Abstract
In this study, the effect of human erythropoietin Δ (Epo) on smooth muscle cell (SMC)–rich lesions was evaluated. Mice, of which the left carotid artery was ligated, were treated with suberythropoietic as well as erythropoietic doses of Epo and both doses of Epo enhanced SMC-rich lesion formation. No association was observed between hemoglobin levels and lesion size. Moreover, endothelial progenitor cell (EPC) numbers in the peripheral blood increased only in the erythropoietic dosing group, indicating that EPC numbers did not correlate with lesion size. Immunohistochemical analysis revealed that Epo-mediated enhancement of lesion formation correlates with increased signal transducer and activator of transcription 5 (Stat5) phosphorylation in the vessel wall. Experiments performed in cultured vascular cells demonstrated that Epo robustly induced phosphorylation of Stat5 in human umbilical vein endothelial cells (HUVECs), but only very weakly in SMCs. In tumor necrosis factor-α (TNFα)–activated HUVECS, Epo induced expression of platelet-derived growth factor B (PDGF-B), which was at least partially responsible for the induction of Stat5 phosphorylation in SMCs by HUVEC-conditioned medium. In conclusion, in mice Epo accelerates SMC-rich neointima formation, which correlates with increased Stat5 phosphorylation in the vessel wall but is independent of erythrocyte and EPC numbers.
Introduction
Erythropoietin (Epo) is a cytokine that is produced primarily in adult kidney and is essential for the differentiation of red blood cell progenitors into mature red blood cells, which function principally to transport oxygen from the lungs to tissues. Epo expression is induced by anemia or hypoxic stress to increase the number of red blood cells in the circulation. Human recombinant Epo (rHuEpo) became available in the early 1980s and is administered to patients with anemia, in particular patients with chronic renal failure and anemic cancer patients, to increase their hematocrit to near normal levels. In addition to red blood cell progenitors, other cell types express Epo receptors, implicating that other tissues may respond to this circulating factor. For instance, administration of high doses of rHuEpo has been shown to protect tissues such as the myocardium and brain against damage in response to ischemia.1 More recently, it has been shown that Epo treatment increases the number of endothelial progenitor cells in the bone marrow, spleen, and peripheral blood.2 It is well established that endothelial cells express Epo receptors and respond to Epo.3,4 Treatment of endothelial cell cultures with Epo resulted in the activation of intracellular signaling, enhanced proliferation and migration, and inhibition of apoptosis.4-6 In line with these observations, Epo has been shown to promote angiogenesis in vivo.6 Similar to endothelial cells, Epo activates intracellular signaling in vascular smooth muscle cells (SMCs) and stimulates their proliferation.7-10
In the current study, we evaluated the effect of treatment with erythropoietin delta on the formation of SMC-rich neointimal hyperplasia upon ligation of the carotid artery in mice. In this model, the endothelial cell monolayer remains intact and repair of the provoked injury is therefore not dependent on healing of the endothelium involving endothelial progenitor cells. Both suberythropoietic and erythropoietic doses of Epo accelerate neointima formation, independent of hemoglobin levels or the population of endothelial progenitor cells. Epo treatment and enhanced neointima size correlate with increased signal transducer and activator of transcription 5 (Stat5) phosphorylation in the vessel wall. Our experiments performed in cultured vascular cells revealed that Epo-mediated expression of platelet-derived growth factor BB (PDGF-BB) in activated endothelial cells is at least partially responsible for Stat5 phosphorylation in SMCs, which may explain the observed increase in lesion formation.
Methods
Reagents
Erythropoietin Δ (Dynepo) was provided in solution (10 000 IU/mL) by Shire. The PDGFR-specific tyrosine kinase inhibitor CP-673,45111 was obtained as a pure substance from Pfizer and diluted in dimethyl sulfoxide to a stock concentration of 10mM. Stock solutions of PDGF-BB (Peprotech) and vascular endothelial-derived growth factor (VEGF; R&D Systems) were diluted in sterile phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA).
Mice and experimental protocol
Male wild-type FVB mice were obtained from Charles River Laboratories Inc. All mice were maintained on a standard chow diet. The mice were 12 weeks old at the start of the study and weighed approximately 30 g. The mice were divided into 3 different groups that received intraperitoneal injections of solvent, 150 IU/kg Epo or 500 IU/kg Epo 3 times per week. In preliminary experiments, 3 times weekly 150 IU/kg Epo was suberythropoietic, whereas 500 IU/kg significantly stimulated erythropoiesis (data not shown). Treatment with 1000 IU/kg Epo induced the Epo-inhibiting antibodies in some mice, resulting in severe anemia, which was not observed at lower doses. One week after treatment with Epo, ligation of the left carotid artery, near the distal bifurcation, was performed as described previously.12 The mice were killed at 4 weeks after ligation of the carotid artery. All mice were operated on and killed under ketamine/xylazine anesthesia and subsequently fixed by perfusion with 1:5 Shandon Formal-fixx (Thermo Scientific) in PBS. The carotid arteries were excised, immersion-fixed in 1:5 Shandon Formal-fixx in PBS, and embedded in paraffin. Serial sections of 8 μm were cut for morphometric analysis. After sectioning and mounting on Superfrost Plus slides (Menzel-Gläser), the specimens were subjected to hematoxylin-eosin staining or (immuno)histochemical analyses. Animal care and experimental procedures were approved by the University of Amsterdam Ethical Committee on Animal Experiments.
