Abstract
We tested whether cyclooxygenase 2 (COX-2) expression and unacetylated COX-1 in newly formed platelets might contribute to persistent thromboxane (TX) biosynthesis in aspirin-treated essential thrombocythemia (ET). Forty-one patients on chronic aspirin (100 mg/day) and 24 healthy subjects were studied. Platelet COX-2 expression was significantly increased in patients and correlated with thiazole orange–positive platelets (r = 0.71, P < .001). The rate of TXA2 biosynthesis in vivo, as reflected by urinary 11-dehydro-TXB2 (TXM) excretion, and the maximal biosynthetic capacity of platelets, as reflected by serum TXB2, were higher in patients compared with aspirin-treated healthy volunteers. Serum TXB2 was significantly reduced by the selective COX-2 inhibitor NS-398 added in vitro. Patients were randomized to adding the selective COX-2 inhibitor, etoricoxib, or continuing aspirin for 7 days. Etoricoxib significantly reduced by approximately 25% TXM excretion and serum TXB2. Fourteen of the 41 patients were studied again 21 (± 7) months after the first visit. Serum TXB2 was consistently reduced by approximately 30% by adding NS398 in vitro, while it was completely suppressed with 50μM aspirin. Accelerated platelet regeneration in most aspirin-treated ET patients may explain aspirin-persistent TXA2 biosynthesis through enhanced COX-2 activity and faster renewal of unacetylated COX-1. These findings may help in reassessing the optimal antiplatelet strategy in ET.
Introduction
Essential thrombocythemia (ET) is a myeloproliferative neoplasm characterized by high platelet generation, whose incidence is estimated to be around 1 to 2.5 per 100 000 subjects, although this estimate is likely to increase in the near future due to the continuous rise of “occasional,” asymptomatic diagnoses.1-4 ET usually occurs in 50- to 60-year-old patients and has a longer life-expectancy compared with other myeloproliferative neoplasm.4-7 However up to 60% of all ET patients experience a minor or major thrombotic event in their life, mainly arterial, such as myocardial infarction, stroke, or transient ischemic attack.8,9 Thrombotic complications significantly increase morbidity and impair survival.7,10,11
Annual thrombotic event rates range from 2% to 7% in ET patients treated with cytoreductive agents with or without antiplatelet drugs,7-9,11-13 with estimates up to 13%, in the absence of cytoreduction.12 In the Primary Thrombocythemia 1 randomized trial, the annual rate of a composite cardiovascular endpoint was 4% in patients randomized to anagrelide, and 2.7% in the hydroxyurea arm, on a background of aspirin (98% of enrolled patients were on 75 mg of aspirin daily).13 The annual recurrence rate of thrombosis after a first event has been estimated to be approximately 6% to 8% on antiplatelet drugs.14 Hemorrhagic complications are less frequent, approximately 0.33% per year,9 possibly due to an acquired von Willebrand-like defect, associated with the highest platelet counts.15,16
In addition to the clinical evidence of a predominantly arterial thrombotic diathesis, several groups have reported evidence of platelet activation in vivo or ex vivo in ET.17,18 We have previously reported enhanced urinary excretion of thromboxane (TX) metabolites (TXMs) in untreated ET patients.19 TXM excretion is a validated index of in vivo platelet activation, and it has been reported to be consistently increased in different clinical settings at high cardiovascular risk.20
On the basis of the arterial thrombotic diathesis and of the evidence of ongoing platelet activation, low-dose aspirin (75-100 mg once daily) is currently used not only in secondary, but also in primary cardiovascular prevention of ET patients.4-6,8,9,21 Despite the absence of controlled trials formally assessing the efficacy and safety of aspirin in ET, low-dose aspirin is currently recommended in ET, independently of the underlying cardiovascular risk, including young patients without prior vascular events.5,6,9,21 This recommendation is mainly based on retrospective analyses and extrapolation from polycythemia vera,5,6,9 for which a randomized clinical trial of aspirin was performed.22 Aspirin achieves its antithrombotic effect by permanently and selectively inactivating platelet cyclooxygenase 1 (COX-1), thus blocking TXA2 biosynthesis.23 It has been shown that COX-2 is up-regulated during megakaryopoiesis,24 is expressed by normal megakaryocytes and circulating young platelets,24-29 and it is overexpressed in bone marrow megakaryocytes of ET patients.30 Under conditions of physiologic thrombopoiesis, platelet COX-2 does not appear to contribute to TXA2 production.24,31,32 However, under conditions of accelerated thrombopoiesis, such as after bone marrow transplantation, platelet COX-2 can contribute to TXA2 production, as assessed by selective COX-2 inhibition in vitro.24 Thus, it is conceivable that, due to accelerated thrombopoiesis, ET platelets might express relatively higher levels of COX-2, whose contribution to in vivo TXA2 biosynthesis is currently unknown.
The present study was designed to evaluate in ET patients whether (1) the disease is associated with increased platelet COX-2 expression; (2) platelet COX-2 is enzymatically active, contributing to enhanced TXA2 biosynthesis; and (3) the residual TXA2 production in aspirin-treated patients can be fully suppressed by selective COX-2 inhibition and/or by further inactivation of COX-1.
Methods
Design of the study
We enrolled 41 ET patients diagnosed by established World Health Organization criteria.33 Exclusion criteria were: recent (< 6 mo) major vascular event (myocardial infarction or stroke), bleeding history, gastroduodenal ulcer, aspirin intolerance, obesity, diabetes mellitus, dyslipidemia, hypertension, ongoing or planned pregnancy, use of anticoagulants or antiplatelet agents other than low-dose aspirin, need for chronic nonsteroidal anti-inflammatory drugs (NSAIDs), platelet count more than 1 000 000/μL on at least 2 consecutive occasions, major kidney or liver disease. Vitamin supplements, NSAIDs (with the exception of paracetamol), other antiplatelet drugs, or anticoagulants were not allowed at any time during the study. All enrolled patients were on low-dose aspirin, according to current recommendations.9
The present study included a baseline evaluation, a randomized open-label intervention, and a follow-up re-examination of one-third of the patients. The protocol was approved by the Ethics Committee (approval 816, May 10, 2005, Spirito Santo Hospital, Pescara, Italy). Written informed consent was obtained from each participant. The study was conducted in accordance with the Declaration of Helsinki. The study was performed between March 2006 and December 2008, including the reexamination of 14 patients.
