TFC186S/C209S plasmid constructs made it difficult to accurately compare the specific activities of the mutants with that of wild-type TF. Furthermore, regulation of TF expression and its activity in CHO cells may differ from that of human cell types that express TF in vivo. Therefore, we next investigated whether mutant TF lacking the Cys186-Cys209 disulfide bond could be expressed in endothelial cells and, if so, resolve the functional status of the expressed TF mutant protein. HUVECs were transduced with various numbers of recombinant adenoviral particles (1-50 MOI/cell) encoding wild-type TF, TFC186S, TFC209S, TFC186S/C209S, or β-gal adenovirus (as a control). TF protein expression levels were evaluated by immunoblot analysis, and the cell-surface TF procoagulant activity in a FX activation assay. Both wild-type TF and TF mutant proteins were expressed in a viral dose-dependent manner, but the expression of TF mutant protein was markedly lower compared with wild-type TF at all concentrations of the virus (Figure 3A). Similar to TF protein expression, TF procoagulant activity at the cell surface was also dependent on the number of viral particles used for the infection; however, TF procoagulant activity in HUVECs infected with viral particles encoding TFC186S, TFC209S, or TFC186S/C209S was markedly lower at all infection levels compared with HUVECs infected with the same number of viral particles expressing the wild-type TF (Figure 3B).
TF expression in endothelial cells transduced with various concentrations (MOI/cell) of adenovirus-carrying wild-type TF or TF mutant. HUVECs were transduced with various concentrations (MOI/cell) of adenovirus encoding wild-type TF, TFC186S, TFC209S, TFC186S/C209S, or control adenovirus (β-Gal). After culturing cells for 48 hours, (A) the cell lysates were harvested and subjected to immunoblot analysis; (B) intact monolayers were used to measure cell-surface TF procoagulant activity in FX activation assay by adding FVIIa (10nM) and FX (175nM) and measuring the amount of FXa generated in a chromogenic assay (n = 3-4; mean ± SEM).
TF expression in endothelial cells transduced with various concentrations (MOI/cell) of adenovirus-carrying wild-type TF or TF mutant. HUVECs were transduced with various concentrations (MOI/cell) of adenovirus encoding wild-type TF, TFC186S, TFC209S, TFC186S/C209S, or control adenovirus (β-Gal). After culturing cells for 48 hours, (A) the cell lysates were harvested and subjected to immunoblot analysis; (B) intact monolayers were used to measure cell-surface TF procoagulant activity in FX activation assay by adding FVIIa (10nM) and FX (175nM) and measuring the amount of FXa generated in a chromogenic assay (n = 3-4; mean ± SEM).
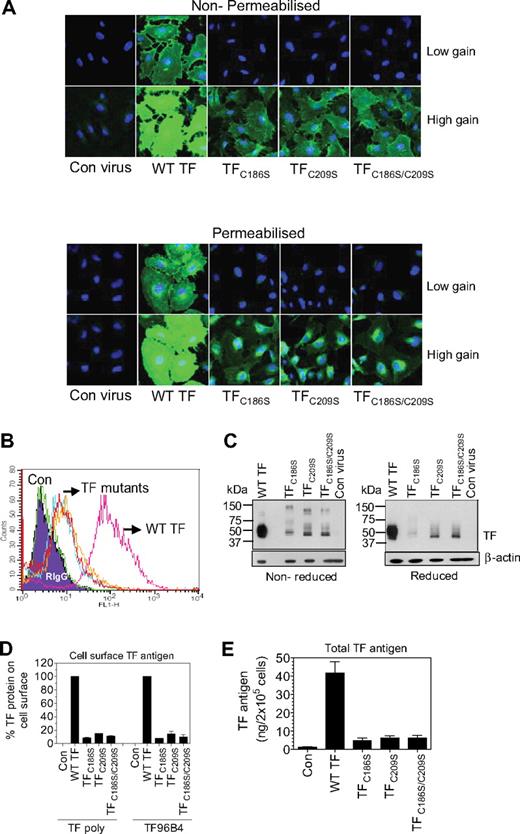
Next, we evaluated TF protein expression and the procoagulant activity of HUVECs transduced with the same number of viral particles encoding wild-type TF or mutant TF (25 MOI/cell) to correlate TF activity with protein level to test whether the TF mutant, once expressed, exhibits similar specific procoagulant activity as that of the wild-type TF. First, we evaluated TF protein expression in HUVECs transduced with wild-type, TFC186S, TFC209S, or TFC186S/C209S by immunofluorescence confocal microscopy. With the use of image capture settings when expression of wild-type TF is clearly evident, expression of mutant TF, either at the cell surface or intracellularly, was undetectable (Figure 4A). The expression of TF mutant is only evident at higher gain settings of the image capture (Figure 4A), suggesting that HUVECs transduced with TFC186S, TFC209S, or TFC186S/C209S does express TF protein but at low levels. Fluorescence-activated cell sorting analysis of cell-surface expression of TF confirmed a reduced expression of TF disulfide mutants at the cell surface (Figure 4B). Immunoblot analysis of cell lysates also showed lower total TF expression in HUVECs infected with viral particles encoding TFC186S, TFC209S, or TFC186S/C209S (Figure 4C). Under nonreducing conditions, the mutant TF, unlike wild-type TF, was heterogeneous and migrated at various molecular weights (45 kDa or higher). At present it is unclear whether the high molecular weight species represent TF dimers or multimers or TF in mixed disulfide bonds with other proteins. On reduction, most of the mutant TF migrated as a single band, at a slightly lower molecular weight than the wild-type TF. Determination of TF antigen at cell surfaces and in cell lysates by TF-specific ELISA confirmed that TF protein was markedly lower in HUVECs transduced to express TFC186S, TFC209S, or TFC186S/C209S compared with HUVECs transduced with wild-type TF adenovirus (Figure 4D-E).
