Abstract
The cellular and molecular mechanisms orchestrating the complex process by which bone marrow megakaryocytes form and release platelets remain poorly understood. Mature megakaryocytes generate long cytoplasmic extensions, proplatelets, which have the capacity to generate platelets. Although microtubules are the main structural component of proplatelets and microtubule sliding is known to drive proplatelet elongation, the role of actin dynamics in the process of platelet formation has remained elusive. Here, we tailored a mouse model lacking all ADF/n-cofilin–mediated actin dynamics in megakaryocytes to specifically elucidate the role of actin filament turnover in platelet formation. We demonstrate, for the first time, that in vivo actin filament turnover plays a critical role in the late stages of platelet formation from megakaryocytes and the proper sizing of platelets in the periphery. Our results provide the genetic proof that platelet production from megakaryocytes strictly requires dynamic changes in the actin cytoskeleton.
Introduction
The formation of anucleated blood platelets from their bone marrow resident precursors, the megakaryocytes (MKs), is a unique process in mammalian physiology.1 Terminally differentiated MKs, the largest cells evolving from hematopoietic stem cells, are polyploid and contain a highly organized cytoplasm with a membranous network, the demarcation membrane system (DMS), which converts into cytoplasmic protrusions during proplatelet formation.1,2 According to the current model, the tips of these proplatelet protrusions extend into the lumen of a vessel in bone marrow sinusoids and are shed by shear forces generated by the blood flow. Recently, the first direct visualization of this process in situ in the bone marrow of mice using 2-photon microscopy revealed that the released fragments are larger in size than normal platelets. In addition, beaded proplatelets and barbell-shaped platelet pairs were observed in the blood, leading to the hypothesis that final shaping and sizing of platelets may occur in the circulation.3 In line with this, very recent observations indicate that platelet progeny formation occurs in whole blood, suggesting that thrombopoiesis continues in the bloodstream.4
Proplatelet formation relies on coordinated cytoskeletal rearrangements with an essential involvement of tubulin-dependent processes.5 The extension of cytoplasmic protrusions from MKs is mechanically driven by tubulin sliding and assembly. In contrast, the role of actin, the other major cytoskeletal component in platelets, has remained elusive in platelet biogenesis from MKs. In mature platelets, the importance of actin filament dynamics, mainly the uncapping and elongation of actin filaments, for platelet activation and shape changes, has been well documented.6 A mouse model for the filamentous actin (F-actin) severing protein gelsolin provided in vivo evidence for a physiologic role of actin dynamics in platelet activation.7 However, surprisingly, gelsolin-driven actin filament turnover was found not to be required for platelet formation itself, although extension of proplatelet processes and proplatelet shaft bifurcation are clearly actin dependent.5 To date, the molecular basis and the key regulators of actin dynamics during platelet formation and maturation have not been identified.
Actin filament disassembly has been recognized to be essential for a wide variety of cellular processes, including cytokinesis, cell polarization, and migration.8-10 Among the known F-actin severing proteins, the members of the actin-depolymerizing factor (ADF)/cofilin family play a pivotal role by spatially and temporally regulating actin turnover in all species.11 In mammals, 3 highly homologous isoforms of the ADF/cofilin family exist that have arisen from 2 sequential gene duplications, namely, ADF, cofilin-1 (nonmuscle cofilin [n-cofilin]), and cofilin-2 (muscle cofilin [m-cofilin]). ADF is expressed in epithelial cells, n-cofilin can be found ubiquitously expressed in most tissues, and m-cofilin is restricted to muscle cells.12,13 The activity of ADF and cofilin is tightly regulated via (de)phosphorylation of a regulatory serine residue.14 On dephosphorylation, ADF/cofilin associate preferentially in a cooperative manner with ADP-bound F-actin.15,16 This binding induces a twist in the actin filament and enhances actin turnover by filament severing17,18 and by dissociation of actin monomers from the minus (pointed) end.15,16,18,19
Studies in different model organisms revealed an essential role for ADF/cofilin during development. Deficiency of the single isoform of ADF/cofilin in yeast or Drosophila (twinstar) resulted in lethality,20-22 and in Caenorhabditis elegans the single ADF/cofilin gene UNC-60 is essential for muscle assembly.23,24 In mice, n-cofilin deficiency leads to defects in neural crest cell migration, neural tube closure, and early embryonic lethality.13 Conditional gene targeting in mice revealed an important role of n-cofilin for cell-cycle control and neuronal migration in the cerebral cortex.25 Interestingly, mice lacking ADF are viable and display no obvious developmental defects,25 apart from an increased proliferation of epithelial cells in the cornea and consequently a pathologic thickening of the cornea, leading to blindness.26
The role of ADF/cofilin in platelet physiology and particularly in platelet biogenesis is not known. Here, we took advantage of a genetic approach to investigate the role of ADF- and n-cofilin–driven actin turnover in MK maturation and platelet production. Furthermore, we were able to dissect the specific functions that can be attributed to either ADF or n-cofilin in platelet morphology and function. Our results show, for the first time, that in vivo actin filament turnover is a critical step in the terminal phase of platelet formation, as well as in the maturation and sizing of early platelets to produce the homogeneous mature platelet population.
Methods
Mice
Animal studies were approved by the local authorities (Bezirksregierung Unterfranken). Adf−/− and n-cofilinfl/fl mice were generated as described previously.13,25 N-cofilinfl/fl mice were crossed with mice carrying the Cre recombinase under the platelet factor 4 (PF4) promoter.27 In the manuscript, n-cofilinfl/fl, PF4-Cre and Adf−/−/n-cofilinfl/fl, PF4-Cre mice are referred to as n-cofilin–null and ADF/n-cofilin–null mice, respectively. N-cofilinfl/fl mice were used as control.
Reagents
Thrombin (Boehringer), enhanced chemiluminescence solution (GE Healthcare), thrombopoietin (BioSource), phalloidin–fluorescein isothiocyanate (FITC), human fibrinogen, Ficoll (Sigma-Aldrich), glutaraldehyde, cacodylate (AppliChem), fetal calf serum (Perbio), Iscove Modified Dulbecco medium, and penicillin/streptomycin (Invitrogen) were used. Anti–α-tubulin (clone DM1A) and 4,6-diamidino-2-phenylindole were purchased from Sigma and phalloidin from Invitrogen.