Patients were studied on 2 separate occasions (V0 and V1), 1 week apart, before randomization and instructed to take aspirin at the same time of the day (late afternoon/evening). At V1 patients were randomized to either continue aspirin (Cardioaspirina; Bayer) 100 mg once daily or to add etoricoxib (Tauxib; Sigma-Tau) 90 mg, once daily for 7 days, to aspirin therapy. Patients were studied again after 7 days of randomized treatment (V2 visit). Blood and urine samples were collected at each visit after an overnight fast. Aspirin and etoricoxib were provided at randomization (V1) for the following 7 days. Compliance was measured by pill count. Complete blood counts were measured using a Sysmex XE 2100 haemocytometer (TOA Medical Electronics). Fourteen patients were studied again 13 to 32 months after the initial evaluation, to assess the stability of platelet TXB2 production and its COX-isozyme dependence.
We also studied 24 healthy subjects (9 men, 15 women; mean age, 42.5 ± 14 years). Emocromocytometric values of these subjects were within the normal range (platelets, 253 285 ± 93 702/μL; hemoglobin, 13.2 ± 2 mg/dL; hematocrit, 40.1 ± 5%; leukocytes, 6100 ± 833/μL [means ± SD]).
Blood and urine TX metabolite measurements
To assess the adequacy of platelet COX-1 inactivation, we measured whole blood TXB2 production.34 One milliliter of peripheral venous blood without anticoagulant was transferred into a glass tube, allowed to clot at 37°C for 1 hour, and centrifuged at 1200 g for 10 minutes.34 The supernatant serum was stored at −20°C until assayed for TXB2 using a commercial enzyme immunoassay (Cayman Chemicals). For the in vitro experiments with COX-inhibitors, 1μM NS-398, 50μM aspirin (both from Cayman Chemicals), or vehicle (ethanol) were dried into glass tubes that were used for whole blood TXB2 production, as detailed above. To investigate the rate of TXA2 biosynthesis in vivo, we measured the urinary excretion of its major enzymatic metabolite, 11-dehydro-TXB2 (TXM).35 Urinary TXM was measured by a previously validated radioimmunoassay.35 Both serum TXB2 and urinary TXM measurements were preformed blindly with respect to randomized treatment.
Platelet immunophenotyping and reticulated platelets
Platelet COX-2 expression was assessed by flow cytometry (fluorescence-activated cell sorter, FACS) and immunocytochemistry on washed platelets. For FACS analysis, platelets were collected in 3.8% sodium citrate and processed within 2 hours. Briefly, after centrifugation (550 rpm, 15 minutes), platelet-rich plasma was aspirated, incubated with 10μM prostaglandin E1 (Cayman Chemicals), and washed twice with Ca/Mg-free Dulbecco phosphate-buffered saline (PBS)/0.5% BSA. An aliquot (107 platelets) was permeabilized (BD FACS Permeabilizing Solution 2; BD Biosciences) for 10 minutes at room temperature, centrifuged, resuspended in Dulbecco PBS/bovine serum albumin and incubated for 3 hours at 4°C with the following antibodies: fluorescein isothiocyanate–conjugated mouse anti–COX-1 (Cayman Chemicals), phycoerythrin-conjugated anti–COX-2 (Cayman Chemicals), peridinin-chlorophyll-protein-–conjugated mouse anti-CD61 (BD Biosciences), and isotype- and fluorochrome-matched irrelevant mouse immunoglobulin Gs as negative controls. Platelets were examined on a FACSCalibur cytometer (BD Biosciences) on a log scale. The platelet population was identified on the basis of forward and side scatter distribution and CD61 positivity, and 30 000 CD61-positive platelets were acquired and analyzed with the CellQuest software (BD Biosciences). Data were analyzed as mean fluorescence intensity (MFI). For each sample, the values of corresponding negative controls were subtracted from the positively stained sample and expressed as ΔMFI.
Reticulated platelets were determined by flow cytometry using the thiazole orange (TO) method as previously described.24 Nonpermeabilized washed platelets were kept in TO (Retic-Count; BD Biosciences) 10 minutes at room temperature or PBS as control, or incubated with anti-CD61 for 1 hour, then spun down, washed, and resuspended in PBS and immediately analyzed on FACSCalibur, with 30 000 events being counted in the CD61-positive gate.
Immunocytochemistry was performed on washed platelets as previously described,24 using an anti-CD61 monoclonal antibody (Dako) and/or an anti–COX-2 noncommercial polyclonal antibody.36 In single-stain experiments, COX-2 was revealed using the ABC peroxidase kit with a diaminobenzidine chromogen (ScyTec Laboratories). In double staining experiments, CD61 was detected with biotinylated secondary antibodies (Vector Laboratories) revealed with streptavidin horseradish peroxidase and AEC chromogen (ScyTec Laboratories), while COX-2 was detected with secondary antibodies revealed with alkaline phosphatase streptavidin (Vector Laboratories) and Sigma FAST 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium chromogen (Sigma-Aldrich). Platelets were lightly counterstained with eosin. In each slide, 6 different fields were analyzed by light microscopy (Axioskop microscope; Zeiss), counting 100 platelets per field, and positive platelets were recorded. A mean of 6 counts was considered for each sample.
Western blottings were performed on washed platelets as previously described.37 Briefly, platelets were washed as detailed for flow cytometry, lysed in ice-cold radioimmunoprecipitation assay buffer containing 1% Nonidet P-40/0.5% sodium deoxycholate/0.1% sodium dodecyl sulfate (SDS), protease inhibitors (Sigma-Aldrich), and mechanically homogenized. Samples were electrophoresed in 10% SDS polyacrilyamide gel under reducing conditions. Gels were blotted onto nitrocellulose membranes, which were incubated with monoclonal antibodies against COX-1 or COX-2.36 Positivity was revealed by anti–mouse horseradish peroxidase–conjugated antibodies (Calbiochem) and enhanced chemiluminescence detection reagent (GE Healthcare). Protein bands were visualized using Kodak BioMax light film (Sigma-Aldrich).