Impaired TF protein expression in endothelial cells transduced with adenovirus carrying TF mutant, TFC186S, TFC209S, or TFC186S/C209S. HUVECs were transduced with control adenovirus (β-Gal), adenovirus encoding wild-type TF, TFC186S, TFC209S, or TFC186S/C209S (25 MOI/cell), and TF expression levels were analyzed by various methods: confocal microscopy (A), fluorescence-activated cell sorting analysis (B), immunoblot analysis (C), in situ ELISA to measure cell-surface TF (D), and ELISA to measure total TF antigen levels (E). (A) HUVECs, nonpermeabilized (top) or permeabilized with 0.05% Triton X-100 for 10 minutes (bottom) were immunostained with polyclonal anti-TF IgG (10 μg/mL) followed by Oregon Green–conjugated secondary antibodies. Immunofluorescence was analyzed by confocal microscopy at 2 different gain settings (high gain, 840; low gain, 630) to capture differences in TF expression in cells expressing wild-type TF or TF mutant. (B) Intact, nonpermeablized HUVECs were stained with anti-TF polyclonal antibodies, followed by FITC-conjugated secondary antibodies, and the cells were analyzed for the fluorescence by flow cytometry. Wild-type TF, pink; TFC186S, cyan; TFC209S, red; TFC186S/C209S, orange; β-gal, green. (C) Cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, either nonreducing (left) or reducing (right) conditions, and immunoblotted for TF with the use of polyclonal anti-TF antibodies. (D) TF expression levels at the cell surface were measured by in situ ELISA with the use of polyclonal anti-TF IgG or TF mAb, TF96B4 (n = 4; mean ± SEM). (E) Total TF antigen levels in cell lysates were measured by ELISA (n = 3, mean ± SEM).
Impaired TF protein expression in endothelial cells transduced with adenovirus carrying TF mutant, TFC186S, TFC209S, or TFC186S/C209S. HUVECs were transduced with control adenovirus (β-Gal), adenovirus encoding wild-type TF, TFC186S, TFC209S, or TFC186S/C209S (25 MOI/cell), and TF expression levels were analyzed by various methods: confocal microscopy (A), fluorescence-activated cell sorting analysis (B), immunoblot analysis (C), in situ ELISA to measure cell-surface TF (D), and ELISA to measure total TF antigen levels (E). (A) HUVECs, nonpermeabilized (top) or permeabilized with 0.05% Triton X-100 for 10 minutes (bottom) were immunostained with polyclonal anti-TF IgG (10 μg/mL) followed by Oregon Green–conjugated secondary antibodies. Immunofluorescence was analyzed by confocal microscopy at 2 different gain settings (high gain, 840; low gain, 630) to capture differences in TF expression in cells expressing wild-type TF or TF mutant. (B) Intact, nonpermeablized HUVECs were stained with anti-TF polyclonal antibodies, followed by FITC-conjugated secondary antibodies, and the cells were analyzed for the fluorescence by flow cytometry. Wild-type TF, pink; TFC186S, cyan; TFC209S, red; TFC186S/C209S, orange; β-gal, green. (C) Cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, either nonreducing (left) or reducing (right) conditions, and immunoblotted for TF with the use of polyclonal anti-TF antibodies. (D) TF expression levels at the cell surface were measured by in situ ELISA with the use of polyclonal anti-TF IgG or TF mAb, TF96B4 (n = 4; mean ± SEM). (E) Total TF antigen levels in cell lysates were measured by ELISA (n = 3, mean ± SEM).
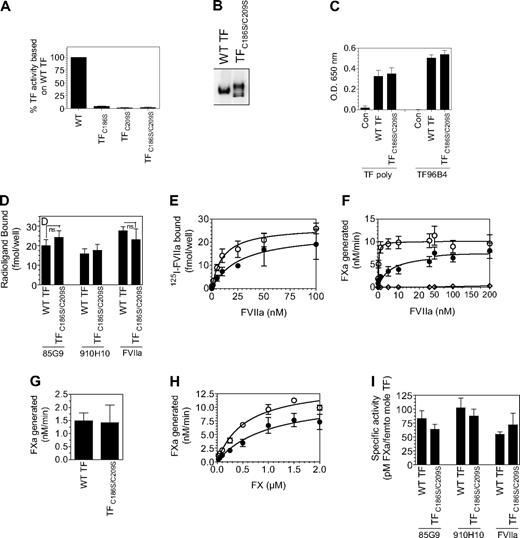
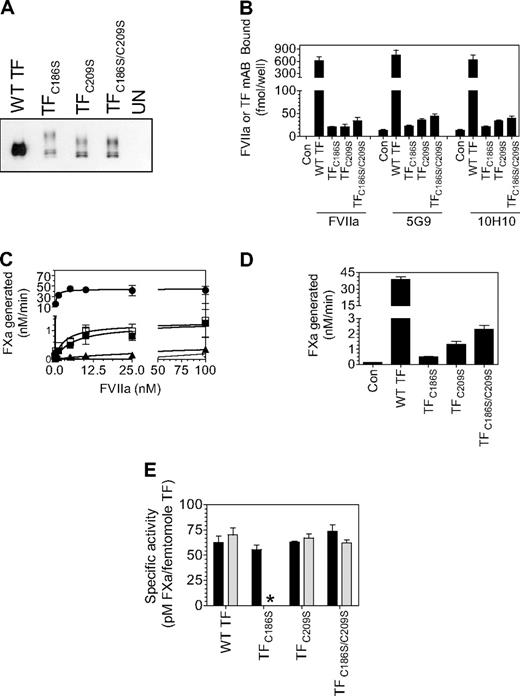
Next, we investigated whether the reduced TF procoagulant activity associated with HUVECs expressing TF mutants is solely the result of diminished TF protein expression and reduced affinity for FVIIa or whether reflects the cryptic nature of the TF mutant. For this, HUVECs were infected with the wild-type or TF mutant (25 MOI/cell), and the cell-surface TF antigen levels were evaluated by cell-surface biotin labeling and quantified by radioligand binding assays with the use of saturating concentrations of 125I-labeled TF mAb (10H10 or 5G9). The number of TF-specific FVIIa binding sites was determined by 125I-FVIIa binding to the cells in the presence and absence of polyclonal anti-TF antibodies; TF procoagulant activity was measured in FX activation assay. Surface biotinylation studies showed that endothelial cells infected with equal MOI of the mutant adenovirus expressed low TF protein on the surface compared with HUVECs infected with wild-type TF (Figure 5A). The number of TF mAb and FVIIa binding sites in HUVECs transduced with TF mutants were also markedly lower (Figure 5B), approximately 1% to 7% of that found in HUVECs transduced with wild-type TF. HUVECs transduced with TFC186S expressed the least amount of TF antigen (1%-2%). As observed with HUVECs expressing wild-type TF, the amount of TF mAb and FVIIa bound to HUVECs expressing TF mutants was similar, indicating that 10H10 and 5G9 TF mAbs are capable of binding to TF mutants in our studies (limited experiments performed with a third mAb, TF96B4, yielded similar results). Kinetic analysis of FVIIa interaction with TF showed higher concentrations of FVIIa were required to saturate TF mutant compared with wild-type TF (Figure 5C; KD values as follows: wild-type TF, 0.4nM; TFC186S, 30.0nM; TFC209S, 5.9nM; TFC186SC209S, 3.1nM). In the presence of saturating concentrations of FVIIa, HUVECs transduced with TF mutants exhibited approximately 1% to 6% of TF activity compared with HUVECs transduced with wild-type TF (Figure 5D), indicating that TF activity of the mutants correlates well with the amount of TF antigen present on the cell surface. No significant differences in the specific activity of TF mutants and wild-type TF were detected when cell-surface TF activity was normalized relative to TF antigen levels or TF-FVIIa complexes formed at the cell surface (Figure 5E). These data clearly show that impaired TF protein expression and reduced affinity for FVIIa associated with the Cys186 and Cys209 mutation are responsible for the lowered procoagulant activity of these mutants.