Morphometry
The ligated carotid artery was sectioned from the ligature toward the aortic arch. A standardized reference point was set at which location the ligature did not distort the vessel and where elastic laminae were intact. The reference point is situated between 0.05 mm and 0.13 mm from the ligation. Cross-sections between 0.5 and 0.6 mm from the reference point were stained with Lawson solution (Sigma) and morphometrically analyzed using the QWin software (Leica Microsystems) on digital images of the vessel, obtained with a Leica DFC420 digital video camera. The circumferences of the lumen, internal elastic lamina (IEL), and external elastic lamina (EEL) were measured by tracing along the luminal surface, IEL, and EEL using QWin software. The medial area (M) was calculated by subtracting the area within the IEL from the area defined by the EEL. Similarly, the intimal area (I) was calculated by subtracting the luminal area from the IEL area.
Hematology
Hemoglobin reagent (Hemiglobincyanide; Instruchemie) was used to measure hemoglobin levels in plasma samples at the end of the study. Therefore, 10 μL of full blood was diluted in 2.5 mL of hemoglobin reagent. Subsequently, absorbance at 540 nm was measured in triplicate in 96-well microtiter plates. Standards were included during analysis to determine accurate hemoglobin concentrations.
Flow cytometry
The population of (endothelial) progenitor cells in the blood of mice was analyzed by flow cytometry using FACSCanto (Becton Dickinson Immunocytometry Systems). A total of 700 μL of EDTA (ethylenediaminetetraacetic acid)–blood was used. Erythrocytes were lysed in a solution containing 155mM NH4Cl, 10mM KHCO3, and 1mM EDTA 2 times for 5 minutes on ice. Subsequently, cells were washed in PBS containing 0.5% BSA. Immunostaining for cell surface molecules was performed for 30 minutes at 4°C using directly labeled antibodies against CD45 (CD45-phycoerythrin [PE]; PharMingen), Sca1 (Sca1–PE–cyanin 7), Ter119 (Ter119–PE–cyanin 5.5), and c-Kit (c-Kit–allophycocyanin–Alexa750; eBioscience). All antibodies were used in concentrations recommended by the manufacturer. After staining, cells were fixed in PBS containing 0.5% paraformaldehyde at 4°C overnight.
Immunohistochemistry
Paraffin sections (8 μm) were deparaffinized and rehydrated, and antigen retrieval was performed by boiling for 15 minutes in 1mM EDTA (pH 8). The sections were incubated with 3% hydrogen peroxide and blocked with 5% (vol/vol) preimmune goat serum (DAKO). Subsequently, sections were incubated overnight at 4°C with a primary antibody against phosphorylated Stat5 (catalog no. 9359; Cell Signaling Technology), followed by incubation with biotinylated secondary goat antirabbit antisera, which were detected with streptavidin-horseradish peroxidase conjugates (DAKO) and, subsequently, with amino-ethylcarbazole and hydrogen peroxide. After counterstaining with hematoxylin, sections were embedded in pertex (Histolab).
Cell culture
Human SMCs were explanted from umbilical cord arteries. Cells were cultured in Medium 199 (Invitrogen) with 10% (vol/vol) fetal bovine serum (FBS) with penicillin and streptomycin (Invitrogen). Cells were used at passages 5 to 7 and culture surfaces were gelatin coated. SMCs were characterized with a monoclonal antibody directed against smooth muscle α-actin (1A4; DAKO), and SMCs demonstrated uniform fibrillar staining. Human umbilical vein endothelial cells (HUVECs) were isolated as described (Jaffe, JCI 1973). HUVEC culture medium was composed of Medium 199 (Invitrogen) supplemented with 20% (vol/vol) fetal bovine serum, 50 μg/mL heparin (Sigma), 12.5 μg/mL endothelial cell growth supplement (Sigma), and 100 U/mL penicillin/streptomycin (Invitrogen). HUVECs were used at passages 1 to 3, and culture surfaces were fibronectin-coated.
Western blotting
Cell lysates were prepared in a buffer containing 20mM tris(hydroxymethyl)aminomethane (pH 7.4), 50mM β-glycerophosphate, 50mM KCl, 0.2mM EDTA, 1% (wt/vol) Triton X-100, and 10% (wt/vol) glycerol. A protease inhibitor mixture (Roche), 1mM Na3VO4, and 1mM NaF were freshly added to the lysis solution before each experiment. Protein concentrations were determined according to Bradford, using the Protein Assay Dye Reagent Solution (Bio-Rad) with bovine serum albumin (BSA) as a standard. Whole-cell lysates were denatured in sample buffer containing sodium dodecyl sulfate, and equal amounts of total protein were separated on 8% to 15% sodium dodecyl sulfate–polyacrylamide gels and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk, the membranes were incubated overnight at 4°C with the primary antibodies as indicated. The following antibodies were used: anti–phospho-Stat5 (Tyr694) (C11C5), anti-Stat5, anti–phospho–Janus kinase 2 (JAK2; Tyr1007/1008), anti–phospho-Akt (Ser473), anti–phospho–extracellular signal-related kinase 1/2 (ERK1/2; Thr202/Tyr204), anti–phospho-p38 (all from Cell Signaling Technology), and anti–α-tubulin (Cedarlane). The following day, the membranes were incubated with the appropriate IRDye secondary antibodies (Westburg) and infrared detection was performed with the Odyssey imager (LI-COR Biosciences). Alternatively, membranes were incubated with appropriate horseradish peroxidase conjugates, and detection was carried out using Lumi-light substrate (Roche) and the LAS3000 imaging system (Fujifilm).