Western blotting
Proteins of lysed platelets were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes. After blocking, the membrane was incubated with an anti-ADF or anti–n-cofilin antibody overnight at 4°C.13,25 Goat anti–rabbit IgG horseradish peroxidase (HRP; 1 hour at room temperature, Dako Germany) and enhanced chemiluminescence were used for visualization.
Flow cytometry
To measure platelet size and counts, heparinized blood was diluted 1:20 and incubated with the appropriate fluorophore-labeled antibody for 15 minutes at room temperature and subsequently analyzed on a FACSCalibur (BD Biosciences).
Platelet spreading
Thrombin (0.001 U/mL) activated washed platelets (30 000/μL) were immediately allowed to spread on a fibrinogen-coated (1 mg/mL) rectangular coverslip. Bound platelets were fixed with 4% paraformaldehyde at the indicated time points and counted. Platelet spreading was monitored by taking pictures every 5 seconds for 20 minutes. For analysis with stimulated emission depletion microscopy (STED) microscopy, spread platelets on fibrinogen were stained with phalloidin-Atto 647N (Sigma-Aldrich) and observed using a Leica SP5 microscope.
Actin polymerization
Washed platelets were incubated with a Dylight-649–labeled anti-GPIX antibody derivative. Subsequently, platelets were either left unstimulated or were stimulated with 1 U/mL thrombin for 2 minutes. The platelets were fixed with 0.55 volume of 10% paraformaldehyde and treated with 0.1 volume 1% Triton X-100. After this, the platelets were stained with 10μM phalloidin-FITC for 30 minutes and analyzed on a FACSCalibur.
TEM of platelets
For transmission electron microscopy (TEM), washed platelets were fixed with 2.5% glutaraldehyde in 0.1M cacodylate buffer (pH 7.2) containing 2% sucrose and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and examined under a CM120 transmission electron microscope (FEI).
Replica of intact resting cytoskeleton of platelets
To visualize the intracellular cytoskeletal filaments of resting platelets, platelets were placed in a solution of 0.75% Triton X-100 in piperazine-N,N-bis-2-ethanesulfonic acid, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, ethyleneglycoltetraacetic acid, and MgCl2 (PHEM) containing 0.1% glutaraldehyde, 5μM phalloidin, and 30μM taxol, and attached to the surface of poly-L-lysine–coated coverslips by centrifugation at 280g for 5 minutes. The cytoskeleton was rinsed in PHEM solution lacking Triton X-100 and fixed for 10 minutes in 1% glutaraldehyde in PHEM. Coverslips were extensively washed in distilled water, rapidly frozen in a liquid helium-cooled copper block, transferred to a liquid nitrogen-cooled stage, freeze-dried at −90°C, and metal cast with 1.2 nm of tantalum-tungsten with rotation at 45° and 3 nm of carbon at 90° without rotation. Replicas were floated, picked up on formvar-carbon-coated grids, and examined in a JEOL 1200-EX transmission electron microscope at 80 kV.
SEM of the cytoskeleton of spread platelets
To visualize the intracellular cytoskeletal filaments of spread platelets on fibrinogen, platelets were incubated for 2 minutes with 0.75% Triton X-100 in PHEM containing 0.1% glutaraldehyde, 5μM phalloidin, and 30μM taxol. After rinsing, the cytoskeleton was fixed overnight in 2% paraformaldehyde.
The samples were treated with 0.2% tannic acid for 20 minutes followed by 0.5% osmium for 5 minutes and prepared for scanning electron microscopy (SEM) as follows. The samples were dehydrated in graded ethanol solutions, air-dried with hexamethyldisilazane, sputtered with gold, and examined under a scanning electron microscope (Sirion, FEI).
TEM of bone marrow megakaryocytes
For TEM, the femura of mice were cut in pieces using scissors and then fixed overnight at 4°C with 0.1M sodium cacodylate (pH 7.2) containing 2.5% glutaraldehyde and 2% formaldehyde. The bone was removed with forceps. The remaining bone marrow was washed with 50mM sodium cacodylate (pH 7.2) and subsequently fixed for 2 hours at 4°C with 2% osmium tetroxide in 50mM sodium cacodylate (pH 7.2). Samples were washed with distilled water and stained overnight with 0.5% aqueous uranyl acetate, dehydrated with ethanol, and embedded in Epon 812. Ultrathin sections were stained with 2% uranyl acetate (in 100% ethanol) followed by lead citrate.
Sections were inspected with an EM900 electron microscope (Carl Zeiss). Negatives were digitalized by scanning and processed with Adobe Photoshop Version 7.
Staining of histologic sections
Five-micron sections of paraffin-embedded or cryoconserved samples were stained with hematoxylin and eosin or with HRP-labeled anti-GPIb antibody, respectively.
In vitro megakaryocyte differentiation
Fetal liver cells of embryos at day 13.5 to 14.5 were cultured in medium (Iscove Modified Dulbecco medium, 10% fetal calf serum, and 1% penicillin/streptomycin) containing 50 ng/mL thrombopoietin (TPO). On day 3, megakaryocytes were enriched by gradient density filtration with 1.5% and 3% bovine serum albumin. Day 4 megakaryocytes were analyzed and counted for proplatelet formation under a light microscope. For confocal microscopy (Leica SP5), cells were spun onto an object slide, fixed in 4% paraformaldehyde, and permeabilized with Triton X-100. Tubulin was stained with anti–α-tubulin (clone DM1A, Sigma-Aldrich), and polymerized actin was visualized using Alexa-labeled phalloidin (Invitrogen). Nuclei were stained with 4,6-diamidino-2-phenylindole (Sigma-Aldrich).
Determination of ploidy from bone marrow MKs
Bone marrow was harvested and MKs were stained with the megakaryocyte-specific antibody (anti-GPIIb) and DNA with propidium iodide. DNA distribution was determined by flow cytometric analyses on a FACSCalibur.
Bone marrow explants time-lapse analysis
Analysis of bone marrow explants was performed as described.29
Data analysis
The results shown are mean plus or minus SD. Statistical analysis was performed using the Mann-Whitney U test, with P less than .05 indicating significant differences and P less than .001 indicating highly significant differences.
