Abstract
Enzyme replacement therapy is the standard of care for symptomatic Gaucher disease. Velaglucerase alfa is a human β-glucocerebrosidase produced in a well-characterized human cell line. A 9-month phase 1/2 open-label, single-center trial and ongoing extension study were conducted to evaluate safety and efficacy of velaglucerase alfa. Twelve symptomatic adult type 1 Gaucher patients (intact spleens) received velaglucerase alfa (60 U/kg per infusion) during phase 1/2. An extension study was offered to patients completing the trial; step-wise dose reduction (to 30 U/kg per infusion) was instituted. Eleven patients completed phase 1/2; 10 entered the extension; 9 patients reached 39 months of extension. No drug-related serious adverse events or withdrawals, and no antibodies were observed. Home therapy was successfully implemented during the extension. Statistically significant improvements (P < .004) were noted in mean percentage change from baseline to 9 months and baseline to 48 months for hemoglobin (+19.2%, +21.7%, respectively), platelet counts (+67.6%, +157.8%, respectively), normalized liver volume (−18.2%, −42.8%, respectively), and normalized spleen volume (−49.5%, −79.3%, respectively). These significant clinical changes and safety profile led to phase 3 trials and highlight the potential of velaglucerase alfa as alternative therapy for type 1 Gaucher disease. The extension trial is registered at http://www.clinicaltrials.gov as NCT00391625.
Introduction
Gaucher disease (GD) is a multisystem disorder involving the liver, spleen, bone marrow, skeleton, lungs, and occasionally the central nervous system. GD is an autosomal recessive, lysosomal storage disease caused by the deficiency of β-glucocerebrosidase.1 Enzyme replacement therapy (ERT) is currently the standard of care for the treatment of symptomatic Gaucher disease. Alglucerase injection (Ceredase; Genzyme Corporation), a mannose-terminated placental-derived β-glucocerebrosidase was the first enzyme formulation approved by the Food and Drug Administration (FDA) for the treatment of type 1 GD. Imiglucerase for injection (Cerezyme; Genzyme Corporation), a recombinant analog of β-glucocerebrosidase produced in Chinese hamster ovary cells, received FDA approval in 1994 and gradually replaced alglucerase. Both enzymatic preparations have been generally well tolerated and efficacious in the improvement of type 1 Gaucher–related hematologic abnormalities and reduction of hepatosplenomegaly.1-3
Velaglucerase alfa (formerly known as gene-activated human glucocerebrosidase [GA-GCB]) is an investigational human β-glucocerebrosidase, produced in a human cell line using proprietary Gene Activation technology (Shire Human Genetic Therapies Inc [Shire HGT]). It is a monomeric glycoprotein (∼ 63 kDa, containing 5 potential N-linked glycosylation sites) that targets macrophages via mannose receptors, and acts to degrade accumulated glucocerebroside within the macrophages.4 The amino acid sequence of velaglucerase alfa is identical to that of the human, wild-type enzyme, unlike imiglucerase, which differs from the wild-type human enzyme sequence by a single amino acid substitution at position 495.2 Another distinguishing structural feature is that velaglucerase alfa has higher α-mannosyl content than imiglucerase.
To evaluate the safety and efficacy of velaglucerase alfa, a phase 1/2 trial was performed. The primary objective was to assess the safety of velaglucerase alfa administered intravenously at a dose of 60 U/kg every other week for 9 months in adult patients with symptomatic type 1 (nonneuronopathic) Gaucher disease (GD1). The secondary objective of this trial was to assess the clinical activity of velaglucerase alfa on key disease features.1 The extension study was similarly designed to evaluate the long-term safety and assess the effects of velaglucerase alfa on 4 disease measures: hemoglobin concentration, platelet count, liver volume, and spleen volume.1 The results of the 9-month phase 1/2 open-label, single-center study of velaglucerase alfa and its 39-month extension, through 48 total months, are reported here.
Methods
The phase 1/2 and extension study were conducted in a single center (Gaucher Clinic, Shaare Zedek Medical Center). These studies were conducted in compliance with US Food and Drug Administration regulations and approved by the Gaucher Clinic Institutional Helsinki (Ethics) Committee and the Israeli Ministry of Health. The start of the phase 1/2 trial (April 2004) predated the July 2005 requirement for registration with National Institutes of Health. The extension trial was registered as NCT00391625 (http://www.clinicaltrials.gov/ct/show/NCT00391625?order=8).