RNA extraction, cDNA synthesis, and RT-PCR analysis
RNA was extracted from cells using the Aurum Total RNA Mini Kit (Bio-Rad), after which cDNA was synthesized from 1 μg of total RNA (iScript; Bio-Rad). Semiquantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed using iQ SYBR-Green Super-Mix in the MyiQ RT-PCR system (Bio-Rad) using gene-specific primers. Primer sequences were obtained from literature or designed with Primer 3 software (Howard Hughes Medical Institute, National Institutes of Health, National Human Genome Research Institute, http://frodo.wi.mit.edu/primer3). All RT-PCR data were corrected for housekeeping gene ribosomal protein P0.
Results
Epo accelerates intima formation after carotid-artery ligation in mice
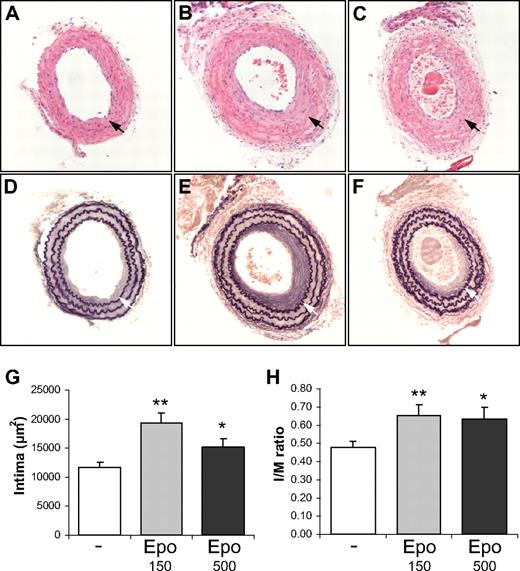
To study the effect of Epo on the formation of SMC-rich lesion formation, we applied the carotid artery ligation model in which the endothelial monolayer remains intact. Consequently, healing of the endothelial cell layer does not determine the extent of lesion formation. Significant neointima formation was observed 4 weeks after ligation in carotid arteries of control and Epo-treated mice (Figure 1A-F). The neointimal area was quantified in ligated carotid arteries and treatment with suberythropoietic (150 IU/kg 3 times per week) and erythropoietic (500 IU/kg 3 times per week) doses of Epo resulted in a significant increase in mean neointimal area by 1.66-fold and 1.30-fold, respectively (Figure 1G). The intima-media (I/M) ratios were calculated for low- and high-dose Epo groups and were 1.38-fold and 1.34-fold higher, respectively, compared with the control group.
Epo accelerates SMC-rich lesion formation in mice. Comparison of the intima formation of ligated carotid arteries of wild-type FVB mice treated with solvent (A,D), 150 IU/kg Dynepo (B,E), or 500 IU/kg Dynepo (C,F). The mean intimal area (F) and intima/media (I/M) ratio (G) reveal significantly enhanced intima formation in Epo-treated groups after 4 weeks of ligation. Photomicrographs of hematoxylin-eosin–stained (A-C) and Lawson solution–stained (D-F) sections: bright-field microscopy, original magnification ×200. Mean and SEM of different treatment groups are shown (G-H). Student t test vs control: *P < .05; **P < .01.
Epo accelerates SMC-rich lesion formation in mice. Comparison of the intima formation of ligated carotid arteries of wild-type FVB mice treated with solvent (A,D), 150 IU/kg Dynepo (B,E), or 500 IU/kg Dynepo (C,F). The mean intimal area (F) and intima/media (I/M) ratio (G) reveal significantly enhanced intima formation in Epo-treated groups after 4 weeks of ligation. Photomicrographs of hematoxylin-eosin–stained (A-C) and Lawson solution–stained (D-F) sections: bright-field microscopy, original magnification ×200. Mean and SEM of different treatment groups are shown (G-H). Student t test vs control: *P < .05; **P < .01.
Epo at 500 IU/kg stimulates hemoglobin levels
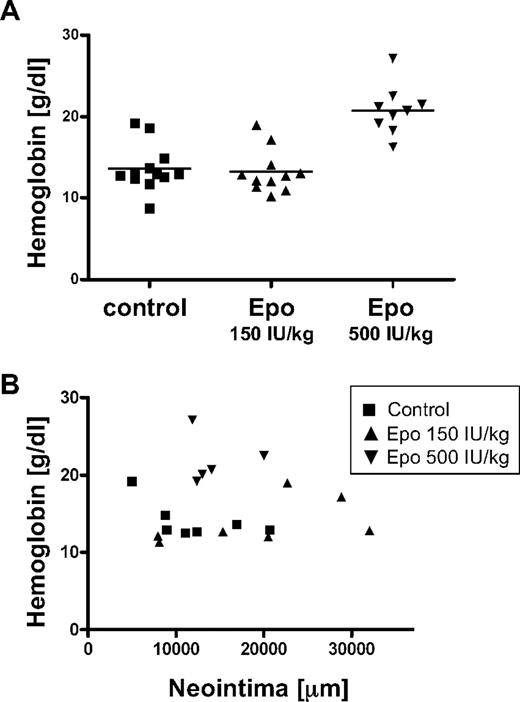
At the end of the study, peripheral blood was collected to evaluate hemoglobin levels. Compared with the control group, the 500 IU/kg Epo group displayed significantly elevated hemoglobin levels, 1.36 plus or minus 0.286 g/L versus 2.08 plus or minus 0.303 g/L (13.6 ± 2.86 g/dL vs 20.8 ± 3.03 g/dL, P < .001), whereas the 150 IU/kg Epo group showed comparable hemoglobin levels as the control group, 1.32 plus or minus 0.266 g/L (13.2 ± 2.66 g/dL; Figure 2A). To evaluate whether hemoglobin levels and intimal area were correlated, we performed regression analysis, but the 2 parameters did not correlate (R = −0.01; Figure 2B).