Correlation between TF antigen and TF activity levels at the cell surface in endothelial cells expressing wild-type TF or TF lacking Cys186-Cys209 disulfide bond. HUVECs cultured in 6- or 24-well plates were transduced with adenovirus encoding wild-type TF, TFC186S, TFC209S, TFC186S/C209S, or a control virus (β-Gal; 25 MOI/cell) and cultured for 48 hours. (A) To measure TF antigen at the cell surface, the monolayers were labeled with cell-impermeant NHS-SS-biotin (0.5 mg/mL) for 30 minutes at 4°C. Total cell extracts were then subjected to immunoprecipitation with neutravidin-agarose and immunoblotted with anti-TF polyclonal antibodies. (B) Monolayers were incubated with 125I-labeled TF mAb or FVIIa (100nM) for 2 hours at 4°C, and the amount of TF mAb and FVIIa associated with the cell surface was determined. To determine TF-specific FVIIa binding, parallel experiments were conducted in which cells were preincubated with polyclonal anti-TF IgG (100 μg/mL) for 30 minutes before the addition of 125I-FVIIa, and the radioactivity associated with cells in the presence of anti-TF was subtracted from the values obtained in the absence of anti-TF (total binding). (C) Higher concentrations of FVIIa were required to saturate TF Cys186 and Cys209 mutants compared with wild-type TF. Monolayers of HUVECs expressing wild-type TF or TF mutant were incubated with various concentrations of FVIIa (0.05-100nM) for 5 minutes at room temperature before substrate FX (1μM) was added to the cells. The amount of FXa generated was measured in a chromogenic assay. The symbols are as follows: HUVECs expressing wild-type TF, ●; TFC186S, ▲; TFC209S, ■; TFC186S/C209S, □; control virus (β-gal), ○ (n = 4, mean ± SEM). (D) HUVEC monolayers were incubated with FVIIa (100nM) for 5 minutes at room temperature, and then substrate FX (1μM) was added to the cells. The amount of FXa generated was measured in a chromogenic assay (n = 3-4; mean ± SEM). (E) TF-specific activity at the surface was determined as the amount of FXa formed (pM)/fmol of TF, as measured by TF mAb binding studies (■) or fmol FVIIa bound (▩), as determined by TF-specific binding of 125I-FVIIa. The concentrations of FVIIa and FX used were 100nM and 1μM, respectively. *Because of low expression of TF mutant, TFC186S, it was difficult to measure FVIIa binding to these cells accurately; therefore, TF-specific activity in relation to FVIIa bound to cells was not calculated.
Correlation between TF antigen and TF activity levels at the cell surface in endothelial cells expressing wild-type TF or TF lacking Cys186-Cys209 disulfide bond. HUVECs cultured in 6- or 24-well plates were transduced with adenovirus encoding wild-type TF, TFC186S, TFC209S, TFC186S/C209S, or a control virus (β-Gal; 25 MOI/cell) and cultured for 48 hours. (A) To measure TF antigen at the cell surface, the monolayers were labeled with cell-impermeant NHS-SS-biotin (0.5 mg/mL) for 30 minutes at 4°C. Total cell extracts were then subjected to immunoprecipitation with neutravidin-agarose and immunoblotted with anti-TF polyclonal antibodies. (B) Monolayers were incubated with 125I-labeled TF mAb or FVIIa (100nM) for 2 hours at 4°C, and the amount of TF mAb and FVIIa associated with the cell surface was determined. To determine TF-specific FVIIa binding, parallel experiments were conducted in which cells were preincubated with polyclonal anti-TF IgG (100 μg/mL) for 30 minutes before the addition of 125I-FVIIa, and the radioactivity associated with cells in the presence of anti-TF was subtracted from the values obtained in the absence of anti-TF (total binding). (C) Higher concentrations of FVIIa were required to saturate TF Cys186 and Cys209 mutants compared with wild-type TF. Monolayers of HUVECs expressing wild-type TF or TF mutant were incubated with various concentrations of FVIIa (0.05-100nM) for 5 minutes at room temperature before substrate FX (1μM) was added to the cells. The amount of FXa generated was measured in a chromogenic assay. The symbols are as follows: HUVECs expressing wild-type TF, ●; TFC186S, ▲; TFC209S, ■; TFC186S/C209S, □; control virus (β-gal), ○ (n = 4, mean ± SEM). (D) HUVEC monolayers were incubated with FVIIa (100nM) for 5 minutes at room temperature, and then substrate FX (1μM) was added to the cells. The amount of FXa generated was measured in a chromogenic assay (n = 3-4; mean ± SEM). (E) TF-specific activity at the surface was determined as the amount of FXa formed (pM)/fmol of TF, as measured by TF mAb binding studies (■) or fmol FVIIa bound (▩), as determined by TF-specific binding of 125I-FVIIa. The concentrations of FVIIa and FX used were 100nM and 1μM, respectively. *Because of low expression of TF mutant, TFC186S, it was difficult to measure FVIIa binding to these cells accurately; therefore, TF-specific activity in relation to FVIIa bound to cells was not calculated.