Results
Adf−/− mice are viable, whereas mice lacking n-cofilin die during embryonic development.13,25 To study n-cofilin function in MKs and platelets, mice carrying a conditional floxed n-cofilin allele25 were crossed with mice expressing Cre recombinase under the control of the megakaryocyte-specific PF4 promoter (n-cofilinfl/fl, PF4-Cre, later referred to as n-cofilin–null).27 Western blot analysis confirmed that both ADF and n-cofilin are strongly expressed in control platelets (Figure 1A). The absence of both proteins in platelets from knockout mice was confirmed by Western blot demonstrating efficient gene deletion in both systems.
n-cofilin–null platelets are markedly increased in size. (A) Whole platelet proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-ADF or anti–n-cofilin antibodies. GPIIIa was used as a loading control. Results from 2 individual mice per group (control, Adf−/−, n-cofilin–null) are shown. For the analysis of ADF/n-cofilin–null platelets, pooled platelet lysates were prepared from 6 mice. (B-C) Fluorescence-activated cell sorter analyses. (B) Peripheral platelet counts in control, Adf−/− and n-cofilin–null mice. Values are mean ± SD (n = 6). n.s. indicates not significant. *P < .05. (C) Platelet size of control, Adf−/− and n-cofilin–null platelets. Values are mean ± SD (n = 6). ***P < .001. FSC indicates forward scatter. (D) TEM analysis of resting platelets (top panel). Scale bar represents 2 μm. Visualization of the cytoskeleton of resting platelets on poly-L-lysine (bottom panel). Scale bar represents 1 μm.
n-cofilin–null platelets are markedly increased in size. (A) Whole platelet proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-ADF or anti–n-cofilin antibodies. GPIIIa was used as a loading control. Results from 2 individual mice per group (control, Adf−/−, n-cofilin–null) are shown. For the analysis of ADF/n-cofilin–null platelets, pooled platelet lysates were prepared from 6 mice. (B-C) Fluorescence-activated cell sorter analyses. (B) Peripheral platelet counts in control, Adf−/− and n-cofilin–null mice. Values are mean ± SD (n = 6). n.s. indicates not significant. *P < .05. (C) Platelet size of control, Adf−/− and n-cofilin–null platelets. Values are mean ± SD (n = 6). ***P < .001. FSC indicates forward scatter. (D) TEM analysis of resting platelets (top panel). Scale bar represents 2 μm. Visualization of the cytoskeleton of resting platelets on poly-L-lysine (bottom panel). Scale bar represents 1 μm.
n-cofilin, but not ADF, is essential for platelet size and shape determination
Adf−/− mice had normal platelet counts and size (Figure 1B-C), whereas mice lacking n-cofilin displayed moderately reduced platelet counts (60%-80% of controls; Figure 1B). Remarkably, the size of n-cofilin–null platelets was markedly increased as revealed by flow cytometric determination of the forward scatter signal (Figure 1C) and TEM (Figure 1D top panel). Furthermore, n-cofilin–null platelets displayed an ovoid shape, whereas the characteristic discoid shape was observed in control and Adf−/− platelets. Detailed analysis revealed that the platelet width of n-cofilin–null platelets was markedly increased (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Approximately 80% of n-cofilin–null platelets displayed a width of more than 0.66 μm, the maximal width observed in control platelets (supplemental Figure 1B). Ultrastructural analysis of the cytoskeleton revealed no major differences between control and single mutant platelets, except that the cytoskeleton of Adf−/− platelets appeared less dense and robust (Figure 1D bottom panel). In agreement with their increased size, expression levels of prominent surface receptors were increased in n-cofilin–deficient platelets (supplemental Figure 2A), whereas no differences were found between control and Adf−/− platelets (supplemental Figure 2B). The ultrastructure of n-cofilin–deficient giant platelets was not disordered, but consistent with their increased size they contained more granules than control platelets. In line with this observation, n-cofilin–null platelets exposed slightly increased levels of α granule–derived P-selectin in response to strong agonists, such as thrombin or collagen-related peptide compared with control platelets (supplemental Figure 3A left). These results demonstrated that n-cofilin, but not ADF, is critical for the formation of normally sized and discoid-shaped platelets.
Platelets of increased size and altered morphology are frequently found in conditions of increased platelet destruction, such as autoimmune thrombocytopenia or platelet-consuming disease states.30,31 To test the possibility that the increased platelet size and altered shape of n-cofilin–deficient platelets was a result of an enhanced platelet turnover, we determined their in vivo life span. For this, circulating platelets were labeled with a fluorescent noncytotoxic anti-GPIX antibody derivative, and the labeled platelet population was monitored over time.32 After 1 hour (day 0), more than 90% of the circulating platelets in control, Adf−/−, and n-cofilin–null mice were labeled, and this platelet population gradually decreased over 5 days in all 3 mouse lines with comparable kinetics, which is in agreement with the approximate 5-day life span of mouse platelets (supplemental Figure 4). Moreover, no correlation of different platelet sizes (supplemental Figure 1B) and altered life span was found in n-cofilin–deficient mice (data not shown).
These data suggest that the lack of n-cofilin in MKs directly impairs the production of correctly sized and shaped platelets rather than indirectly by increasing platelet production resulting from a reduced platelet life span.
Delayed spreading of n-cofilin–null platelets
To test the effect of deficiency of the actin severing/depolymerizing proteins ADF or n-cofilin on platelet cytoskeletal dynamics, we allowed control and mutant platelets to adhere and spread on immobilized fibrinogen. Spreading of thrombin-activated platelets was assessed by differential interference contrast microscopy at predetermined time points (Figure 2A; supplemental Videos 1-3). Adf−/− platelets spread with the same kinetics as control platelets, with more than 90% of the cells having formed lamellipodia after 20 minutes. In contrast, although the kinetics of adherence of n-cofilin–null platelets to the fibrinogen surface was unaltered, filopodia and subsequent lamellipodia formation was profoundly delayed. After 20 minutes, lamellipodia formation had started in only approximately 40% of the n-cofilin–null cells, whereas after 60 minutes virtually all n-cofilin–null platelets were able to form lamellipodia (Figure 2A). This was also shown by SEM (Figure 2B) and STED microscopy (Figure 2C), which revealed normal reorganization of the cytoskeleton in spread n-cofilin–deficient platelets at that time point.