No correlation between hemoglobin levels and intima size. (A) Hemoglobin levels in the blood of mice were determined at the end of the study. Data of individual mice and means of different treatment groups are indicated. (B) Regression analysis was performed on hemoglobin levels and intimal area.
No correlation between hemoglobin levels and intima size. (A) Hemoglobin levels in the blood of mice were determined at the end of the study. Data of individual mice and means of different treatment groups are indicated. (B) Regression analysis was performed on hemoglobin levels and intimal area.
Erythropoietic dose Epo induces the population of endothelial progenitor cells
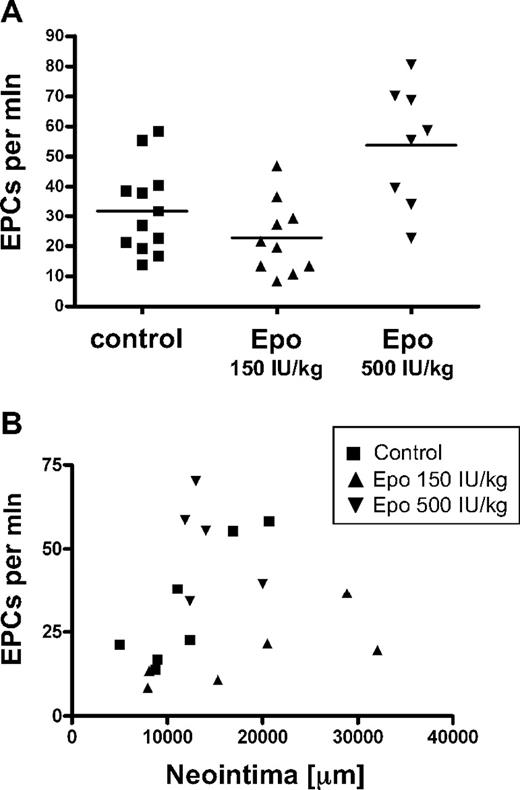
Previously, it has been shown that a single, high dose of Epo provokes release of endothelial progenitor cells in the circulation, affecting lesion formation, when a model is applied in which the endothelial monolayer is disrupted by wire injury.13 To evaluate a potential effect of the Epo doses applied in our study on progenitor cell numbers, we assayed endothelial progenitor cell numbers in the peripheral blood by flow cytometry. We stained for the leukocyte marker CD45, the erythroid marker Ter119, and the stem cell markers c-Kit and Sca1, and quantified the number of endothelial progenitor cells as the number of Ter119−/CD45lo/c-Kit+/Sca1+ cells within the mononuclear cell population. Mice treated with an erythropoietic dose of Epo (500 IU/kg) displayed significantly elevated numbers of progenitor cells compared with the control group (53.8 ± 20.0 and 31.9 ± 14.6 cells/million, respectively; P = .01), whereas mice treated with the suberythropoietic dose of Epo (150 IU/kg) had no increased progenitor cell numbers (22.73 ± 12.4 cells/million; Figure 2A). Clearly, regression analysis revealed that endothelial progenitor cell numbers did not significantly correlate with neointimal area (R = 0.19; Figure 3B).
No correlation between endothelial progenitor cell numbers and intima size. (A) The number of endothelial progenitor cells (EPCs; Ter119−/CD45lo/c-Kit+/Sca1+) in peripheral blood was determined at the end of the study. Data of individual mice and means of different treatment groups are indicated. (B) Regression analysis was performed on endothelial progenitor cell numbers and intimal area.
No correlation between endothelial progenitor cell numbers and intima size. (A) The number of endothelial progenitor cells (EPCs; Ter119−/CD45lo/c-Kit+/Sca1+) in peripheral blood was determined at the end of the study. Data of individual mice and means of different treatment groups are indicated. (B) Regression analysis was performed on endothelial progenitor cell numbers and intimal area.