As shown in Figures 3 through 5, HUVECs infected with the same number of viral particles encoding wild-type and TF mutant express vastly different amounts of TF antigen on their cell surfaces. This would result in differences in TF density and TF/anionic phospholipid ratio at the cell surface in HUVECs expressing wild-type TF and TF mutant. It is unclear how these differences could influence the aforementioned findings. To overcome this potential problem, HUVECs were transduced with different amounts of viral particles encoding wild-type TF or TF mutants (wild-type TF, 2 MOI/cell; TF mutants, ≥ 50 MOI/cell) to express similar levels of TF protein at the cell surface. When TF procoagulant activity was measured in a clotting assay, TF mutants expressing similar levels of TF as that of wild-type TF exhibited low procoagulant activity compared with wild-type TF (< 5%; Figure 6A). However, this is not totally surprising because FVII(a) binding to TF mutants is severely impaired; thus, physiologic concentrations of FVII(a) are not sufficient to saturate TF mutants. In further studies, we examined the effect of various concentrations of FVIIa on binding to TF mutant and the resultant procoagulant activity. Because of difficulties in expressing TF mutants, particularly TFC186S mutant, consistently to similar levels as that of wild-type TF and for other practical limitations, we have limited our additional studies to TFC186S/C209S.
TF mutant lacking Cys186-Cys209 disulfide bond has reduced affinity for factor VIIa but retains coagulant function. (A) HUVECs were transduced with adenovirus encoding wild-type TF (2 MOI/cell) or TFC186S, TFC209S, or TFC186S/C209S (50 MOI/cell) to express similar levels of TF antigen and were cultured for 48 hours before they were used. Cells were detached from the dish, and TF activity of intact cells was measured in a clotting assay. (B-I) HUVECs were transduced with adenovirus encoding wild-type TF (2 MOI/cell) or TFC186S/C209S (50 MOI/cell), and TF protein expression levels at the cell surface were evaluated by cell-surface biotinylation (B), in situ ELISA (C), and the binding of radiolabeled TF mAb or FVIIa (D) as described in the legend to Figure 5. (E) Specific binding of 125I-FVIIa to TF. Various concentrations of 125I-FVIIa were incubated with HUVEC monolayers expressing wild-type TF (○) or TFC186S/C209S (●) in the presence or absence of anti-TF IgG for 2 hours at 4°C. The amount of FVIIa bound to TF was determined as described in “Determination of 125I-FVIIa and 125I-TF monoclonal antibody binding to cells.” (F) TF coagulant function. HUVECs expressing wild-type TF (○), TFC186S/C209S (●), or uninfected HUVECs (◇) were incubated with various concentrations of FVIIa (0.0-200nM) for 5 minutes, then FX (1μM) was added to the cells, and the rate of FX activation was determined by measuring the amount of FXa generated in a chromogenic assay. (G) TF activity of HUVECs expressing wild-type TF and TFC186S/C209S in the presence of saturating concentration of FVIIa. HUVECs were incubated with FVIIa (100nM) and FX (1μM), and the rate of FXa generation was measured in a chromogenic assay. These assays were performed in parallel to the experiments in which TF antigen levels were determined. (H) Rate of FXa generation by cells expressing wild-type TF or TFC186S/C209S at various concentrations of FX and a saturating concentration of FVIIa (100nM). Wild-type TF, ○; TFC186S/C209S, ● (n = 3; mean ± SEM). (I) Specific activity derived, based on FVIIa or TF mAb bound to the cell surface.
TF mutant lacking Cys186-Cys209 disulfide bond has reduced affinity for factor VIIa but retains coagulant function. (A) HUVECs were transduced with adenovirus encoding wild-type TF (2 MOI/cell) or TFC186S, TFC209S, or TFC186S/C209S (50 MOI/cell) to express similar levels of TF antigen and were cultured for 48 hours before they were used. Cells were detached from the dish, and TF activity of intact cells was measured in a clotting assay. (B-I) HUVECs were transduced with adenovirus encoding wild-type TF (2 MOI/cell) or TFC186S/C209S (50 MOI/cell), and TF protein expression levels at the cell surface were evaluated by cell-surface biotinylation (B), in situ ELISA (C), and the binding of radiolabeled TF mAb or FVIIa (D) as described in the legend to Figure 5. (E) Specific binding of 125I-FVIIa to TF. Various concentrations of 125I-FVIIa were incubated with HUVEC monolayers expressing wild-type TF (○) or TFC186S/C209S (●) in the presence or absence of anti-TF IgG for 2 hours at 4°C. The amount of FVIIa bound to TF was determined as described in “Determination of 125I-FVIIa and 125I-TF monoclonal antibody binding to cells.” (F) TF coagulant function. HUVECs expressing wild-type TF (○), TFC186S/C209S (●), or uninfected HUVECs (◇) were incubated with various concentrations of FVIIa (0.0-200nM) for 5 minutes, then FX (1μM) was added to the cells, and the rate of FX activation was determined by measuring the amount of FXa generated in a chromogenic assay. (G) TF activity of HUVECs expressing wild-type TF and TFC186S/C209S in the presence of saturating concentration of FVIIa. HUVECs were incubated with FVIIa (100nM) and FX (1μM), and the rate of FXa generation was measured in a chromogenic assay. These assays were performed in parallel to the experiments in which TF antigen levels were determined. (H) Rate of FXa generation by cells expressing wild-type TF or TFC186S/C209S at various concentrations of FX and a saturating concentration of FVIIa (100nM). Wild-type TF, ○; TFC186S/C209S, ● (n = 3; mean ± SEM). (I) Specific activity derived, based on FVIIa or TF mAb bound to the cell surface.