JAK2 mutational analysis
A quantitative real-time polymerase chain reaction (qRT-PCR)–based allelic discrimination assay was used to detect the V617F (JAK2) mutation, using TaqMan real-time technology on an AB7900. Genomic DNA was amplified in 40 cycles at a T annealing of 61°C, in a final volume of 25 μL containing 1× PCR Master Mix (Applied Biosystems), 900nM forward and reverse primers and 100nM each probe. For each DNA sample a control gene was amplified to test DNA amount. Allele relative frequency was calculated as previously described.38
Statistical analyses
The sample size of the randomized intervention study was calculated to evaluate the impact of selective COX-2 inhibition on in vivo TXA2 biosynthesis. We estimated that 20 patients per treatment arm (etoricoxib plus aspirin vs aspirin alone) would be required to yield 85% power to detect a 20% reduction in urinary TXM in the etoricoxib arm versus aspirin alone, with a 2-tailed α of 0.05. The results were analyzed by parametric or nonparametric methods, according to their distribution. Comparisons were made using analysis of variance or the Kruskal-Wallis test. Correlations were assessed by the Pearson or Spearman rank test, as appropriate. Differences between pre and postrandomization treatment values were analyzed with the t test or Wilcoxon signed-rank test. Data are reported as mean plus or minus SD or as median and interquartile range (IQR), according to their distribution. Multiple linear regression analysis was conducted to assess independent predictors of TXM levels. Significance was defined as P < .05. All tests were 2-tailed, and analyses were performed using SigmaStat 3.1 (Systat Software).
Results
Phenotypic and enzymatic studies of platelets isolated from ET patients
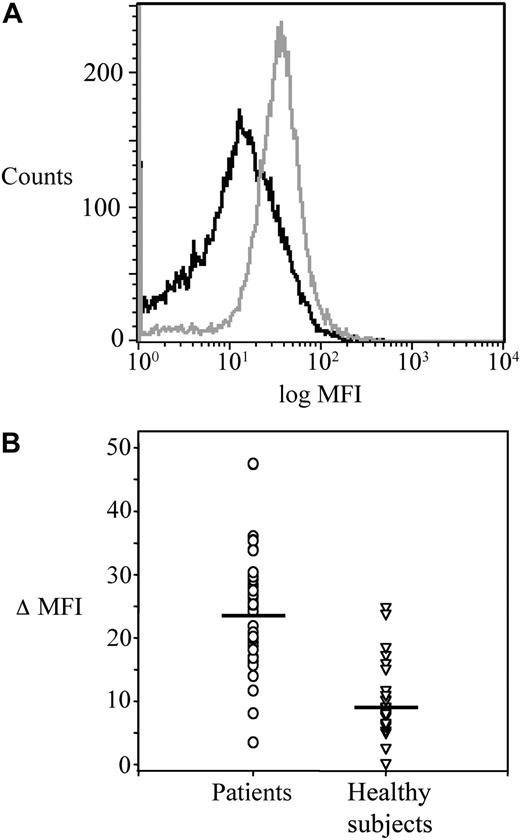
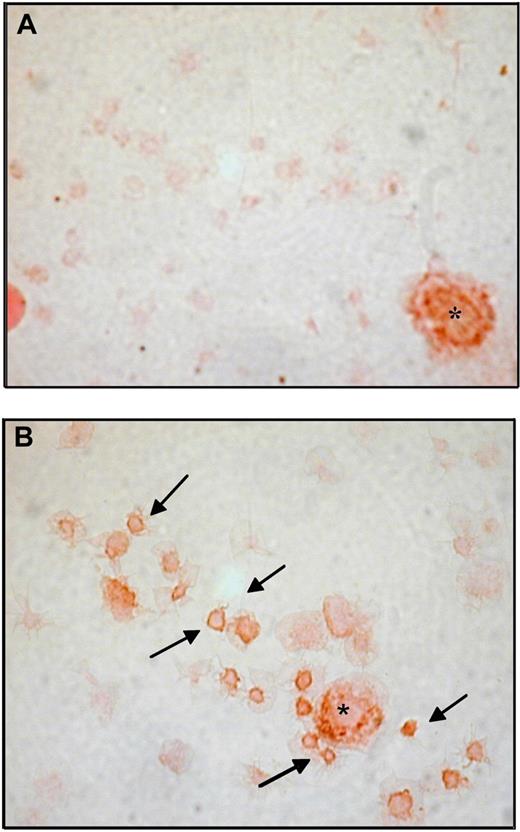
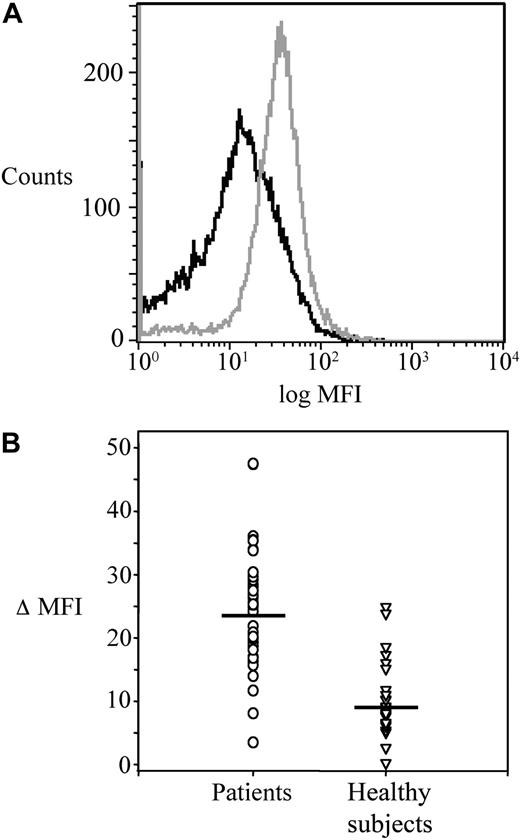
The main clinical and haematologic characteristics of the 41 patients are detailed in Table 1. Platelet COX-1, COX-2, and CD61 expression was studied by flow cytometry in patients before randomization, and in 24 age- and sex-matched healthy subjects. MFI values of platelet COX-2 (gated on the CD61+ population) were significantly (P < .001) increased in patients compared with controls (Figure 1A-B), while the expression of CD61 and COX-1 was comparable in the 2 groups (CD61, 46 [IQR 30-85] vs 64 [IQR 44-95] ΔMFI in patients and controls, respectively, P = .24; COX-1, 25.1 [IQR 17.5-35.7] vs 36.1 [IQR 24.4-46.4] ΔMFI, in patients and controls, respectively, P = .10). Using immunocytochemistry of washed platelets and light microscopy, COX-2 expression in circulating platelets was consistently stronger and detectable in a significantly (P < .001) higher percentage of the whole platelet population in samples from patients (18.8% [IQR 16-29]), compared with controls (6.5% [IQR 4-7]; Figure 2 and data not shown).