Epo treatment stimulates Stat5 phosphorylation in the injured vessel wall
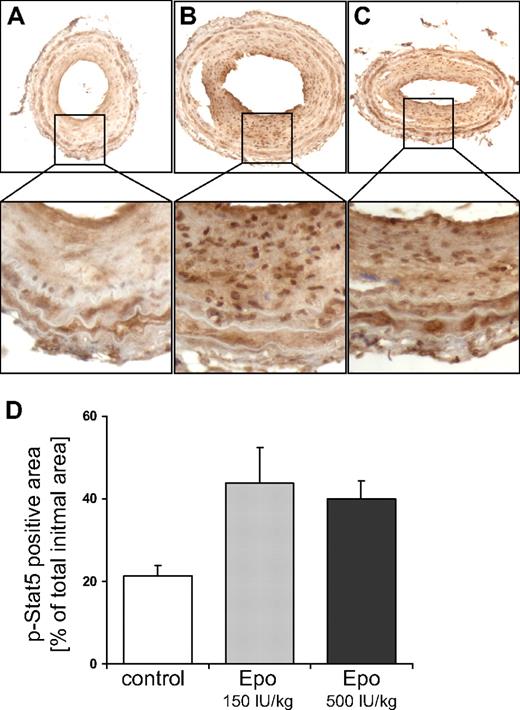
Epo induces dimerization of Epo receptors that are expressed on the cell membrane of target cells. Receptor dimerization results in activation of JAK2, a kinase that is intrinsically bound to the intracellular domain of the Epo receptor. In turn, JAK2 activates downstream signaling molecules, in particular Stat5,14 which stimulates cell growth and motility of SMCs.15 We investigated whether systemic Epo treatment induces the activation of Stat5 in the ligated carotid artery. Therefore, we performed immunohistochemical staining using an antibody that specifically recognizes the phosphorylated, activated form of Stat5, which is localized predominantly in the nucleus. Compared with the control group, Stat5 phosphorylation was significantly increased in the SMC-rich lesions of mice treated with both doses of Epo (Figure 4A-D).
Epo stimulates Stat5 phosphorylation in the injured vessel wall. Stat5 phosphorylation was investigated by immunohistochemical staining of sections from ligated carotid arteries of mice treated with solvent (A), 150 IU/kg Dynepo (B), or 500 IU/kg Dynepo (C). Representative pictures are shown, and were captured with bright-field microscopy at an original magnification of ×200. (D) The percentage of total intima surface that stained positive for phosphorylated Stat5. Mean and SEM of 4 sections per treatment group are shown. Student t test versus control: **P < .01.
Epo stimulates Stat5 phosphorylation in the injured vessel wall. Stat5 phosphorylation was investigated by immunohistochemical staining of sections from ligated carotid arteries of mice treated with solvent (A), 150 IU/kg Dynepo (B), or 500 IU/kg Dynepo (C). Representative pictures are shown, and were captured with bright-field microscopy at an original magnification of ×200. (D) The percentage of total intima surface that stained positive for phosphorylated Stat5. Mean and SEM of 4 sections per treatment group are shown. Student t test versus control: **P < .01.
Expression of receptors for Epo in cultured human endothelial cells and SMCs
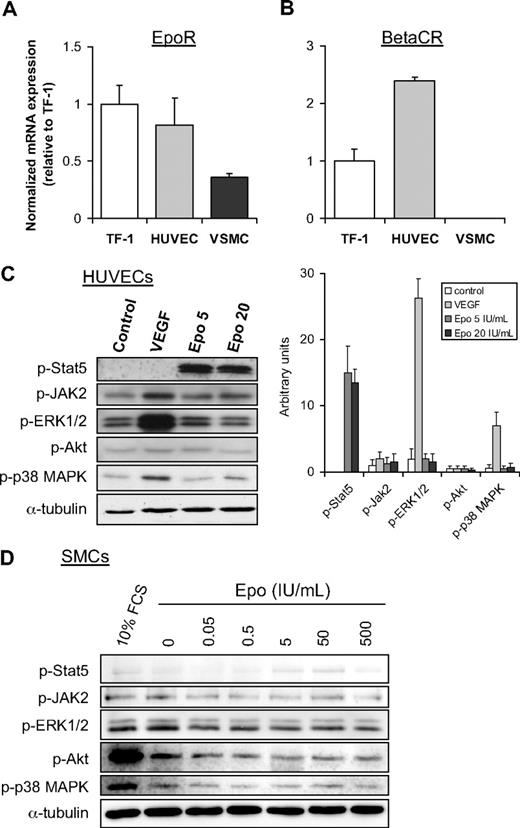
To investigate the functionality of Epo signaling in cultured human endothelial cells (HUVECs) and human SMCs, we first assessed the mRNA expression levels of receptors for Epo, the Epo receptor (EpoR), and the common β receptor (βcR), by semiquantitative RT-PCR. EpoR expression was detected in both cell types, although HUVECs showed approximately 2-fold higher expression of EpoR compared with SMCs. HUVECs, however, expressed only slightly lower levels of EpoR mRNA than the Epo-responsive erythroleukemia TF-1 cell line, which has high expression levels of EpoR.16 In comparison with TF-1 cells, relatively high βcR expression was found in HUVECs, whereas SMCs do not express the βcR (Figure 5B).
Epo signaling is functional in endothelial cells but not in SMCs. Analysis of mRNA expression of EpoR (A) and βCR (B) in TF-1 cells, HUVECS, and SMCs was performed by semiquantitative RT-PCR. Normalized expression levels of TF-1 cells were set to 1. Mean and SD of 2 independent experiments are shown. (C) Effect of Epo and VEGF on phosphorylation of Stat5, JAK2, and ERK1/2, Akt, and p38 MAPK in HUVECs (C) and SMCs (D) was analyzed by Western blotting with phospho-specific antibodies. Serum-starved cells were stimulated for 15 minutes with VEGF (C), different doses of Epo (C-D), or 10% fetal calf serum (D). As a control for equal protein loading, membranes were reprobed with antibodies against α-tubulin. The intensity of the bands in panel C was quantitatively analyzed as indicated in the right panel (expressed in arbitrary units).