Delayed spreading of n-cofilin–null, but not Adf−/− platelets on fibrinogen. (A) Thrombin-activated (0.001 U/mL) platelets were allowed to spread on immobilized human fibrinogen (1 mg/mL). Platelets were fixed at different time points and counted. Differential interference contrast images were recorded after 20 minutes. Bar represents 4 μm. (B) SEM after plasma membrane denudation of platelets spread for 60 minutes on fibrinogen. Scale bar represents 2 μm. (C) STED microscopy of platelets spread for 60 minutes on fibrinogen and stained with phalloidin. Scale bar represents 3 μm.
Delayed spreading of n-cofilin–null, but not Adf−/− platelets on fibrinogen. (A) Thrombin-activated (0.001 U/mL) platelets were allowed to spread on immobilized human fibrinogen (1 mg/mL). Platelets were fixed at different time points and counted. Differential interference contrast images were recorded after 20 minutes. Bar represents 4 μm. (B) SEM after plasma membrane denudation of platelets spread for 60 minutes on fibrinogen. Scale bar represents 2 μm. (C) STED microscopy of platelets spread for 60 minutes on fibrinogen and stained with phalloidin. Scale bar represents 3 μm.
These results indicate that n-cofilin can functionally compensate for the loss of ADF in platelets, which was also confirmed by the observation that Adf−/− platelets were fully functional and could be activated by all tested agonists as assessed by flow cytometry (supplemental Figure 3B) and aggregometry (supplemental Figure 5A for activation with thrombin; and data not shown). Interestingly, the lack of n-cofilin also had no effect on activation (supplemental Figure 3A) and aggregation responses to major agonists (supplemental Figure 5A for activation with thrombin; and data not shown). Furthermore, thrombin-activated n-cofilin–null platelets showed normal granule centralization 15 seconds after stimulation (supplemental Figure 5B), supporting the previous notion that n-cofilin–mediated accelerated actin dynamics are important for platelet outside-in signaling through integrins but dispensable for initial agonist-induced activation.33
Lack of ADF and n-cofilin in megakaryocytes virtually abolishes platelet formation
To further assess the role of ADF/n-cofilin in the process of platelet formation, we generated mice lacking both ADF and n-cofilin in megakaryocytes (Adf−/−/n-cofilinfl/fl, PF4-Cre, referred to as ADF/n-cofilin–null). Remarkably, platelet counts in the double-mutant mice were dramatically reduced to less than 5% of control mice (Figure 3A); however, the animals did not show any signs of spontaneous bleeding (not shown). TEM (Figure 3B) revealed that the few circulating platelets displayed a striking variability in size and morphology. We observed both giant and microparticle-like platelets. In most cases, an aberrant platelet ultrastructure was observed that was characterized by the presence of organelles, such as centrioles that are normally not found in platelets (not shown). In contrast to single-mutant platelets, ADF/n-cofilin–null platelets contained large accumulations of filaments in the cytoplasmic and the peripheral zone as shown by TEM on resting cells (Figure 3C). Further analyses revealed marked functional deficits in the double-mutant platelets, including defective spreading as shown by SEM (Figure 3D, for control see Figure 2B), as well as impaired integrin activation and degranulation (data not shown).
Markedly reduced peripheral platelet counts and abnormal platelet ultrastructure in ADF/n-cofilin–null mice. (A) Peripheral platelet counts were determined by flow cytometry. Results are mean ± SD (n = 6). ***P < .001. (B left, C) TEM analysis of resting platelets. Boxed area in panel C shows accumulation of filamentous material (see also higher magnification). (B right) Visualization of the cytoskeleton of resting platelets on poly-L-lysine. (D) SEM after denudation of the membrane of an ADF/n-cofilin–null platelet spread for 60 minutes on fibrinogen. Scale bars represent 2 μm (B left, D), 1 μm (B right), and 0.5 μm (C).
Markedly reduced peripheral platelet counts and abnormal platelet ultrastructure in ADF/n-cofilin–null mice. (A) Peripheral platelet counts were determined by flow cytometry. Results are mean ± SD (n = 6). ***P < .001. (B left, C) TEM analysis of resting platelets. Boxed area in panel C shows accumulation of filamentous material (see also higher magnification). (B right) Visualization of the cytoskeleton of resting platelets on poly-L-lysine. (D) SEM after denudation of the membrane of an ADF/n-cofilin–null platelet spread for 60 minutes on fibrinogen. Scale bars represent 2 μm (B left, D), 1 μm (B right), and 0.5 μm (C).
Together, these findings demonstrate that deletion of all ADF/n-cofilin activity results in a severe block of platelet production in vivo and in a complete absence of functional platelets.
No detectable actin polymerization in ADF/n-cofilin–null platelets
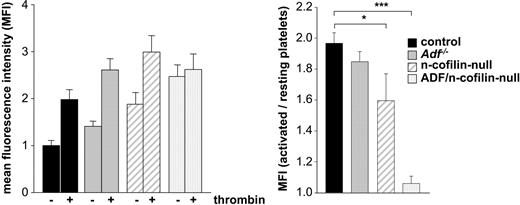
In vitro, ADF and n-cofilin enhance the severing and depolymerization rate of F-actin and the release of globular actin monomers (G-actin) at the pointed end of filaments, thereby promoting actin turnover and providing G-actin for the de novo actin polymerization. To directly analyze actin polymerization responses in mutant platelets, F-actin levels were measured in thrombin-activated and resting platelets using a flow cytometric approach. Baseline levels of F-actin in resting platelets were significantly increased in n-cofilin mutants and in ADF/n-cofilin double mutants (Figure 4 left panel). However, the markedly increased F-actin level in resting n-cofilin–deficient platelets can be assigned to the increased platelet size (Figure 1C-D). Taking the ratio of F-actin levels in thrombin-activated versus resting platelets as a measure for stimulated actin assembly, we observed a complete block in stimulus-induced actin polymerization in ADF/n-cofilin–deficient platelets (Figure 4 right panel). In contrast, stimulated F-actin assembly in Adf−/− platelets was comparable with control platelets, whereas the deletion of n-cofilin alone led to a significant reduction, but not to a complete block of F-actin assembly.