Platelet COX-2 expression in ET patients and healthy subjects. (A) Flow cytometric histograms of fluorescence intensity on platelets stained for COX-2 in a patient (gray) and a control subject (black). The plot of the patient is shifted to the right, indicating a higher expression of COX-2. (B) Individual values of ΔMFI (see “Platelet immunophenotyping and reticulated platelets” for details) for COX-2 in platelets from patients (n = 41) and controls (n = 22). Horizontal lines indicate medians.
Platelet COX-2 expression in ET patients and healthy subjects. (A) Flow cytometric histograms of fluorescence intensity on platelets stained for COX-2 in a patient (gray) and a control subject (black). The plot of the patient is shifted to the right, indicating a higher expression of COX-2. (B) Individual values of ΔMFI (see “Platelet immunophenotyping and reticulated platelets” for details) for COX-2 in platelets from patients (n = 41) and controls (n = 22). Horizontal lines indicate medians.
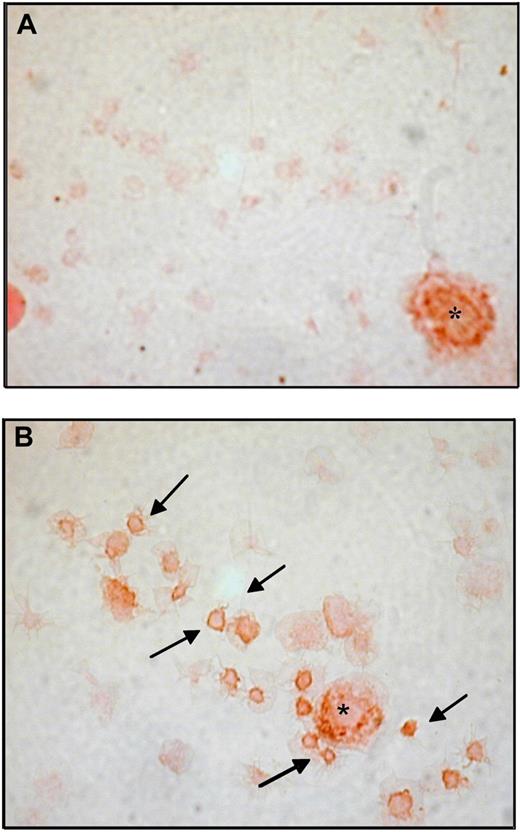
Representative immunohistochemistry for COX-2 in washed platelets from ET patients and healthy subjects. Immunoperoxidase of washed platelets from a control subject (A) and a patient (B), stained for COX-2 revealed with diaminobenzidine chromogen; platelets were lightly counterstained with eosin. Examples of COX-2-positive platelets are indicated by. In each panel a COX-2-positive leukocyte is visible and indicated by the asterisk as intensity staining control. Samples were analyzed by light microscopy using an Axioskop microscope (Zeiss), with an objective lens type Plan-neo Fluar (Zeiss) at a numerical aperture of 1.30, in oil. Original magnification, ×1000. Images were photographed by a CoolPix 950 digital camera (Nikon).
Representative immunohistochemistry for COX-2 in washed platelets from ET patients and healthy subjects. Immunoperoxidase of washed platelets from a control subject (A) and a patient (B), stained for COX-2 revealed with diaminobenzidine chromogen; platelets were lightly counterstained with eosin. Examples of COX-2-positive platelets are indicated by. In each panel a COX-2-positive leukocyte is visible and indicated by the asterisk as intensity staining control. Samples were analyzed by light microscopy using an Axioskop microscope (Zeiss), with an objective lens type Plan-neo Fluar (Zeiss) at a numerical aperture of 1.30, in oil. Original magnification, ×1000. Images were photographed by a CoolPix 950 digital camera (Nikon).
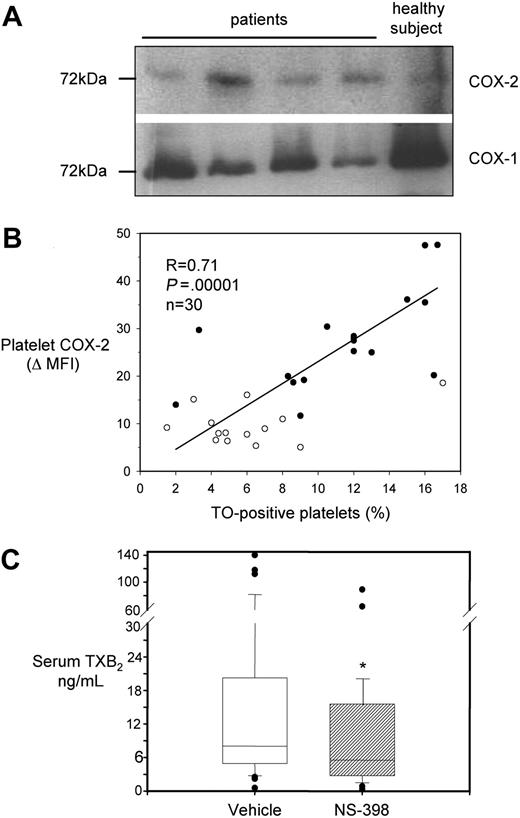
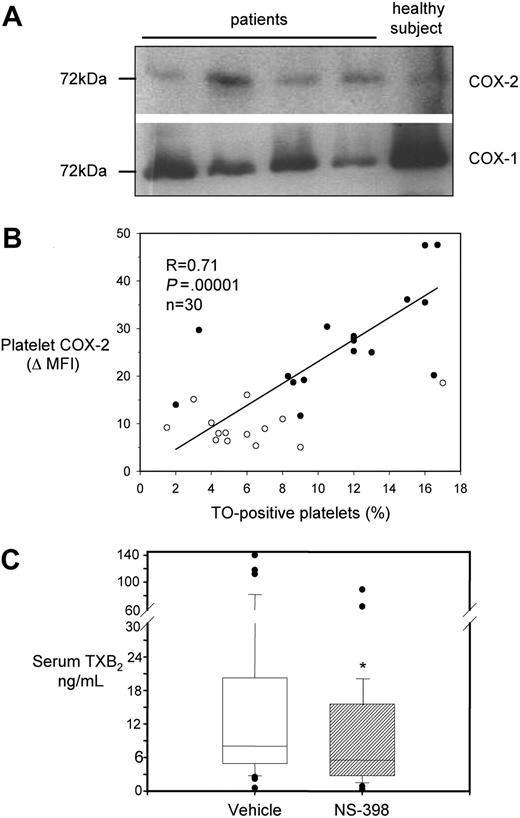
We investigated whether COX-2, detectable in platelets by flow cytometry or immunocytochemistry, had a shift in molecular weight, as previously reported in a different clinical condition, characterized by enhanced platelet COX-2 expression.39 By Western blot analysis, we observed bands of the expected molecular mass (∼ 72 kDa) in platelet extracts from both patients and controls (Figure 3A). Notably, antibodies used for flow cytometry, immunohistochemistry, and Western blottings were from different sources (poly- or monoclonal, commercial or not), and they all detected COX-2 expression in platelets.