Epo signaling is functional in endothelial cells but not in SMCs. Analysis of mRNA expression of EpoR (A) and βCR (B) in TF-1 cells, HUVECS, and SMCs was performed by semiquantitative RT-PCR. Normalized expression levels of TF-1 cells were set to 1. Mean and SD of 2 independent experiments are shown. (C) Effect of Epo and VEGF on phosphorylation of Stat5, JAK2, and ERK1/2, Akt, and p38 MAPK in HUVECs (C) and SMCs (D) was analyzed by Western blotting with phospho-specific antibodies. Serum-starved cells were stimulated for 15 minutes with VEGF (C), different doses of Epo (C-D), or 10% fetal calf serum (D). As a control for equal protein loading, membranes were reprobed with antibodies against α-tubulin. The intensity of the bands in panel C was quantitatively analyzed as indicated in the right panel (expressed in arbitrary units).
Epo induces Stat5 phosphorylation in HUVECs, but not in cultured SMCs
Next, we investigated whether Epo activates intracellular signaling in HUVECs and SMCs. As expected, Epo induces phosphorylation of several signal transduction molecules, including JAK2, Stat5, Akt, and Erk in the control TF-1 cell line (data not shown). Similarly, phosphorylation of Stat5 is strongly induced by Epo in HUVECs. In contrast, JAK2 and p38-mitogen-activated protein kinase (MAPK) phosphorylation were only marginally induced, whereas phosphorylated Erk and Akt remain largely unaffected in HUVECs upon stimulation with Epo (Figure 5C). In contrast to endothelial cells, Stat5 phosphorylation is only marginally induced by Epo in SMCs (Figure 5D). Moreover, Epo does not stimulate phosphorylation of JAK2, Erk, Akt, or p38-MAPK in these cells, indicating that human SMCs are largely unresponsive to Epo. Similarly, Epo did not induce activation of these signal transduction molecules in murine SMCs, which were explanted from aorta (data not shown).
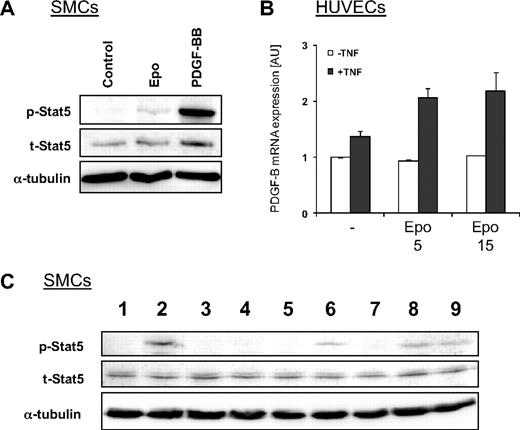
PDGF-BB strongly stimulates Stat5 phosphorylation in SMCs and is expressed by endothelial cells under the influence of Epo and TNFα
Based on the data we obtained from the signaling experiments with cultured cells, we concluded that the strong Stat5 phosphorylation observed in SMCs in the vessel wall of Epo-treated mice is not directly induced by Epo. We therefore hypothesized that endothelial cells stimulated by Epo secrete growth factors or cytokines, which are responsible for the induction of Stat5 phosphorylation in SMCs. As previously reported,15 we found that stimulation of SMCs with PDGF-BB significantly induces phosphorylation of Stat5 (Figure 6A). Consequently, Epo-mediated expression of PDGF-B by endothelial cells may explain the induction of phosphorylated Stat5 in SMCs in vivo. However, stimulation of cultured endothelial cells with Epo alone did not affect mRNA expression levels of PDGF-B (Figure 6B white bars). Vascular lesion formation, however, is an inflammation-driven process and in models of stenosis tumor necrosis factor-α (TNFα) is highly up-regulated.17 Hence, we anticipated that endothelial cells in vivo are exposed to Epo and inflammatory cytokines such as TNFα. In line with our hypothesis, cotreatment of cultured endothelial cells with TNFα and Epo results in a significant induction of PDGF-B expression (Figure 6B). These data indicate that PDGF-B is secreted by endothelial cells in response to Epo under inflammatory conditions, which may subsequently induce Stat5 phosphorylation in SMCs.
Epo induces expression of PDGF-BB in activated endothelial cells, which is partially responsible for Stat5 phosphorylation in SMCs. (A) Effect of PDGF-BB on phosphorylation of Stat5 in SMCs. Serum-starved cells were stimulated for 15 minutes with Epo or PDGF-BB and Stat5 phosphorylation was investigated by Western blotting with phospho-specific antibodies. Total levels of Stat5 and α-tubulin remained unchanged. (B) PDGF-B mRNA expression was induced by Epo in TNFα-activated HUVECs. HUVECS were treated for 4 hours with Epo or TNFα (25 ng/mL) and mRNA expression was investigated with quantitative RT-PCR. (C) Serum-starved SMCs were untreated (lane 1), stimulated with PDGF-BB (lane 2), or treated with the PDGFR-specific tyrosine kinase inhibitor CP-673,451 before stimulation with PDGF-BB (lane 3). SMCs were stimulated for 15 minutes with medium conditioned for 24 hours by HUVECs that were untreated (lanes 4-5) or treated with Epo (lane 6), TNFα (lane 7), or Epo and TNFα (lanes 8-9). SMCs were pretreated with the PDGFR-specific tyrosine kinase inhibitor CP-673,451 before stimulation with conditioned medium from HUVECs that were untreated (lane 5) or treated with Epo and TNFα (lane 9). Phosphorylated and total Stat5 was analyzed by Western blotting. Membranes were reprobed with antibodies against α-tubulin to check equal protein loading.