HUVECs were transduced with wild-type TF (2 MOI/cell) or TFC186S/C209S (50 MOI/cell), and cell-surface TF expression levels were analyzed by a variety of methods. Cell-surface biotinylation followed by the detection of biotinylated TF by Western blot analysis showed similar expression levels of wild-type TF and TF mutant at the cell surface (Figure 6B). Evaluation of cell-surface TF expression by in situ ELISA with the use of either TF polyclonal antibodies or TF96B4 also showed that wild-type TF and TF mutants were expressed at similar levels at the cell surface (Figure 6C). Quantification of TF antigen levels by radioligand binding studies with saturating concentrations of TF mAb or FVIIa confirmed that wild-type and TF mutants were expressed at similar levels (Figure 6D). HUVECs expressing similar levels of either wild-type or TF mutant were then used to characterize the TF mutant. As shown in Figure 6E, radioligand binding studies showed that the affinity of FVIIa for TF mutant was reduced by approximately 3-fold compared with its affinity for wild-type TF (Kd, 27nM vs 10nM). The maximal number of FVIIa binding sites (Bmax) on HUVECs expressing TF mutant was slightly lower (∼ 15%) compared with HUVECs expressing wild-type TF; however, this difference was not statistically significant. The procoagulant function of TF mutant expressed at the cell surface was analyzed by measuring TF-FVIIa–mediated activation of FX in the presence of various concentrations of FVIIa (0.05-200nM; Figure 6F). At low concentrations of FVIIa, TFC186S/C209S exhibited low procoagulant activity compared with the wild-type TF. However, at saturating concentrations of FVIIa, both the wild-type TF and TFC186S/C209S activated FX at a similar rate (Figure 6F-G), indicating that TF mutant is as active as wild-type TF once it is complexed with FVIIa. Consistent with this notion, FX dose-dependence experiments showed no significant differences in apparent Km between the mutant and wild-type TF (Figure 6H; Kmapp, wild-type TF, 0.54 ± 0.10μM; TFC186S/C209S, 0.99 ± 0.46μM). Finally, determination of TF-specific activities, based on cell-surface TF antigen levels estimated by TF mAb binding or the amount of TF-FVIIa complexes formed at the cell surface determined by 125I-FVIIa binding, showed that TFC186S/C209S exhibited a similar specific activity as that of wild-type TF (Figure 6I).
Cys186-Cys209 disulfide bond formation does not play a role in TF de-encryption
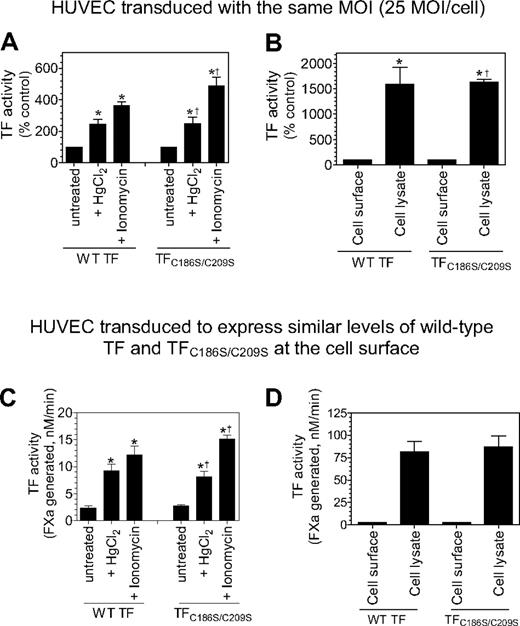
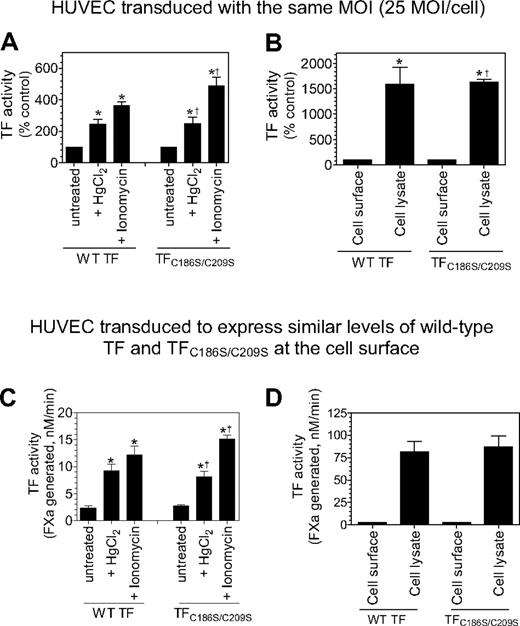
As one expects with HUVECs expressing wild-type TF, it is probable that TF disulfide mutants at the cell surface are segregated into 2 populations, a minor population of active TF and a major population of cryptic TF. TF activity measured in the aforementioned studies, for both wild-type TF and TF mutant, reflects the activity of the active (decrypted) TF population. TF activity at the cell surface can be increased several fold by decrypting the cryptic TF. Various cellular perturbations, including thiol-oxidizing agent HgCl2, ionomycin, and cell lysis, were known to decrypt TF. To investigate the importance of the Cys186-Cys209 disulfide bond formation in TF de-encryption, HUVECs were transduced with the same number of viral particles encoding wild-type TF or TFC186S/C209S, or with a different number of viral particles to express similar levels of TF protein at the cell surface. HUVECs expressing wild-type TF or TFC186S/C209S were treated with HgCl2 or ionomycin to de-encrypt TF. As shown in Figure 7A and C, HgCl2 treatment increased TF activity at the cell surface by approximately 3-fold over the treatment with control vehicle in cells expressing wild-type TF. HgCl2 treatment also increased TF activity to the same fold in cells expressing TFC186S/C209S. Similarly, ionomycin treatment increased TF activity of cells expressing both wild-type and TFC186S/C209S by 5- to 6-fold. Disruption of cells with freeze/thaw, which fully de-encrypts TF, increased the procoagulant activity of TFC186S/C209S by 20- to 25-fold, the same level as observed with wild-type TF (Figure 7B,D). We obtained similar data with HUVECs that were transduced with the same number of viral particles encoding wild-type TF or TFC186S/C209S, or cells expressing similar levels of wild-type or TF mutant proteins. These results clearly show that formation of the Cys186-Cys209 disulfide is not essential for TF de-encryption.