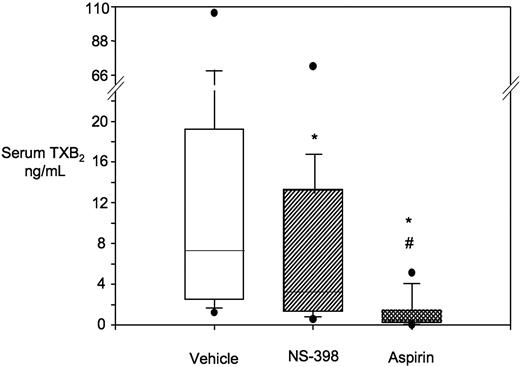
Characterization of COX-2 expression and activity in platelets from ET patients. (A) Western blot analysis of platelet protein extracts for COX-1 and COX-2 in 4 patients and 1 healthy subject. Proteins were extracted from washed platelets and electrophoresed in 10% SDS polyacrylamide gel under reducing conditions. Gels were blotted onto nitrocellulose membranes, which were incubated with monoclonal antibodies against COX-1 or COX-2. Positivity was revealed by anti–mouse horseradish peroxidase–conjugated antibodies and ECL detection reagent. Protein bands were visualized using Kodak Biomax light film. (B) Correlation between COX-2 expression in platelets, expressed as ΔMFI, and the percentage of TO-positive platelets in 16 patients (●) and 14 healthy subjects (○). (C) Box-whisker plots representing whole blood TXB2 production in vitro, as reflected by serum TXB2, in samples from 41 ET patients incubated with vehicle (open box) or NS-398 (striped box) added in vitro, at V0. *P < .001 versus vehicle.
Characterization of COX-2 expression and activity in platelets from ET patients. (A) Western blot analysis of platelet protein extracts for COX-1 and COX-2 in 4 patients and 1 healthy subject. Proteins were extracted from washed platelets and electrophoresed in 10% SDS polyacrylamide gel under reducing conditions. Gels were blotted onto nitrocellulose membranes, which were incubated with monoclonal antibodies against COX-1 or COX-2. Positivity was revealed by anti–mouse horseradish peroxidase–conjugated antibodies and ECL detection reagent. Protein bands were visualized using Kodak Biomax light film. (B) Correlation between COX-2 expression in platelets, expressed as ΔMFI, and the percentage of TO-positive platelets in 16 patients (●) and 14 healthy subjects (○). (C) Box-whisker plots representing whole blood TXB2 production in vitro, as reflected by serum TXB2, in samples from 41 ET patients incubated with vehicle (open box) or NS-398 (striped box) added in vitro, at V0. *P < .001 versus vehicle.
In a subgroup of 16 patients (4 women, 12 men; mean age, 53.6 ± 12 years) and 14 healthy subjects (4 women, 10 men; mean age, 45.4 ± 15 years), we studied both platelet COX-2 expression and the fraction of TO-positive platelets. TO-positive, RNA-containing platelets represent the youngest fraction of peripheral platelets.40 The percentage of the whole platelet population staining for TO was positively correlated with COX-2 expression both in patients (r = 0.62, P = .007), and in the entire population under study (Figure 3B), while in the 14 control subjects only a nonsignificant trend toward a positive correlation was found (r = 0.42, P = .14, data shown in Figure 3B). There was no statistically significant correlation between the TO fraction and platelet COX-1 expression in patients (r = −0.19, P = .5) or in the whole studied subjects (r = -0.25, P = 0.3). Interestingly, neither platelet COX-2 expression nor TO-positive platelet fraction significantly correlated with absolute platelet counts (r = 0.15 for COX-2 ΔMFI and r = −0.08 for the percentage of TO-positive platelets).
We assessed in vitro whether platelets from ET patients had detectable COX-2 enzymatic activity, as measured by whole blood TXB2 production34 in the presence of a selective COX-2 inhibitor, NS-398.24 At V0, serum TXB2 was measured after incubation of 1-mL blood samples with 1μM NS-398 or vehicle (Figure 3C). NS-398 significantly reduced serum TXB2 values by approximately 30% compared with vehicle-treated samples, from 8 [IQR 4.9-20] to 5.6 [IQR 3-14.9] ng/mL.
In vivo and ex vivo TX measurements
Blood and urine samples were taken twice before randomization, at V0 and at V1, to assess the intrasubject variability of TX biosynthesis as well as to maximize adherence to low-dose aspirin intake before randomization.
No statistically significant differences in TXM excretion were found between the 2 repeated measurements 1 week apart: V0, 325 [IQR 243-404.7] pg/mg creatinine; V1, 322 [IQR 233-507] pg/mg creatinine, P = .945. Similarly, serum TXB2 values did not differ between the 2 baseline visits, being 8 [IQR 4.9-20] ng/mL at V0 and 7.5 [IQR 4-11.7] ng/mL at V1 (p = 0.31). Both the rate of TXA2 biosynthesis in vivo, as reflected by urinary TXM excretion, and the maximal biosynthetic capacity of blood platelets, as reflected by serum TXB2, were markedly increased in ET patients compared with values measured with the same methodology in 48 healthy volunteers treated with the same dose of aspirin for 1 to 8 weeks (Figure 4).32
Effects of randomized treatments on TX biosynthesis in vivo and ex vivo in ET patients. (A-B) Box-whisker plots of urinary TXM excretion values in ET patients before (visit 1) and after (visit 2) 1 week of randomized treatment with etoricoxib 90 mg daily added to low-dose aspirin (A) or low-dose aspirin alone (B). (C-D) Serum TXB2 values in patients before (visit 1) and after (visit 2) randomization to etoricoxib addition (C) or low-dose aspirin continuation (D). *P < .05 versus visit 1. The horizontal dotted line in each panel represents the median values previously reported by our group in healthy subjects taking 100 mg of aspirin daily,32 and namely in these subjects serum TXB2 median was 1.18 [IQR 0.52-2.38] ng/mL (n = 120 determinations), while urinary TXM median was 135.5 [IQR 106-170] pg/mg creatinine (n = 120 determinations).