Patients
Adult, symptomatic, enzymatically confirmed patients with GD1 were screened. Eligibility criteria included age of 18 years or older, an intact spleen, disease-related anemia (hemoglobin values at least 10 g/L [1 g/dL] below lower limit of normal for sex), thrombocytopenia (platelet counts below the lower limit of normal), and a negative result for hepatitis B and C antigen and human immunodeficiency virus. Patients were eligible if they were naive to ERT or had not received imiglucerase within the 12 months prior to enrollment and were imiglucerase antibody–negative. Female patients of child-bearing potential were required to use a medically acceptable form of contraception during both studies and a negative serum pregnancy test at enrollment and prior to each infusion was required.
Patients were excluded if they had received an investigational therapy for any other indication 30 days or less prior to enrollment or if they could not comply with the protocol for either medical or nonmedical reasons. Patients who successfully completed the phase 1/2 trial were eligible to transition to the extension study.
Preparation and dosing
The formulation of velaglucerase alfa includes sodium citrate, polysorbate 20, and sucrose. Velaglucerase alfa was supplied by Shire HGT as a lyophilized product and shipped at 2°C to 8°C. The product was reconstituted with preservative-free, sterile water for injection. The appropriate amount of velaglucerase alfa (based on body weight) was slowly mixed with normal saline to a final volume of 100 mL. The diluted velaglucerase alfa was administered intravenously across a 0.2-μm filter (standard practice for all Shire HGT enzymatic, intravenous preparations) for a period of 60 minutes (maximum rate of 1.5 mg/kg per hour; 1 U/kg per minute).
During the phase 1/2 study, the first 3 patients received velaglucerase alfa on an every other week schedule at the trial site. Dose escalation was undertaken for the first 3 patients whereby dosing doubled from an initial dose of 15 U/kg until a final dose of 60 U/kg was achieved. The second and third patients received their initial 15-U/kg infusion only after a 7-day observation period was completed for the first and second patients, respectively. Once the third patient received a single dose of 60 U/kg and was observed for a period of 7 days, 9 additional patients were enrolled and received infusions of 60 U/kg every other week for a total of 20 doses. The patients who had undergone dose escalation were continued on an every other week schedule for 17 further infusions at 60 U/kg for a total of 20 infusions.
During the extension study, all patients were continued at 60 U/kg per infusion every other week. After approximately 6 to 9 months of the extension study, patients who achieved at least 2 of the 4 therapeutic goals for improvement in anemia, thrombocytopenia, hepatomegaly, and/or splenomegaly5 were transitioned to 45 U/kg per infusion every other week for 3 months and then transitioned to 30 U/kg per infusion every other week. This convention of dose reduction based on achievement of therapeutic goals is in accordance with recommendations for individualization of ERT in patients on imiglucerase.6
In addition, the 7 patients residing in Israel were transitioned to home therapy during the extension phase beginning in January 2006.
Safety assessments
Safety was evaluated throughout the study by every other week assessments of adverse events (including infusion-related reactions), concomitant medications, and vital signs performed before, during, and after infusions. Additional safety assessments were conducted approximately every 12 weeks and included physical examinations, clinical laboratory tests (hematology, serum chemistry, urinalysis, and pregnancy test), 12-lead electrocardiograms, and echocardiograms at the trial site. Determination of the presence of anti–velaglucerase alfa antibodies was conducted at 3-month intervals at Shire HGT.