Agonist-induced actin polymerization is abolished in ADF/n-cofilin–deficient platelets. After activation of washed platelets with thrombin (1 U/mL for 2 minutes at 37°C), platelets were fixed, permeabilized, stained with phalloidin-FITC, and analyzed by flow cytometry. The mean fluorescence intensity of resting control platelets was set to 1. (Left panel) The mean fluorescence intensity of resting and activated platelets was measured. (Right panel) The ratio of polymerized actin in activated vs resting platelets was determined. *P < .05; ***P < .001.
Agonist-induced actin polymerization is abolished in ADF/n-cofilin–deficient platelets. After activation of washed platelets with thrombin (1 U/mL for 2 minutes at 37°C), platelets were fixed, permeabilized, stained with phalloidin-FITC, and analyzed by flow cytometry. The mean fluorescence intensity of resting control platelets was set to 1. (Left panel) The mean fluorescence intensity of resting and activated platelets was measured. (Right panel) The ratio of polymerized actin in activated vs resting platelets was determined. *P < .05; ***P < .001.
These results are in accordance with the in vitro findings concerning the different activities of ADF/n-cofilin toward actin.34 Our results show that ADF/n-cofilin are essential for the maintenance of physiologic F-/G-actin ratios in resting platelets and for agonist-induced F-actin assembly. Another important conclusion is that no other actin severing/depolymerizing protein in platelets, such as gelsolin or adseverin, can functionally compensate for the role of ADF/n-cofilin in platelet morphogenesis.
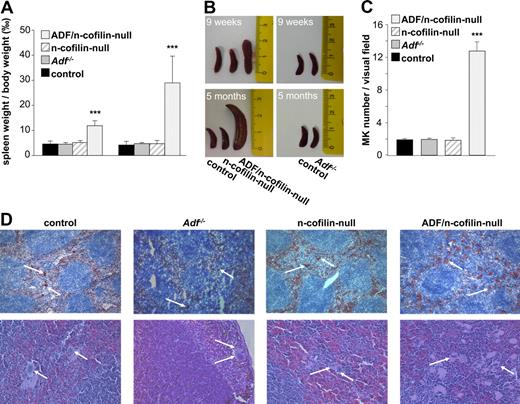
Hypermegakaryoplasia in ADF/n-cofilin–deficient mice
The lack of platelets in ADF/n-cofilin double-mutant mice could either be the result of a defect in MK maturation or a later defect in proplatelet formation resulting from impaired actin dynamics. To investigate the potential reason for the severe thrombocytopenia in ADF/n-cofilin–null mice, we analyzed MKs in spleen and bone marrow. A severe splenomegaly was evident in the double-mutant mice already 9 weeks after birth, which further developed to reach an approximately 6-fold increased spleen/body weight ratio compared with control at the age of 5 months (Figure 5A-B). Notably, n-cofilin–null and Adf−/− mice showed normal spleen size at all ages. The enlarged spleens of ADF/n-cofilin–null mice contained markedly increased numbers of MKs (visual field: 328 × 246 μm; control: 1.93 ± 0.14; ADF/n-cofilin–null 12.8 ± 1.06; Figure 5C) as revealed by analysis of cryosections stained with a platelet/MK–specific anti-GPIb antibody and hematoxylin and eosin–stained paraffin sections (Figure 5D).
Splenomegaly and elevated MK numbers in ADF/n-cofilin–null mice. (A) Spleen and body weight was measured after 9 weeks or after 5 months. Results are mean ± SD (n = 6). ***P < .001. (B) Representative spleens are shown from 9-week- or 5-month-old mice. Scale of ruler is in centimeters. (C) Numbers of MKs per visual field (328 × 246 μm) of hematoxylin and eosin stainings were determined. ***P < .001. (D) Cryo- (top panel, visual field: 1310 × 983 μm) or paraffin-embedded (bottom panel, visual field: 328 × 246 μm) sections (5 μm) were stained with anti-GPIb-HRP/hematoxylin and hematoxylin/eosin, respectively. White arrows represent MKs.
Splenomegaly and elevated MK numbers in ADF/n-cofilin–null mice. (A) Spleen and body weight was measured after 9 weeks or after 5 months. Results are mean ± SD (n = 6). ***P < .001. (B) Representative spleens are shown from 9-week- or 5-month-old mice. Scale of ruler is in centimeters. (C) Numbers of MKs per visual field (328 × 246 μm) of hematoxylin and eosin stainings were determined. ***P < .001. (D) Cryo- (top panel, visual field: 1310 × 983 μm) or paraffin-embedded (bottom panel, visual field: 328 × 246 μm) sections (5 μm) were stained with anti-GPIb-HRP/hematoxylin and hematoxylin/eosin, respectively. White arrows represent MKs.
Together, these results suggest that the severe thrombocytopenia in ADF/n-cofilin–deficient mice was not caused by a lack of MKs, but rather by a block of the subsequent steps of platelet formation.