Effects of randomized treatments on TX biosynthesis in vivo and ex vivo in ET patients. (A-B) Box-whisker plots of urinary TXM excretion values in ET patients before (visit 1) and after (visit 2) 1 week of randomized treatment with etoricoxib 90 mg daily added to low-dose aspirin (A) or low-dose aspirin alone (B). (C-D) Serum TXB2 values in patients before (visit 1) and after (visit 2) randomization to etoricoxib addition (C) or low-dose aspirin continuation (D). *P < .05 versus visit 1. The horizontal dotted line in each panel represents the median values previously reported by our group in healthy subjects taking 100 mg of aspirin daily,32 and namely in these subjects serum TXB2 median was 1.18 [IQR 0.52-2.38] ng/mL (n = 120 determinations), while urinary TXM median was 135.5 [IQR 106-170] pg/mg creatinine (n = 120 determinations).
On multiple regression analysis including TXM as the dependent variable and haematologic parameters (leukocytes, hemoglobin, hematocrit, erythrocytes, polymorphonucleates, and platelet counts) as well as serum TXB2 as independent factors, serum TXB2 was the sole predictor of TXM excretion (n = 82, β = 1.48, SE = 0.6, t = 2.13, P = .036). In addition, serum TXB2 was weakly, but significantly correlated with the platelet count (r = 0.31, P = .002).
As expected, cytoreductive treatment with hydroxyurea (HU) was associated with statistically significant reductions in both platelet (HU-treated patients, 381 000 [IQR 329 500-535 000] platelets/μL, n = 38 determinations; non-HU patients, 496 000 [IQR 437 000-864 000] platelets/μL, n = 44 determinations, P = .004) and leukocyte counts (HU-treated patients, 5720 [IQR 5155-7070] leukocytes/μL, n = 38 determinations; non-HU patients, 7520 [IQR 7040-8640] leukocytes/μL, n = 44 determinations, P = .009). HU treatment was also associated with a 20% statistically significant increase in TXM excretion (HU treated, 371 [IQR 267-642] pg/mg creatinine, n = 37 determinations; non-HU treated, 303 [IQR 202-395] pg/mg creatinine, n = 44 determinations, P = .025). The apparent association between HU treatment and increased TXM excretion needs to be interpreted with caution, given the nonrandomized nature of this comparison. At variance with TXM, serum TXB2 values were not significantly different among the 2 groups, being 7 [IQR 3.6-13.8] ng/mL in HU-treated patients and 8 [IQR 4.9-17.7] in the non-HU-treated group (P = .18).
TXM levels were comparable in patients with (n = 12) or without a previous thrombotic event (314 [IQR 240-371] vs 357 [IQR 253-489] pg/mg creatinine, respectively). Serum TXB2 was also similar in the 2 groups (8.11 [IQR 5-15] vs 7.6 [IQR 3.9-17.1] ng/mL, respectively).
Thirty-eight of 41 patients were screened for the JAK2 V617G mutation, and 24 (63%) were found positive. TXM levels both at V0 and V1 were comparable in JAK2-positive versus -negative patients, with values of 304 [IQR 213-379] n = 47 determinations) and 354 [IQR 240-504] n = 28 determinations) pg/mg creatinine, respectively (P = .20). Whole blood TXB2 production was also not affected by the JAK2 mutation.
Effects of selective COX-2 inhibition in vivo
To assess the contribution of COX-2 activity to the biosynthesis of TXA2, ET patients were randomized to continue aspirin or to add etoricoxib, a highly selective COX-2 inhibitor,42 on top of aspirin, for 7 days. Urinary TXM and serum TXB2 were measured before and after a week (V2) of randomized treatment. One dropout occurred at day 3 of etoricoxib intake for causes unrelated to the study drug (flue-like syndrome and need of NSAID therapy). No adverse effects of the randomized treatment were recorded during the study.
Etoricoxib caused a statistically significant 25% reduction in TXA2 biosynthesis in vivo, as reflected by lower TXM excretion at V2 compared with V1 (Figure 4). It also determined a similar reduction in whole blood TXA2 production ex vivo, as reflected by decreased levels of serum TXB2 (Figure 4). This finding is consistent with the results of adding the selective COX-2 inhibitor NS-398 in vitro to whole blood samples from the same patients (Figure 3C).
Patients continuing aspirin alone showed no statistically significant changes between V1 and V2 in either urinary TXM excretion or whole blood TXB2 production (Figure 4).
Altogether, the results of etoricoxib dosing in vivo (Figure 4) and NS-398 in vitro (Figure 3C) showed that selective COX-2 inhibition was unable to completely suppress residual TXA2 biosynthesis in aspirin-treated ET patients.
We next examined whether aspirin-insensitive TXA2 production in ET and its COX-2-dependent component were stable over time. Fourteen of the 41 ET patients, without major clinical complications or changes in therapy during follow-up, were studied again 21 (± 7) months after their first visit. No statistically significant changes were found in serum TXB2 levels over this interval, with a significant correlation between the 2 sets of repeated measurements (r = 0.685, P = .009, vs V1; r = 0.655, P = .015, vs V0). Moreover, consistently with the results of the first in vitro experiment, we found a statistically significant reduction in serum TXB2 concentration by approximately 30%, after the addition of NS-398 to whole blood in vitro (Figure 5).