Antibody assays
All participants were screened for circulating anti–velaglucerase alfa antibodies using a validated indirect enzyme-linked immunosorbent assay (ELISA). Microwell plates were coated with velaglucerase alfa, washed, and blocked with bovine serum albumin to limit nonspecific antibody binding. They were incubated with patient serum samples diluted 100-fold in phosphate-buffered saline containing 0.05% Tween 20 for 60 minutes at 37°C. The microwells were washed and then incubated with the appropriate horseradish peroxidase (HRP)–conjugated secondary antibody. They were separately probed with the following HRP antibody, isotype-specific conjugates: (1) goat anti–human immunoglobulin G (IgG) Fc, (2) goat anti–human IgA α-chain, (3) goat anti–human IgM μ-chain, or (4) goat anti–human IgE ϵ-chain secondary antibodies. The microwells were washed one final time and incubated with the HRP chromogenic substrate 3,3′,5,5′ tetramethyl benzidine. The reaction was stopped by the addition of 2N sulfuric acid, and the absorbance of each well was quantified at 450 nm (A450) using a Molecular Devices SPECTRAmax Plus 384 plate reader and SOFTMax PRO software. Antibody-positive serum samples were obtained from patients receiving imiglucerase. These patient antibodies cross-reacted with velaglucerase alfa and were used as human positive controls in the screening assay for anti–velaglucerase alfa antibodies. These sera were therefore both anti-imiglucerase and anti–velaglucerase alfa–positive. Negative, as well as positive, human serum controls were included within every assay plate.
A robust ELISA antibody–positive cutpoint absorbance for anti–velaglucerase alfa antibodies was established from the mean absorbance of ERT-naive Gaucher patient serum samples (N = 108). Parametric analysis of ELISA absorbance data was used to calculate the antibody-positive lower limit (mean + 1.645 × SDs; where 1.645 is the 95th percentile of the normal distribution, thus potentially accepting a 5% false-positive rate) for each antibody isotype.7 The ELISA A450 background for ERT-naive Gaucher serum samples was calculated to be 0.040 for all anti–velaglucerase alfa antibody isotype assays. The ELISA antibody–positive cutoff was established as a ratio higher than 2.0 and an A450 higher than 0.040, where ratio is the A450 of a patient sample taken at any time point, divided by the A450 of the patient sample taken at baseline prior to the first ERT treatment. Any sample exceeding the ELISA-positive cutoff would have been confirmed by a quantitative radioimmunoprecipitation assay and tested for neutralizing antibodies; however, no sample achieved the established cutoff criteria.7
Clinical activity
The main efficacy assessments of hemoglobin concentration and platelet counts were evaluated at predetermined trimonthly intervals. Liver and spleen volumes were measured using quantitative abdominal magnetic resonance imaging (MRI; on the same model apparatus) performed at baseline, and 6 and 9 months (at the Hadassah-Hebrew University Medical Center), and at 24, 33, and 45 months (at the MOR-MAR Imaging unit) during the extension study. Liver and spleen volumes were assessed at the end of the trial by a radiologist blinded to the patient's identity and the sequence by which the quantitative abdominal MRIs were performed. Chitotriosidase and CCL18 were measured at the Academic Medical Center (Amsterdam, The Netherlands).
Statistical analysis
The safety population, which was also the intent-to-treat population, was defined as all enrolled patients receiving at least one infusion (partial or full) of velaglucerase alfa, and was used for all clinical activities. No imputation was used.
For the primary clinical activity parameters of hemoglobin concentration, platelet count, and liver and spleen volumes, the null hypothesis was that there was no difference between the baseline value and end-of-the study value (9 months), and baseline value and end-of-48 months value (ie, the difference between the members of each pair of observation has median value of zero). Comparisons were performed using 2-tailed hypothesis testing at the 5% level of significance. Percent differences between baseline and end-of-period values were analyzed using the Wilcoxon signed-rank test.
Funding source
Initially Transkaryotic Therapies (TKT) and then Shire HGT (who bought TKT) supported all facets of the studies, including funding local laboratory evaluations, transportation of patients, and some of the statistical analyses. Protocols were designed together with investigators from this site (A.Z. and D.E.) who also evaluated the data and wrote the original article.
Results
Demographics and disposition
A total of 13 patients were screened and all consented to participate in this study; 1 patient (0004) was excluded because of imiglucerase antibodies. All the patients were treatment naive at advent according to the protocol by virtue of not having been exposed to any Gaucher-specific therapy in the 12 months prior to enrollment although in the more distant past, 2 patients (0008 and 0009) had each received 3 infusions of imiglucerase, 1 patient (0003) had been exposed to miglustat, and 2 patients (0005 and 0007) had been exposed to both miglustat and imiglucerase.