Altered ultrastructure of ADF/n-cofilin–null bone marrow megakaryocytes
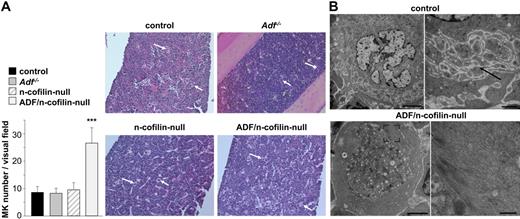
Similar to the spleen, MK numbers in the bone marrow of ADF/n-cofilin–null mice were markedly increased compared with the control mice (visual field: 328 × 246 μm; control: 8.46 ± 2.13; ADF/n-cofilin–null: 26.6 ± 5.6; Figure 6A), whereas no alterations were found in the single mutant mice. A similar percentage of MKs was located at the vascular sinus in ADF/n-cofilin–deficient and control mice (supplemental Figure 6A-B). Moreover, because of increased numbers of MKs in double-mutant mice, absolute numbers of ADF/n-cofilin–deficient MKs located at the vascular sinus were even 3.5-fold increased compared with the control (data not shown). This indicates that the severe thrombocytopenia in double-mutant mice is not caused by mislocation of bone marrow MKs. The overall structure of the bone marrow was not obviously altered in single- or double-mutant mice, but TEM analysis revealed marked morphologic abnormalities in ADF/n-cofilin–null MKs. The typically highly organized structure of the cytoplasm was consistently perturbed. Only fragments of the DMS were observed, whereas the granule distribution was dramatically altered (Figure 6B; supplemental Figure 7B). In contrast, no such defects were seen in Adf−/− or n-cofilin–null MKs (data not shown). These results indicate that ADF/n-cofilin activity is essential for the formation of proplatelet territories in MKs. The peripheral zone was enlarged because of enrichment of actin filaments (Figure 6B) as confirmed by actin immunolocalization (supplemental Figure 7A). In addition, significant accumulation of actin filaments in other areas of the cytoplasm was frequently seen in ADF/n-cofilin–null MKs (supplemental Figure 7B). However, these large amounts of filamentous actin did not result in any detectable increase in apoptosis of ADF/n-cofilin–null MKs (data not shown).
Morphologic abnormalities in bone marrow MKs of ADF/n-cofilin–null mice. (A) Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin (visual field: 655 × 491 μm). White arrows indicate MKs. Numbers of MKs per visual field (328 × 246 μm) were determined. ***P < .001. (B) TEM of control and ADF/n-cofilin–deficient bone marrow MKs. Right image (higher magnification of left image) of control MKs shows the DMS (black arrow). In ADF/n-cofilin–deficient bone marrow MKs, high amounts of fibrillar material are present in the peripheral cytoplasm (see higher magnification of the boxed area). Note that this zone is clearly devoid of organelles. Scale bars represent (from left to right) 5 and 1 μm (control), and 2 and 0.5 μm (ADF/n-cofilin–null).
Morphologic abnormalities in bone marrow MKs of ADF/n-cofilin–null mice. (A) Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin (visual field: 655 × 491 μm). White arrows indicate MKs. Numbers of MKs per visual field (328 × 246 μm) were determined. ***P < .001. (B) TEM of control and ADF/n-cofilin–deficient bone marrow MKs. Right image (higher magnification of left image) of control MKs shows the DMS (black arrow). In ADF/n-cofilin–deficient bone marrow MKs, high amounts of fibrillar material are present in the peripheral cytoplasm (see higher magnification of the boxed area). Note that this zone is clearly devoid of organelles. Scale bars represent (from left to right) 5 and 1 μm (control), and 2 and 0.5 μm (ADF/n-cofilin–null).
Thus, the presence of both ADF and n-cofilin is required for the formation and organization of the demarcation membrane system as well as normal granule distribution in mature MKs.
Reduced proplatelet formation and absence of proplatelet swellings in ADF/n-cofilin–null differentiated megakaryocytes in vitro
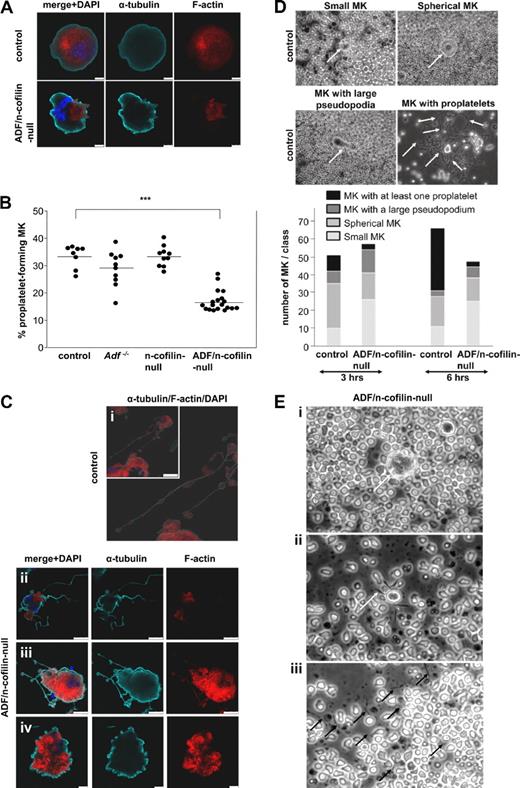
To further study platelet biogenesis, we cultured fetal liver cells of control and mutant mice and allowed them to differentiate into MKs for 4 days as described.35 The localization of actin in undifferentiated and differentiated (proplatelet-forming) MKs was investigated using confocal fluorescence microscopy. Phalloidin staining revealed that actin was homogeneously distributed in undifferentiated control MKs, whereas, in line with the results of TEM, we found patches of F-actin accumulation in the cell body of ADF/n-cofilin–null MKs, underscoring the central role of ADF/n-cofilin for actin turnover (Figure 7A). Although differentiated MKs from Adf−/− or n-cofilin–null mice displayed normal proplatelet formation compared with MKs from control mice, a significant reduction in the number of proplatelet-forming MKs was seen in cultures from double-mutant mice (control: 33.46% ± 4.12%; ADF/n-cofilin–null: 16.98% ± 3.88%; Figure 7B). Taking into consideration the abnormal MK ultrastructure and the severe thrombocytopenia in ADF/n-cofilin–null mice, it was surprising to find that proplatelet formation was not completely blocked. This indicated that additional defects in ADF/n-cofilin–null MKs might account for the absence of platelets in vivo. Therefore, we investigated the differentiated ADF/n-cofilin–null MKs by confocal fluorescence microscopy. Remarkably, whereas control MKs showed the typical structure of proplatelets with periodic swellings (Figure 7Ci), proplatelets of ADF/n-cofilin–null MKs were lacking the swellings, which are normally located at the proplatelet shaft36 (Figure 7Cii-iii). F-actin was partially absent in the double-mutant proplatelets (Figure 7Cii) or, if present, proplatelets appeared shorter than in control MKs (Figure 7Ciii). In addition, ADF/n-cofilin–null MKs frequently displayed a “blebbing” structure (control: 3/84; ADF/n-cofilin–null: 39/99; number of MKs with “blebbing” structure/total number of analyzed MKs), most probably reflecting proplatelet formation that was blocked at an early stage (Figure 7Civ). To assess proplatelet formation of MKs in their native environment, bone marrow explants of control and ADF/n-cofilin–null mice were cultured for 6 hours and MK proplatelet formation was monitored in situ by time-lapse video microscopy (supplemental Videos 4-5). Statistical analysis was performed by categorizing the proplatelet formation process of MKs in the bone marrow explant into 4 morphologic stages: small MKs, spherical MKs, MKs with a pseudopodium, and MKs with at least one proplatelet (Figure 7D, higher magnification of MKs with proplatelets in supplemental Figure 8). This analysis revealed that the majority of control MKs formed proplatelets, whereas only a minor fraction of ADF/n-cofilin–null MKs was able to form proplatelets. Detailed microscopic analysis showed that the cytoplasm and the nuclei of ADF/n-cofilin–deficient MKs were not clearly delineated and that the cellular contour appeared to be irregular (Figure 7Ei) compared with control MKs (Figure 7D, spherical MKs). The few proplatelet-like extensions that were formed by ADF/n-cofilin–null MKs lacked the typical swellings and were shorter (Figure 7Eii). Interestingly, after 6 hours, many thin extension-like structures were observed lying loosely in the chamber of explants of ADF/n-cofilin–null mice (Figure 7Eiii). This finding suggests that proplatelet swellings contribute to the stabilization of proplatelets.