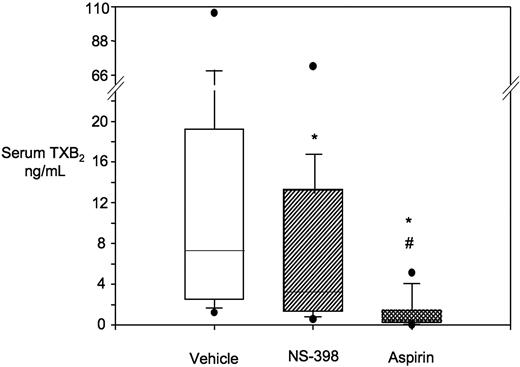
Whole blood TXB2 production and in vitro effect of NS-398 versus aspirin. Box-whisker plots of serum TXB2 values from 14 ET patients after incubation of whole blood samples in vitro with vehicle (n = 14), NS-398 (n = 13), or aspirin (n = 12). *P < .001 versus vehicle; #P < .001 versus NS-398.
Whole blood TXB2 production and in vitro effect of NS-398 versus aspirin. Box-whisker plots of serum TXB2 values from 14 ET patients after incubation of whole blood samples in vitro with vehicle (n = 14), NS-398 (n = 13), or aspirin (n = 12). *P < .001 versus vehicle; #P < .001 versus NS-398.
Effects of aspirin in vitro
In light of the pathogenesis of ET affecting megakaryocytes and the platelet-producing mechanism, we explored whether residual TXA2 production was sensitive to the addition of aspirin in vitro. At variance with NS-398, aspirin (50μM) added to whole blood samples of ET patients was able to completely suppress TXA2 production (Figure 5) to levels similar to those measured in aspirin-treated healthy volunteers, suggesting a major role for unacetylated platelet COX-1 in this phenomenon.
Finally, the absolute reduction in serum TXB2 obtained with aspirin or NS-398 was directly correlated with the basal levels of whole blood TXB2 production (aspirin-treated samples, r = 0.991, n = 12, P < .001; NS-398-treated samples: r = 0.784, P < .001, n = 13).
Discussion
Our study demonstrates that low-dose aspirin, given at the dosing interval uniformly recommended for cardiovascular prevention, is unable to fully inhibit serum TXB2 ex vivo and TXM excretion in vivo in the majority of patients with ET. In fact, the urinary TXM excretion rate while on aspirin was approximately 2.5-fold higher in ET patients compared with the rate measured in healthy subjects. We recently reported a median TXM excretion rate of 135.5 [IQR 106-170] pg/mg creatinine (n = 120 determinations) in 48 aspirin-treated healthy subjects,32 compared with 338 [IQR 247-466.5] pg/mg creatinine (n = 103 determinations) in 41 ET patients under the same antiplatelet regimen in the present study (Figure 4). These levels are also higher than those reported in patients with stable coronary artery disease treated with the same aspirin regimen.41 Serum TXB2 levels in aspirin-treated ET patients were also above the median values of healthy subjects given the same aspirin regimen (shown in Figure 4).32 In fact, only 23 of 104 serum TXB2 measurements in ET patients were below this threshold. Although we could not obtain pre-aspirin measurements, the data of the present study are nevertheless consistent with the less-than-complete inhibition (< 90%) of serum TXB2 that we previously reported in a limited number of untreated ET patients who were given aspirin of 50 or 100 mg,19 at a time when antiplatelet therapy was not uniformly prescribed.
In light of previous evidence of COX-2 expression in mature megakaryocytes from normal24-29 and ET30 bone marrow specimens, and of in vitro data showing that platelet COX-2 might contribute to TXA2 biosynthesis in hematopoietic hyperregenerative conditions (eg, recovery after bone marrow transplantation),24 we sought to explore the contribution of platelet COX-2 to TXA2 biosynthesis in ET. Etoricoxib, a highly selective COX-2 inhibitor,42 was used as a pharmacologic tool to test this hypothesis in vivo, along with experiments adding a selective COX-2 inhibitor, NS-398, in vitro to blood samples from aspirin-treated ET patients. The choice of a 7-day treatment period was dictated by safety considerations as the shortest interval compatible with the achievement of steady-state drug levels.42 The urinary excretion rate of TXM, which is a validated index of whole body TXA2 biosynthesis in vivo,35 as well as the level of serum TXB2, which reflects the maximal biosynthetic capacity of thrombin-stimulated COX activity in platelets,34 were further but incompletely lowered by selective COX-2 inhibition.
To explore whether residual TXA2 production in whole blood was fully suppressible, we added aspirin in vitro at a concentration that fully inhibits both COX-1 and COX-2.43 From a direct comparison of the results obtained with aspirin versus NS-398 in vitro, we can conclude that there is a fraction of enzymatically active COX-1 in platelets isolated from ET patients taking 100 mg of aspirin once daily, and this pool of unacetylated enzyme is normally sensitive to aspirin. We observed a weak positive correlation (r = 0.31) between the platelet count and whole blood TXB2 production. On this basis, we can reasonably assume that the increased platelet mass would not account for incomplete inhibition of COX-1 activity by low-dose aspirin in ET. In fact, the dose of 100 mg represents at least a 3-fold excess over the lowest dose of aspirin necessary and sufficient to fully inactivate platelet COX-144 and protect against thrombosis,45 in healthy subjects and patients with a normal platelet count, that is, 30 mg daily.44-47 Because our ET patients had a 2-fold increase in platelet count at the time of study (Table 1), this is unlikely to require a higher dose of aspirin than 100 mg to produce saturation of its antiplatelet effect. Therefore, an alternative explanation would be required to reconcile our in vitro, ex vivo, and in vivo findings. Based on enhanced thrombopoiesis that characterizes ET, it is conceivable that the duration of platelet COX-1 suppression by low-dose aspirin given once daily is shortened as a consequence of enhanced turnover of the drug target. Because of the short half-life of intact acetylsalicylic acid in the human circulation,46 a 24-hour dosing interval is compatible with full acetylation of platelet COX-1 in subjects with a normal platelet lifespan given daily doses in excess of 30 mg daily,44,46,47 by virtue of the irreversible nature of COX-1 inactivation. Faster platelet regeneration in the majority of ET patients, as suggested by the abnormal serum TXB2 levels in 78% of all samples, accompanied by expression of unacetylated COX-1 and COX-2 in newly formed platelets seem to provide a biologically plausible explanation for aspirin-insensitive TXA2 biosynthesis described in the present study. This hypothesis is also consistent with recent data showing an association between enhanced platelet turnover, expressed as TO-positive platelet fraction, and thrombosis occurrence in ET.48 The extent to which a higher formation of platelet hydroperoxides, such as 12-hydroperoxyeicosatetraenoic acid resulting from persistent platelet activation, may reduce the ability of aspirin to irreversibly inactivate COX-143 is currently unknown and deserves further investigation.