The intent-to-treat population (Table 1) included 12 patients who received at least one dose of velaglucerase alfa; of these, 11 patients (92%) completed the phase 1/2 study (1 patient, 0006, because of a sudden death in the family withdrew consent after receiving 3 infusions). One patient (0012) did not consent to enter the extension study because of the inconvenience of every other week hospital attendance, and 1 patient (0005) withdrew from the extension study because of pregnancy.
At enrollment of the phase 1/2 trial, 7 patients (58%) were female; mean age was 41.7 years (SD ± 17.3; range, 19-70 years); mean weight was 59.6 kg (SD ± 9.1; range, 50-73 kg); and mean height was 169 cm (SD ± 8.0; range, 160-184 cm). Two patients (16.7%) had avascular necrosis (AVN) of the hip joint at enrollment and another had a destructive lesion in each ankle. Table 1 provides the demographic, genotypic, and clinical characteristics at baseline, as well as the clinical findings of each intent-to-treat patient at key data collection points within the phase 1/2 and extension studies.
Safety
In both the phase 1/2 and extension studies there were no drug-related serious adverse events, regardless of infusion setting. No premedications were administered and no patient withdrew because of an adverse event. No patient developed antibodies to velaglucerase alfa. All of the 12 treated patients experienced one or more treatment-emergent adverse events, all of which were mild to moderate, and the majority of which were not drug related.
The most common possibly or probably drug-related treatment-emergent adverse events during the phase 1/2 trial were dizziness, migraines, headaches, nausea, back pain, bone pain, and increased body temperature; and during the extension study were epistaxis and abdominal pain (Tables 2–3). One patient was dose-increased to 60 U/kg per infusion every other week 24 months after dose reduction because of worsening bone pain but experienced no relief after dose increase. During the first year of the extension study, all 7 residents of Israel were successfully transitioned to home therapy.
Clinical activity
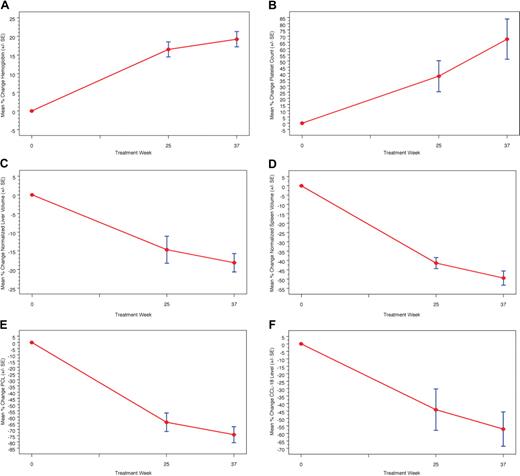
Figure 1 presents the mean percentage change in hematologic parameters, organ volumes, and biomarkers for the phase 1/2 study. Statistically significant improvements (P < .003) were noted in the mean percentage change from baseline to 9 months for hemoglobin concentration (+19.2%) platelet counts (+67.6%), normalized liver volume (−18.2%), and normalized spleen volume (−49.5%), with statistically significant improvements from baseline in both hemoglobin concentration and platelet counts achieved within the first 3 months (Figure 2). Continuous improvement in these clinical parameters was noted throughout the extension study (Figure 2), and normalization of hemoglobin was observed in all patients by 24 months (Table 1). The mean percentage change from baseline to 48 months was statistically significant (P < .004) for hemoglobin concentration (+21.7%), platelet counts (+157.8%), liver volume (−42.8), and spleen volume (−79.3%).
Mean percentage change in hematologic values, organ values, and biomarkers in phase 1/2 trial. A marked increase in hemoglobin concentration (A) and platelet count (B) is observed during weeks 25 and 37 along with a marked reduction in liver (C) and spleen volumes (D). Although biomarker sampling for chitotriosidase (E) and CCL18 (F) was incomplete, a general decrease in both biomarkers relative to baseline is observed per patient over time.