Epo induces expression of PDGF-BB in activated endothelial cells, which is partially responsible for Stat5 phosphorylation in SMCs. (A) Effect of PDGF-BB on phosphorylation of Stat5 in SMCs. Serum-starved cells were stimulated for 15 minutes with Epo or PDGF-BB and Stat5 phosphorylation was investigated by Western blotting with phospho-specific antibodies. Total levels of Stat5 and α-tubulin remained unchanged. (B) PDGF-B mRNA expression was induced by Epo in TNFα-activated HUVECs. HUVECS were treated for 4 hours with Epo or TNFα (25 ng/mL) and mRNA expression was investigated with quantitative RT-PCR. (C) Serum-starved SMCs were untreated (lane 1), stimulated with PDGF-BB (lane 2), or treated with the PDGFR-specific tyrosine kinase inhibitor CP-673,451 before stimulation with PDGF-BB (lane 3). SMCs were stimulated for 15 minutes with medium conditioned for 24 hours by HUVECs that were untreated (lanes 4-5) or treated with Epo (lane 6), TNFα (lane 7), or Epo and TNFα (lanes 8-9). SMCs were pretreated with the PDGFR-specific tyrosine kinase inhibitor CP-673,451 before stimulation with conditioned medium from HUVECs that were untreated (lane 5) or treated with Epo and TNFα (lane 9). Phosphorylated and total Stat5 was analyzed by Western blotting. Membranes were reprobed with antibodies against α-tubulin to check equal protein loading.
Medium conditioned by activated endothelial cells activates PDGF-dependent Stat5 phosphorylation
To investigate whether activation of Stat5 in SMCs is mediated by endothelial cells, conditioned medium from HUVECs was used to stimulate cultured SMCs. In line with our hypothesis, conditioned medium from HUVECs treated with Epo and TNFα induced strong phosphorylation of Stat5 in SMCs (Figure 6C lane 8). Conditioned medium from untreated HUVECs or from cells treated with TNFα alone did not induce Stat5 phosphorylation in SMCs (Figure 6C lanes 4 and 7). In contrast, conditioned medium from Epo-treated HUVECs resulted in a small induction of phosphorylated Stat5 (Figure 6C lane 6), comparable with levels observed in SMCs treated with Epo alone (Figure 5C), suggesting that Epo that was still present in the conditioned medium was responsible for this effect. To determine whether the effect of conditioned medium from HUVECs treated with Epo and TNFα depended on PDGF signaling, SMCs were pretreated with the specific PDGFR inhibitor CP-673,451. As expected, treatment with CP-673,451 completely blocked PDGF-BB–induced Stat5 phosphorylation (Figure 6C compare lanes 2 and 3). Phosphorylation of Stat5 induced by conditioned medium from HUVECs treated with Epo and TNFα was partially inhibited with the PDGFR inhibitor (Figure 6C compare lanes 8 and 9), indicating that Stat5 phosphorylation was at least partially mediated through PDGF signaling.
Discussion
In the present study, the effect of Epo on SMC-rich lesions was evaluated. Mice, whose carotid arteries were ligated, were treated with a suberythropoietic dose of Epo and an erythropoietic Epo dose that significantly increased hemoglobin levels in the blood. Treatment with both doses of Epo enhanced SMC-rich lesion formation in ligated carotid arteries. Thus, no association was observed between hemoglobin levels and neointima size. Similar to our data, Epo induced excessive SMC-rich lesion formation in a study using a carotid artery injury model in rats.18
Conversely, a previous report showed that Epo inhibited neointimal hyperplasia after wire injury of the femoral artery of mice.13 In this study, Urao et al demonstrated that Epo mobilized EPCs, which were involved in repair of the injured vessel. In the carotid artery ligation model that was used in the present study, however, the endothelial cell monolayer remains intact and is therefore not dependent on vascular healing, which involves EPCs. In line with this, we demonstrated that EPC numbers after long-term Epo treatment did not correlate with lesion size. Nevertheless, EPC numbers were stably increased after 5 weeks in mice treated with an erythropoietic dose of Epo (500 IU/kg), but not in mice treated with a suberythropoietic Epo dose (150 IU/kg). Heeschen et al have shown that EPC numbers in the peripheral blood were modestly increased in animals treated with a comparable low Epo dose (100 IU/kg).2 In that study, however, EPC numbers were determined after 3 days of Epo treatment, whereas we determined the EPC population after 5 weeks of treatment. Epo-mediated mobilization of EPCs might be a transient process, which may explain the observation that EPC numbers were not elevated in comparison with controls after 5 weeks in mice treated with 150 IU/kg Epo.