Reduced proplatelet formation and absence of swellings in ADF/n-cofilin–null MKs. Fetal liver cells of embryos at day 14.5 were prepared and cultured in medium containing 50 ng/mL TPO. On day 4, MKs were stained (A,C). Control (Ci), ADF/n-cofilin–null (Cii-iv). Scale bars represent (A) = 10 μm, (Ci) = 50 μm, (Cii-iii) = 25 μm, (Civ) = 10 μm. (B) Fetal liver cells were cultured for 4 days and MKs were analyzed for proplatelet formation under a light microscope using a 20× objective. (●) Average of 20 analyzed visual fields per fetal liver-derived MK culture. The horizontal bars represent the arithmetic mean of each group. ***P < .001. (D) Bone marrow sections (0.5-mm-thick) were incubated with Tyrode buffer supplemented with 5% mouse serum at 37°C. Representative pictures of small MKs, spherical MKs, MKs with protrusion, and MKs with proplatelets are shown. Quantification was performed after 3 and 6 hours of incubation. Arrows indicate MKs (small MKs, spherical MKs, MKs with large pseudopodia). Arrows indicate swellings (MKs with proplatelets). (E) Representative images of bone marrow explants from ADF/n-cofilin–null mice after 6 hours of culture. The morphologies of spherical MKs (i) and proplatelet-like extensions (ii) were compared with control (see also control MKs in panel D) and analyzed. (i) Arrow indicates the MKs. (ii) Arrow indicates short extension of the MKs. (iii) Isolated extensions (black arrows) of ADF/n-cofilin–null MK were observed in the chamber.
Reduced proplatelet formation and absence of swellings in ADF/n-cofilin–null MKs. Fetal liver cells of embryos at day 14.5 were prepared and cultured in medium containing 50 ng/mL TPO. On day 4, MKs were stained (A,C). Control (Ci), ADF/n-cofilin–null (Cii-iv). Scale bars represent (A) = 10 μm, (Ci) = 50 μm, (Cii-iii) = 25 μm, (Civ) = 10 μm. (B) Fetal liver cells were cultured for 4 days and MKs were analyzed for proplatelet formation under a light microscope using a 20× objective. (●) Average of 20 analyzed visual fields per fetal liver-derived MK culture. The horizontal bars represent the arithmetic mean of each group. ***P < .001. (D) Bone marrow sections (0.5-mm-thick) were incubated with Tyrode buffer supplemented with 5% mouse serum at 37°C. Representative pictures of small MKs, spherical MKs, MKs with protrusion, and MKs with proplatelets are shown. Quantification was performed after 3 and 6 hours of incubation. Arrows indicate MKs (small MKs, spherical MKs, MKs with large pseudopodia). Arrows indicate swellings (MKs with proplatelets). (E) Representative images of bone marrow explants from ADF/n-cofilin–null mice after 6 hours of culture. The morphologies of spherical MKs (i) and proplatelet-like extensions (ii) were compared with control (see also control MKs in panel D) and analyzed. (i) Arrow indicates the MKs. (ii) Arrow indicates short extension of the MKs. (iii) Isolated extensions (black arrows) of ADF/n-cofilin–null MK were observed in the chamber.
Together, these in vitro and ex vivo results support our findings that ADF/n-cofilin–null MKs develop significantly fewer proplatelets and that the formed proplatelet-like extensions do not contain periodical swellings.
In summary, our results provide conclusive genetic, cell-biologic, and functional evidence that ADF/n-cofilin–mediated actin filament turnover is critical for the formation of proplatelets and for the elaboration of proplatelet swellings.
Discussion
Our study demonstrates that ADF/n-cofilin–driven actin turnover plays a fundamental role in the terminal step of platelet production. Surprisingly, mice specifically lacking ADF/n-cofilin in MKs are unable to produce functional platelets. To our knowledge, no other defect in MKs has been reported to date that has a similarly severe impact on platelet biogenesis in vivo. For comparison, mice lacking β1-tubulin still produce approximately 40% of normal platelet numbers, although β1-tubulin−/− cells show severe defects in proplatelet formation in vitro.37 Mice harboring a targeted mutation of regulatory elements within the GATA-1 locus that leads to a lineage-specific loss are characterized by severely defective megakaryocytes but can still produce 15% of the platelet counts of controls.38 In contrast, ADF/n-cofilin controls the 2 key events in platelet formation: proplatelet formation and peripheral maturation of platelets. Remarkably, mice deficient for ADF and n-cofilin in MKs and platelets are viable and do not have spontaneous bleeding (not shown), despite the absence of functional platelets. Previous observations suggested that thrombocytopenia alone is not sufficient to cause bleeding.39,40 Interestingly, knockout mice for another actin severing protein, gelsolin, have normal platelet counts,7 demonstrating an unexpected specificity of actin dynamics in platelet biogenesis.