Mean percentage change in hematologic values, organ values, and biomarkers in phase 1/2 trial. A marked increase in hemoglobin concentration (A) and platelet count (B) is observed during weeks 25 and 37 along with a marked reduction in liver (C) and spleen volumes (D). Although biomarker sampling for chitotriosidase (E) and CCL18 (F) was incomplete, a general decrease in both biomarkers relative to baseline is observed per patient over time.
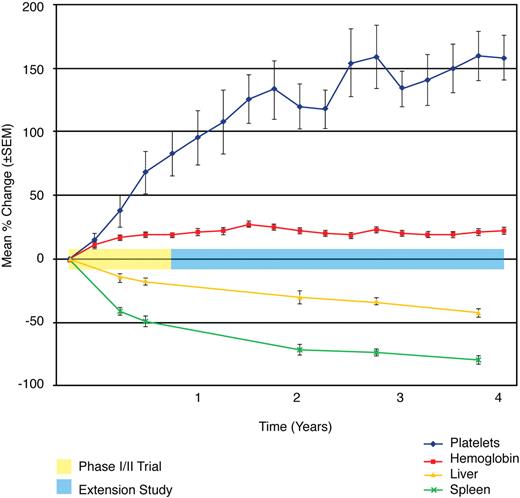
Phase 1/2 and extension trials. Mean percentage change of key clinical parameters. The mean percentage change in hemoglobin concentration, platelet counts, liver volume, and spleen volume is plotted across time and demarcated for both the phase 1/2 and extension studies. A statistically significant change from baseline is observed from baseline to 9 months (P < .003) and baseline to 48 months (P < .004) for each parameter. The most marked changes were observed for platelet count and spleen volume. Hemoglobin values normalized for all patients by 24 months. Liver volumes approached normal.
Phase 1/2 and extension trials. Mean percentage change of key clinical parameters. The mean percentage change in hemoglobin concentration, platelet counts, liver volume, and spleen volume is plotted across time and demarcated for both the phase 1/2 and extension studies. A statistically significant change from baseline is observed from baseline to 9 months (P < .003) and baseline to 48 months (P < .004) for each parameter. The most marked changes were observed for platelet count and spleen volume. Hemoglobin values normalized for all patients by 24 months. Liver volumes approached normal.
Discussion
The introduction of enzyme therapy has significantly impacted the natural history of type 1 Gaucher disease. Unfortunately, the existence of a single therapeutic option represents an inherent vulnerability in the treatment of patients with type 1 Gaucher disease. Approximately 15% of imiglucerase-treated patients have been reported to develop IgG antibodies and approximately half of these patients reported symptoms of hypersensitivity.8 Globally, the dependence on a single product in the treatment of Gaucher disease has been underscored by the recent shortage of imiglucerase.9 Among those receiving imiglucerase infusions, an unknown number of patients require premedication to mitigate a potential immune-mediated response. In some instances, patients require hydrocortisone, which itself is associated with medical risks including AVN. Furthermore, recently published data suggest that 59% of 195 GD1 patients treated with imiglucerase did not achieve all of 6 therapeutic goals after 4 years of treatment regardless of dose and schedule of treatment.10
Velaglucerase alfa is a novel ERT with unique characteristics (wild-type amino acid sequence and high α-mannosyl content) that distinguishes it from imiglucerase. This phase 1/2 clinical trial and extension study were undertaken to evaluate the safety and efficacy of velaglucerase alfa. This is the longest prospective safety and efficacy study of ERT in the treatment of GD1 to date. This was also the first clinical trial involving an ERT to implement dose reduction and home therapy.
The findings reported herein demonstrate that adverse events associated with velaglucerase alfa were generally mild in severity and were mostly unrelated to therapy. Treatment-emergent adverse events were mild to moderate and were mostly not drug related. No patient enrolled in these studies developed antibodies, no drug-related serious adverse events were observed regardless of infusion setting or duration of exposure, and no patient withdrew from the study because of adverse events. After an initial period of observation at the study site, eligible patients were successfully transitioned to home-based, nurse-administered velaglucerase alfa.