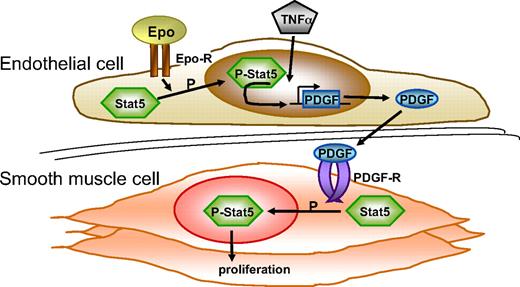
An important finding in the current study is that Epo-mediated enhancement of lesion formation correlates with increased Stat5 phosphorylation in the vessel wall. Activation of Stat5 has been demonstrated to regulate proliferation and motility of cells, including SMCs.14,15 Moreover, it has been reported that suppression of Stat5 signaling in the vessel wall reduces balloon injury–induced lesion formation in rats.15 Our experiments with cultured vascular cells revealed that Epo induces Stat5 activation in endothelial cells but not in SMCs. To understand how Stat5 becomes phosphorylated in lesion SMCs in Epo-treated mice, we rationalized that PDGF-BB is known as a factor that both induces Stat5 phosphorylation in SMCs and is synthesized by endothelial cells. We subsequently demonstrated that Epo indeed induces PDGF-BB synthesis in activated endothelial cells and that the conditioned medium of these endothelial cells provokes Stat5 phosphorylation in SMCs. Specificity of this signal is substantiated by the observation that inhibition of PDGF-receptor signaling reduces Stat5 activation in SMCs. It is important to emphasize that in our in vitro experiments Epo induces excessive PDGF-BB synthesis only in the presence of TNFα. This may imply that Epo has an adverse effect on endothelial cells in vivo only under inflammatory conditions (see Figure 7 for a schematic representation).
Epo activates the vessel wall. Schematic representation of effects of Epo on the vessel wall. Epo induces phosphorylation of Stat5 in endothelial cells (Figure 5C). Endothelial cells that were activated by TNFα and Epo express enhanced levels of PDGF-BB (Figure 6B). PDGF-BB expressed by endothelial cells subsequently stimulates phosphorylation of Stat5 in SMCs (Figure 6C) and induces SMC proliferation.15 See “Discussion” for further details. Epo-R indicates erythropoietin receptor; and PDGF-R, PDGF receptor.
Epo activates the vessel wall. Schematic representation of effects of Epo on the vessel wall. Epo induces phosphorylation of Stat5 in endothelial cells (Figure 5C). Endothelial cells that were activated by TNFα and Epo express enhanced levels of PDGF-BB (Figure 6B). PDGF-BB expressed by endothelial cells subsequently stimulates phosphorylation of Stat5 in SMCs (Figure 6C) and induces SMC proliferation.15 See “Discussion” for further details. Epo-R indicates erythropoietin receptor; and PDGF-R, PDGF receptor.
In line with published data,5 we demonstrated that treatment of endothelial cell cultures with Epo resulted in the activation of intracellular signaling, in particular the JAK2-Stat5 signaling pathway. Unlike experiments performed with rat SMCs,7,8 we demonstrated that Epo did not activate MAPK or phosphoinositide-3 kinase signaling in human SMCs, but weakly induced Stat5 phosphorylation. These dissimilarities may reflect the different species from which the cells were originated, although we obtained similar results with murine SMCs (data not shown).
The difference between endothelial cells and SMCs in responsiveness of Stat5 phosphorylation upon Epo stimulation is striking, because both cell types express Epo receptors. In addition to the induction of a homodimeric complex of 2 EpoRs, however, Epo can induce a heteromeric complex consisting of EpoR and βcR, a coreceptor shared by the granulocyte-macrophage colony-stimulating factor, and the interleukin-3 (IL-3) and IL-5 receptors.19 Of interest, expression of the βcR was detected in HUVECs but not in SMCs, suggesting that the βcR may play an important role in Epo-mediated Stat5 phosphorylation. In support of a key role of the βcR in cytokine-induced Stat5 activation, it was reported that the βcR specifically recruited Stat5 upon IL-3 stimulation of adherent endothelial cells.20
The results of the presented study prompt caution with the use of Epo in certain clinical scenarios. Our observation that Epo induced PDGF expression in cultured endothelial cells only under inflammatory conditions indicates that Epo stimulates the development of SMC-rich lesions in which inflammation is ongoing, rather than the involvement of Epo in the initiation of atherosclerosis. This suggests that patients with existing lesions and who are at risk to develop SMC-rich lesions, such as patients who have received a stent, may have an elevated risk to develop hyperplasia upon Epo treatment; however, clinical studies are warranted to evaluate this potential risk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mariska Vos from the Academic Medical Center for excellent technical assistance.
This work was financially supported by Shire.
Authorship
Contribution: M.L.J., J.L.T.H., A.M.d.B., and A.K. performed experiments; M.L.J., V.d.W., and C.J.M.d.V. designed the research; and M.L.J. and C.J.M.d.V. wrote the paper.
Conflict-of-interest disclosure: M.L.J., J.L.T.H., and C.J.M.d.V. were financially supported by Shire for performing this research. The remaining authors declare no competing financial interests.
Correspondence: Carlie J. M. de Vries, Department of Medical Biochemistry K1-113, Academic Medical Center, Meibergdreef 15, 1105 AZ, Amsterdam, The Netherlands; e-mail c.j.devries@amc.uva.nl.