It has been well established that sliding of microtubules is the primary force for the elaboration of proplatelets,41 although the specific role of dynamic actin turnover has remained elusive in proplatelet formation. So far, in vitro studies using cytochalasin (a fungal metabolite that inhibits actin assembly) have been performed to address the function of actin turnover in proplatelet formation. MKs treated with cytochalasin showed defects in proplatelet branching, resulting in reduced complexity and rolling of proplatelets.1 The genetic approach used here suggests a more sophisticated mode of how actin contributes to platelet morphogenesis. We show that MKs deficient in ADF/n-cofilin display a significant reduction in proplatelet formation in vitro (Figure 7B) and ex vivo (Figure 7D), and F-actin accumulation within the MKs (Figure 7A,C). Although the double-mutant MKs still had a limited capacity to produce proplatelets (Figure 7B), these proplatelets were lacking the typical beaded appearance found in control MKs, and their shape was less complex and shorter (Figure 7C,E). Extensions were found to be devoid of polymerized actin, which is in line with the established role of ADF/n-cofilin for de novo actin assembly and with previous observations that F-actin is particularly enriched in swellings.1 Together, these results reveal a novel role for ADF/n-cofilin–dependent F-actin turnover and actin assembly for proplatelet formation, and show that this function cannot be compensated by any other severing/depolymerizing proteins, such as gelsolin and adseverin.
In contrast, MK differentiation was not impaired in the absence of ADF/n-cofilin, as suggested by high numbers of mature MKs in the spleen and bone marrow of ADF/n-cofilin–null mice (Figures 5C, 6A). Only a minor increase in ploidy levels was observed in MK from ADF/n-cofilin–deficient mice (mean ploidy: control 14.27N ± 0.07N; Adf−/− 13.71N ± 1.36N; n-cofilin–null 13.43N ± 0.97N; ADF/n-cofilin–null 16.38N ± 0.95N), which was most probably a secondary effect of the severe thrombocytopenia (Figure 3A). Similar deviation from mean ploidy levels has been described after induction of chronic thrombocytopenia in mice.42 Interestingly, the increase in MK numbers was not associated with elevated plasma TPO levels (data not shown), an observation that has also been made in other mouse lines with low platelet counts and elevated MK numbers (Stim1Sax/+and Wdr1rd/rd).32,43 One explanation could be that the high MK mass in these mice can compensate for the loss of platelet mass in the regulation of TPO levels.
Mechanistically, the DMS has been linked to proplatelet formation,44 and we show here that ADF/n-cofilin–null bone marrow MKs are virtually devoid of the DMS and enriched for F-actin structures (Figure 6B; supplemental Figure 7B), as also shown by immunolocalization (supplemental Figure 7A). These findings clearly demonstrate that ADF/n-cofilin–dependent actin turnover is crucial for the development of the DMS in vivo and that it is a prerequisite for normal platelet formation. Interestingly, Kile et al described mutant mice for the Wdr1 gene, a cofilin interaction partner, which also displayed a defect in DMS development and granule distribution.43
In the last decade, 2 models of thrombopoiesis have been extensively discussed: (1) platelets are prepacked in platelet territories and final platelets are released into the circulation by MK fragmentation45 ; and (2) mature MKs extrude proplatelets, which contain periodic swellings.46 To date, numerous studies have supported the latter model, mostly by using in vitro megakaryocyte differentiation approaches, and have shown that the entire MK cytoplasm converts into proplatelets. Importantly, Junt et al recently described using in vivo imaging that protrusions from MKs extend into the microvessels and that platelet particles exceeding the size of normal platelets are released into the bloodstream.3 This supports previous reports that had suggested that the final maturation of platelet sizing occurs in the circulation.47 In a very recent publication, Schwertz et al report on platelets that produce functional progeny in whole blood cultures and assume a form of cell division to be involved in this process.4 We show here that loss of n-cofilin alone in MKs leads to the production of large platelets displaying an ovoid rather than a discoid shape (Figure 1C-D). Remarkably, these platelets are fully functional, display a normal life span (supplemental Figure 4), and do not exhibit major defects. This is in contrast to many other genetic defects in cytoskeletal proteins, many of which produce enlarged platelets that exhibit partially severe functional defects. Our data suggest that the altered size and shape of n-cofilin–null platelets are a direct consequence of n-cofilin deficiency in MKs, highlighting a specificity even among the ADF/n-cofilin–depolymerizing factors. This effect was not associated with altered size or morphology of platelet territories in mature MKs in vivo or with altered proplatelet swellings in MKs cultured in vitro (data not shown). This indicates that n-cofilin–dependent platelet sizing normally occurs at a later stage of platelet biogenesis, possibly at the maturation step from preplatelets to final platelets, which could occur in the circulation. This hypothesis is supported by the finding that n-cofilin–null platelets resemble preplatelets that are blocked in the final maturation step. Studies in other cell types have established a critical role for actin filament dynamics and n-cofilin in processes of membrane fission, such as mitosis and cytokinesis,20,48-50 and similar to its function in nucleated cells, cofilin might also be required for orchestrating the “division process” of preplatelets to platelets. Further in vivo studies on the mutant mice will allow us to test this hypothesis in the future.
In conclusion, we demonstrate that ADF/n-cofilin activity is the critical denominator in controlling actin dynamics during the morphogenesis of proplatelets as well as in the terminal step of platelet maturation. We also show that n-cofilin, but not ADF, is a key regulator of platelet size and shape in the periphery.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Radek Skoda for kindly providing us with the PF4-Cre mice; Birgit Midloch, Jean-Yves Rinckel, and Fabienne Proamer for excellent technical assistance; and David Stegner for help with figure preparation.
This work was supported by the Rudolf Virchow Center and the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688; B.N., A.G.). S.G. was supported by the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg.
Authorship
Contribution: M.B. performed experiments, analyzed data, and contributed to the writing of the manuscript; A.E., J.H.H., M.E., I.P., S.G., G.K., and E.J. performed experiments and analyzed data; A.G., C. Gachet, C. Gurniak, and W.W. analyzed data and contributed to the writing of the manuscript; and B.N. planned the project, analyzed data, and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Department of Vascular Medicine, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University Clinic Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.