Cys186-Cys209 disulfide bond formation is not essential for de-encryption of TF. HUVECs were transduced with the same number of adenovirus encoding wild-type TF or TFC186S/C209S (25 MOI/cell; A-B) or different MOI/cell (wild-type TF, 2 MOI/cell; TFC186S/C209S, 50 MOI/cell) to express similar levels of TF (C-D). After culturing the cells for 48 hours, the monolayers were treated with HgCl2 (100μM) for 3 minutes or ionomycin (10μM) for 5 minutes at 37°C, the cells were washed with buffer B, and the cell-surface TF activity was measured in FX activation assay (A,C). (B,D) Cell lysates were made by scraping the cells in buffer B followed by repeated (3) freeze-thaw cycles. The lysates were diluted 1:10 in buffer B before they were used in the assay. TF activity in intact monolayers and cell lysates was determined by adding FVIIa (100nM) and FX (1μM) and measuring the rate of FX activation in a chromogenic assay. The rate of FX activation measured with intact and untreated cells was taken as 100% (A-B). *Significant increase in TF activity in treated cells versus the untreated cells (n = 3-6; mean ± SD; P < .05, t test). †The fold increase did not significantly differ from the fold increase observed with wild-type TF (n = 3-6; mean ± SD; P > .1, t test).
Cys186-Cys209 disulfide bond formation is not essential for de-encryption of TF. HUVECs were transduced with the same number of adenovirus encoding wild-type TF or TFC186S/C209S (25 MOI/cell; A-B) or different MOI/cell (wild-type TF, 2 MOI/cell; TFC186S/C209S, 50 MOI/cell) to express similar levels of TF (C-D). After culturing the cells for 48 hours, the monolayers were treated with HgCl2 (100μM) for 3 minutes or ionomycin (10μM) for 5 minutes at 37°C, the cells were washed with buffer B, and the cell-surface TF activity was measured in FX activation assay (A,C). (B,D) Cell lysates were made by scraping the cells in buffer B followed by repeated (3) freeze-thaw cycles. The lysates were diluted 1:10 in buffer B before they were used in the assay. TF activity in intact monolayers and cell lysates was determined by adding FVIIa (100nM) and FX (1μM) and measuring the rate of FX activation in a chromogenic assay. The rate of FX activation measured with intact and untreated cells was taken as 100% (A-B). *Significant increase in TF activity in treated cells versus the untreated cells (n = 3-6; mean ± SD; P < .05, t test). †The fold increase did not significantly differ from the fold increase observed with wild-type TF (n = 3-6; mean ± SD; P > .1, t test).
Discussion
Blood clotting is initiated when plasma clotting protein FVIIa binds to TF, an integral membrane protein, and the resultant FVIIa-TF complexes activate substrates FX and factor IX. Interestingly, not all of the FVIIa bound to TF on cells is able to activate the substrates because most of TF on cell surfaces exists in an encrypted state, ie, binds to FVIIa but is unable to support FX activation. Cell perturbations, such as damage to the plasma membrane, apoptosis, or exposure to oxidizing agents, transform cryptic TF to active TF.8,9,14,15,22,34,35 It is generally accepted that exposure of PS to the outer leaflet of plasma membrane in response to injury is primarily responsible for transforming the cryptic TF to active TF.10,16 However, recent studies have suggested that the status of Cys186-Cys209 disulfide bond in the membrane-proximal domain of TF, independent of PS, determines the functional status of TF.22,23 A key evidence for this came from the observation that TF mutant, in which Cys186-Cys209 disulfide bond formation was precluded by site-specific mutagenesis, exhibits low or no TF coagulant activity.22,23,30 However, there was no firm evidence that this TF mutant actually mimics the cryptic TF and is resistant to de-encryption. The data presented herein clearly shows that TF mutants lacking the Cys186-Cys209 disulfide bond exhibit the same specific activity as that of the wild-type TF in the presence of saturating concentrations of FVIIa. A severely reduced TF procoagulant activity in cells expressing TF mutants stem from a profound defect in TF synthesis and processing and the reduced affinity of TF mutant for FVIIa. More importantly, our present data show that the TF mutant, which cannot undergo Cys186-Cys209 disulfide bond formation, could undergo transformation to active TF on cell perturbation.
Disulfide bond formation plays an important role in the protein folding process and stabilizing the structure of subdomains.36 Inhibition of disulfide bond formation as a consequence of cellular stress or mutations would result in misfolding of the protein; misfolded proteins have a tendency to form aggregates or get destroyed by the ubiquitin proteasome system.37,38 The extracellular domain of TF contains 4 cysteines, which could form 2 disulfide bonds, one at the amino-terminal half (Cys49-Cys57) and one proximal to the membrane domain (Cys186-Cys209). Cys186-Cys209 disulfide bond has RHStaple configuration and thus has potential to be an allosteric disulfide bond.39 Analysis of human TF purified from brain or transfected mammalian cells showed that 4 cysteines in the extracellular domain participate in forming 2 disulfide bonds, and there were no free sulfhydryl groups.40,41 Recent studies in which 3-(N-maleimidylpropionyl)-biocytin was used to label free thiols yielded conflicting data on whether TF contains free thiols.15,22,26 If TF in cells exists with free thiols, it is likely to form mixed disulfide bonds with other proteins, as we observed in cells transfected with TF cys mutants. Because there is no evidence for the presence of such mixed disulfide-bonded proteins in cells that express TF, it is unlikely that TF contains free thiols.