A low-dose aspirin regimen (75-100 mg once daily) is currently recommended for primary and secondary cardiovascular prevention in ET,4-6,8,9,21 in the absence of direct evidence from randomized clinical trials, largely on the basis of the high vascular risk of ET patients and extrapolation of the results of a low-dose aspirin trial in polycythemia vera.22 Our results provide the pharmacologic basis for questioning the adequacy of such an antiplatelet strategy in ET. Additional studies testing the antiplatelet effects of a shorter dosing interval of aspirin administration and the potential additive effects of a TXA2 receptor (TP) antagonist49 are clearly required to define the optimal antiplatelet strategy for ET patients.
Finally, ET might be considered an extreme paradigm of aspirin-insensitive TXA2 biosynthesis due to accelerated renewal of the drug target, and the lessons learned by the study of ET could be applied to other clinical conditions characterized by enhanced platelet turnover, such as type 2 diabetes mellitus and coronary artery bypass grafting, where evidence of aspirin “resistance” has been reported.39,50
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Prof Stefania Basili for statistical advice and to Daniela Basilico for expert editorial assistance. This work is dedicated to the memory of Drs Nicola Maggiano and Jacques Maclouf.
This study was supported by the European Commission FP6 funding (LSHMCT-2004-005033) to C.P., B.R., and G.D.
Authorship
Contribution: A.D. and S.P. recruited patients and performed all visits and follow-up reassessment; A.R., D.M., S.L., G.P., L.M., E.F., G.C., and B.R. performed experiments; A.H. contributed with noncommercial reagents and supervised analytical measurements; F.O.R. analyzed immunohistochemistry and made the figures; G.D. designed the research; and C.P. and B.R. designed the research and wrote the first draft. All authors contributed to the final version of the manuscript.
Conflict-of-interest disclosure: G.D. has received research grant support from Bayer, Sanofi-Aventis, and Servier. C.P. has received research grants from Bayer and Servier and lecture and consulting fees from AstraZeneca, Bayer, Eli-Lilly, Schering-Plough, Sanofi-Aventis, and Servier. B.R. has received honoraria from Bayer and Merck and lecture fees from Nycomed, CSL-Behring, AstraZeneca, and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Bianca Rocca, Department of Pharmacology, Catholic University School of Medicine, Largo F Vito 1, 00168 Rome, Italy; email: b.rocca@tiscali.it.
References
Author notes
A.D. and S.P. contributed equally to this work.

![Figure 4. Effects of randomized treatments on TX biosynthesis in vivo and ex vivo in ET patients. (A-B) Box-whisker plots of urinary TXM excretion values in ET patients before (visit 1) and after (visit 2) 1 week of randomized treatment with etoricoxib 90 mg daily added to low-dose aspirin (A) or low-dose aspirin alone (B). (C-D) Serum TXB2 values in patients before (visit 1) and after (visit 2) randomization to etoricoxib addition (C) or low-dose aspirin continuation (D). *P < .05 versus visit 1. The horizontal dotted line in each panel represents the median values previously reported by our group in healthy subjects taking 100 mg of aspirin daily,32 and namely in these subjects serum TXB2 median was 1.18 [IQR 0.52-2.38] ng/mL (n = 120 determinations), while urinary TXM median was 135.5 [IQR 106-170] pg/mg creatinine (n = 120 determinations).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/5/10.1182_blood-2009-08-236679/4/m_zh89990946600004.jpeg?Expires=1765133697&Signature=aaZcAg8PQvRiUeoun3RmizVO5UBOJbW2T20C709ZBoWsAZOFAIQSTVBKcXmNBSqZ5DZAO74oRJyGTXaaHBWgcVa~3eb~TcOXgpBV8cASv-UX2ElG5tElEYcmML6tRcc1nYBfEN1LiPTum-BpBX1eQIzO1Ykh0-MD1FKT8q8jqb6sd76nCF87pHrMMPDs9aQyKG-eAH4K8WB2T0~OIEmzcpGa1cWcwpE5sbMFASBwQBNkg0uBFfqxRXhRahuYhkFjX-kGA0N750w33ElOxegK4B99dCFEaNzknlEzmkrHmzAVDqlDtmCsH0EBxUnrSAMFtFNpzkmOznj7ooZ2Csqd-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Effects of randomized treatments on TX biosynthesis in vivo and ex vivo in ET patients. (A-B) Box-whisker plots of urinary TXM excretion values in ET patients before (visit 1) and after (visit 2) 1 week of randomized treatment with etoricoxib 90 mg daily added to low-dose aspirin (A) or low-dose aspirin alone (B). (C-D) Serum TXB2 values in patients before (visit 1) and after (visit 2) randomization to etoricoxib addition (C) or low-dose aspirin continuation (D). *P < .05 versus visit 1. The horizontal dotted line in each panel represents the median values previously reported by our group in healthy subjects taking 100 mg of aspirin daily,32 and namely in these subjects serum TXB2 median was 1.18 [IQR 0.52-2.38] ng/mL (n = 120 determinations), while urinary TXM median was 135.5 [IQR 106-170] pg/mg creatinine (n = 120 determinations).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/5/10.1182_blood-2009-08-236679/4/m_zh89990946600004.jpeg?Expires=1765203564&Signature=uF-o28FzJGxucHPhC7zCcgWavqMpSuYntHUzZYnyM2Lsl6oEf-qVp08a0TT9SIKFaCBQKLnKpR5SNdChFjsPRsIUSA3fnMLQ6hnNRucnFSzZxaTFdJYcX-Z32IUCFqDVbDXIlVd73IOck8YQTzJUlzh33tlq3DqPi-XHNqMeQ-BaZnatGzTjY127lMFIG97a6yQSZ286vmBgzqeE8nS3Wu-gpmuo4Ol-Ggvl61kaAEP4fSG3gB8KN7hZG2HCM4h2tHHqaV-~oEtBUGrisuBRIke1FExhKtARYAkK4L8Uh3Y~CiPqIme2fWJpv~bem87GcNA3C3~ZpsYRnxiKttTQLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)