Velaglucerase alfa demonstrated efficacy in the 4 disease parameters studied with statistically significant and clinically meaningful improvements from baseline observed within the first 6 months of treatment and throughout the course of the trial and extension study. Within 24 months of initiation of therapy, all patients achieved normalization of hemoglobin level, all but 1 patient achieved platelet counts of greater than 100 000/mm3, all patients achieved near normalization in liver volumes, and all patients but one exhibited a reduction of more than 50% in spleen volume. Moreover, these improvements were observed throughout the duration of the studies, including the dose-reduction phase. The only patient who was returned to the original dose of 60 U/kg every other week did so at 39 months, secondary to bone pain after an initial dose reduction at 15 months. This patient had boney destructive lesions in both her ankles at enrollment (imaging pathology could not rule out AVN) and had a prior history of osteomyelitis. The principal investigator (A.Z.) attributed the worsening pain to the pre-existing destructive lesion and prior pathology, indicating that was likely not related to the dose reduction or treatment failure.
The observed safety profile including the transition to home treatment and the significant changes observed in the clinical parameters despite dose reduction have led to 3 further subsequent phase 3 trials for velaglucerase alfa (which allowed children), as well as a global early access program, and an FDA-accepted treatment protocol.
These very promising results have led to additional comprehensive studies of velaglucerase alfa and underscore the potential value of this novel enzyme preparation as an alternative therapeutic option for the treatment of type 1 Gaucher disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The initial study (TKT 025) was initiated by Transkaryotic Therapies, which was bought by Shire HGT, which has continued to support the extension study. D.E. and A.Z. wrote the report, which was approved by the sponsor; no medical writer was employed. The authors acknowledge the contributions of Robert Fram, MD, Candida Fratazzi, and Paul Martha, MD (all formerly of Transkaryotic Therapeutics/Shire HGT), Robert Mensah, PhD, and David Zahrieh (both of Shire HGT) to the design of the study and statistical support. John Gomorri, MD, and Bryon Gomberg, PhD, of the Hadassah-Hebrew University Medical Center and George Blinder, MD, of MOR-MAR Advanced Imaging Ltd, were in charge of the MRI evaluations. The authors also gratefully acknowledge the professionalism and dedication of Sorina Grisaru-Gransovsky, MD, Ehud Lebel, MD, Irith Hadas-Halpern, MD, and Ariel Brautbar, MD, and our nurses, Rozzie Feldman, RN, Yael Greenberg, RN, and Devorah Shapiro, RN, of the Pediatric Out-Patient Service at Shaare Zedek Medical Center (SZMC), and Anat Oz, RN, and Naama Arbel, RN, who provided home therapy. The technical assistance of Ms Gaya Chicco and Nina Lonshekova, MD, at SZMC is also acknowledged. Finally, the authors gratefully acknowledge Maja Djordjevic, MD (Belgrade, Serbia) and Costin Brutaru, MD (Bucharest, Romania) for providing home therapy for the non-Israeli patients.
Authorship
Contribution: A.Z. was principal investigator in the seminal trial (TKT 025) and its extension (TKT 025Ext) from the inception and throughout, helped design the protocols for the trial and extension, monitored patients personally, oversaw all clinical aspects of the trials, and wrote all drafts of the paper together with D.E.; G.A., M.P., D.A., M.J., and M.D. are medical doctors and were subinvestigators during different periods of the trial and extension, each responsible for monitoring patients; N.W. assisted in the statistical analysis used in the current version of the paper; K.B. was medical monitor in the latter part of the extension period; G.M.C. was responsible for overseeing the preparation of the current version of the paper; and D.E. was a subinvestigator and study coordinator of the seminal trial (TKT 025) and its extension (TKT 025Ext) from the inception and throughout, helped design the protocols for the trial and extension, and was responsible for all nonclinical aspects of the trials.
Conflict-of-interest disclosure: A.Z. receives consultancy fees from Shire HGT; receives consultancy fees and has options in Protalix Therapeutics and sits on its Scientific Advisory Board; and receives support from Genzyme Corporation for participation in the International Collaborative Gaucher Group registry. D.E. receives consulting fees from Shire HGT. K.B., G.M.C., and N.W. are employees of Shire HGT. The remaining authors declare no competing financial interests.
Correspondence: Deborah Elstein, Gaucher Clinic, Shaare Zedek Medical Center, PO Box 3235, Jerusalem 91031, Israel; e-mail: elstein@szmc.org.il.