Selective preclusion of disulfide bond formation by site-directed mutagenesis has been used widely to determine the role of specific disulfide bonds in the structure and function of proteins. Earlier studies showed that TF mutants lacking either Cys49-Cys57 or Cys186-Cys209 disulfide bond were expressed at the cell surface, indicating that the disulfide bonds are not essential for TF synthesis and processing.30 Therefore, it is unclear why in the present study we found low levels of TF expression in cells transfected with TFC186S, TFC209S, or TFC186S/C209S. A lower expression of TF mutants in the present study does not appear to be an experimental artifact because we have obtained similar data in cases in which we either mutated cysteine to serine or alanine, transfected the cells transiently or generated stable clones, or used different vectors and cell-model systems. It may be pertinent to note here that close examination of the earlier published data30 did show that the substitution of serine for cysteine 186 and 209 resulted in decreased levels of TF expression,30 although not as drastic as observed in the present study. It is possible that the clonal selection of TF mutants in the earlier study might have yielded an atypical TF mutant clone expressing relatively higher levels of TF. Transduction of endothelial cells with adenovirus TF mutants slightly improved the expression of TF mutants, yet it was very low compared with wild-type TF (1%-5%). Transduction of HUVECs with low number of wild-type TF viral particles (1-2 MOI/cell) and a high number of TF mutant viral particles (≥ 50/cell) was necessary to obtain similar levels of TF expression at the cell surface. When adenoviral transduction of HUVECs was used in earlier studies23 to express similar levels of TFC186A or TFC209A as of wild-type TF, it was unclear whether the same or different number of viral particles were used for the transduction. Our present data clearly suggest that Cys186-Cys209 disulfide bond formation is essential for proper TF synthesis and processing. Because it is not the focus of our present study, we have not investigated the reasons for poor expression of TF mutant lacking the Cys186-Cys209 disulfide bond.
Earlier studies showed that preclusion of the disulfide bond between cys49 and cys57 had no effect on TF function, whereas ablation of the allosteric bond between Cys186 and Cys209 severely impaired the procoagulant activity.22,39 On the basis of these data it was hypothesized that TF mutant lacking Cys186-Cys209 disulfide bond mimics the cryptic form of TF.22 The hallmark feature of cryptic TF is that it can bind to FVIIa with relatively high affinity, but the resultant TF-FVIIa complexes remain nonfunctional.7,10 Thus, increasing FVIIa concentrations beyond 1.0-10nM does not lead to increased TF activity. In contrast to that observed with cryptic TF, addition of high levels of FVIIa (100-300nM) partially restored the coagulant activity of TFC186S/C209S.30 In the present study, the addition of 50 to 100nM FVIIa fully restored the procoagulant activity of TFC186S/C209S. At saturating concentrations of FVIIa, TF mutant exhibited similar specific procoagulant activity compared with wild-type TF. A similar Km of FX activation between wild-type TF and TF mutant in the present study and in the earlier study30 also suggests that TF mutant, unlike cryptic TF, can readily associate with FX. These data clearly indicate that the decreased procoagulant activity of TF mutant at physiologic concentrations of FVII(a) is due to severe impairment of TF mutant to bind to FVIIa, and this should not be viewed as a potential cryptic nature of the TF mutant.
Recent studies also suggest that TF de-encryption involves the formation of the Cys186-Cys209 disulfide bond.22,23,26 However, the evidence for this conclusion is largely circumstantial. If the above mechanism is to be true, then the procoagulant activity of TFC186S/C209S should not increase on exposure of cells to agents that are known to de-encrypt TF, particularly thiol-oxidizing agents. Our present data show clearly that TF mutant in which formation of Cys186-Cys209 disulfide bond is precluded is de-encrypted to the same extent as of wild-type TF on treatment with a variety of agonists, including thiol-oxidizing agent HgCl2. Although the extent of increased TF activity in HUVECs in response to HgCl2 or ionomycin was lower than that which was observed with HL60 cells, it is important to note that these treatments increased the activity of wild-type TF and TFC186S/C209S to a similar fold. Cell lysis, which completely de-encrypts TF, increased the activity of TFC186S/C209S by 20- to 25-fold, to the same level as that observed with wild-type TF. Similar data were obtained in HUVECs transduced with other TF mutants (TFC186S and TFC209S) or CHO cells transfected with TFC186S/C209S, TFC186S, or TFC209S. Because HL60 cells are resistant to adenoviral transduction, we have not used these cells for the aforementioned studies. Overall, the present data clearly show that formation of the Cys186-Cys209 disulfide bond is not essential for de-encryption of TF.
In summary, the present data provide clear evidence that a TF mutant lacking the ability to form Cys186-Cys209 disulfide bond is coagulantly active and that TF de-encryption does not require the formation of Cys186-Cys209 disulfide bond. A lower TF procoagulant activity in cells expressing TF lacking the Cys186-Cys209 disulfide bond is the result of severe impairment in TF protein synthesis/processing and its reduced affinity for FVIIa and not because the mutant TF mimics the cryptic form of TF.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Wolfram Ruf of The Scripps Research Institute for providing TF monoclonal antibodies.
This work was supported by National Institutes of Health grants HL58869 and HL65500.
National Institutes of Health
Authorship
Contribution: H.K. performed most of the experiments included in the manuscript, analyzed the data, compiled the figures, and wrote the first draft of the manuscript; H.K. and R.C.N. performedexperiments involving immunofluorescence confocal microscopy; L.V.M.R. designed the research, analyzed the data, and wrote the paper; and U.R.P. conceived and designed the research, generated or helped H.K. to generate TF mutant plasmids or adenovirus, and contributed to data analysis and the preparation of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Usha R. Pendurthi or L. Vijaya Mohan Rao, Center for Biomedical Research, The University of Texas Health Science Center at Tyler, 11937 US Highway 271, Tyler, TX 75708; e-mail: usha.pendurthi@uthct.edu or vijay.rao@uthct.edu.

![Figure 1. Increased TF procoagulant activity associated with HgCl2 treatment in HL60 cells depended on PS exposure at the cell surface. (A) HL60 cells (1 × 106/mL) were stimulated with PMA (1μM) in serum-free medium for 6 hours at 37°C to induce TF expression. PMA-stimulated HL60 cells (2 × 105 cells in 200 μL) were treated with HgCl2 (100μM) for 30 seconds and 3 minutes at 37°C in the presence or absence of annexin V (400nM). At the end of HgCl2 treatment, FVIIa (10nM) and FX (175nM) were added to the cells, and the amount of FXa generated at the end of the 2-minute activation period was measured in a chromogenic assay (n = 3; mean ± SEM). *P < .05; §P < .001. (B) HL60 cells were stimulated with PMA and treated with HgCl2 as described above, and TF activity was measured in a clotting assay. TF activity is shown in arbitrary units. (C) HL60 cells stimulated with PMA and treated with HgCl2 as described in panel A were stained for cell-surface expression of PS (Alexa Fluor 488 [AF488]–annexin V staining) and TF. Control and treated cells were washed with buffer A and then incubated with AF488–annexin V in the binding buffer at room temperature for 15 minutes. The cells were then washed, fixed, and immunostained with anti-TF antibodies followed by Rhodamine Red–conjugated secondary antibodies. The immunofluorescence was analyzed by confocal microscopy (Axio Observer Z1 microscope, Plan-APOCHROMAT 63×/1.4 NA oil objective lens, Carl Zeiss LSM 510 Meta confocal system). (D) PMA-stimulated HL60 cells were incubated with 400nM annexin V for 30 minutes at 37°C and then the unbound annexin V was removed, and the cells were washed once with buffer B before they were treated with either control buffer or HgCl2 (100μM) for 3 minutes. In one set, annexin V (400nM) was added to cells again at the time of the HgCl2 treatment. TF activity was measured in FX activation assay as described for panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-09-241356/5/m_zh89991052810001.jpeg?Expires=1766057221&Signature=F93n2fJ3cZ-bJHsEdnc85jw3qxGjr6WCoglinCF1-IKqhUHYNw7NMKXSyT~WPwE0B5C59tNAI5mWowHKBhZNnTqlmG6fJot-4cs2dtd081qmmA39ykxvNLITbYl50emDb1H~pGxjTaD8TsNmL2iWUMBE9y36JUrcFLg-Qv3kK4ipdXvF9cIuh5sTWi0YzxdVCsvzPi60KV4FTegQIyP43~CR-sSY98DYAPh9qGm0zjqz9z1D1zdQ41NCzqZO1hjtn-n5tePV3A~NSyNJqvFl6FTueuE6SlHOwau-vAJMqxI-jX6F89lUcKJ3xQqyzhuNcc-hSHOtutuuHN0q7PgzVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Increased TF procoagulant activity associated with HgCl2 treatment in HL60 cells depended on PS exposure at the cell surface. (A) HL60 cells (1 × 106/mL) were stimulated with PMA (1μM) in serum-free medium for 6 hours at 37°C to induce TF expression. PMA-stimulated HL60 cells (2 × 105 cells in 200 μL) were treated with HgCl2 (100μM) for 30 seconds and 3 minutes at 37°C in the presence or absence of annexin V (400nM). At the end of HgCl2 treatment, FVIIa (10nM) and FX (175nM) were added to the cells, and the amount of FXa generated at the end of the 2-minute activation period was measured in a chromogenic assay (n = 3; mean ± SEM). *P < .05; §P < .001. (B) HL60 cells were stimulated with PMA and treated with HgCl2 as described above, and TF activity was measured in a clotting assay. TF activity is shown in arbitrary units. (C) HL60 cells stimulated with PMA and treated with HgCl2 as described in panel A were stained for cell-surface expression of PS (Alexa Fluor 488 [AF488]–annexin V staining) and TF. Control and treated cells were washed with buffer A and then incubated with AF488–annexin V in the binding buffer at room temperature for 15 minutes. The cells were then washed, fixed, and immunostained with anti-TF antibodies followed by Rhodamine Red–conjugated secondary antibodies. The immunofluorescence was analyzed by confocal microscopy (Axio Observer Z1 microscope, Plan-APOCHROMAT 63×/1.4 NA oil objective lens, Carl Zeiss LSM 510 Meta confocal system). (D) PMA-stimulated HL60 cells were incubated with 400nM annexin V for 30 minutes at 37°C and then the unbound annexin V was removed, and the cells were washed once with buffer B before they were treated with either control buffer or HgCl2 (100μM) for 3 minutes. In one set, annexin V (400nM) was added to cells again at the time of the HgCl2 treatment. TF activity was measured in FX activation assay as described for panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/21/10.1182_blood-2009-09-241356/5/m_zh89991052810001.jpeg?Expires=1766844983&Signature=NH~kVAm-gqrWRFXiy82bqxMa1LoIivQLq6BWHqffPyklA407nUTX3BxLw6oi6SrSZS~Tb2fRCOxKtfJ3A2QCulisfWeitTpKsr-huguxX57pIGYKiieQejR2-PthxbGnf6Atvy8p39vKS3YyKMKF-QgaiQsDLUusJxjLIw2wcg88DxERPQecIGCV97dLCcgpgnBPWpTHIVSTW4xwMoE0MmivXdnxcIvCRZteahFU9CAcx1ZlFqWPgpOn-kfINGzIEqqIoqJ4lQsOLzR9qnZUkKfIOpoloiuWCxM5Ep2qolkV9SdxapJ9TpI3FcBYydzBlHNOTQ-3PZk7AzMrTxhbJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)