Most antithrombotic approaches target prevention rather than the more clinically relevant issue of resolution of an existing thrombus. In this issue of Blood, Zhang and colleagues1 describe a novel and effective therapeutic strategy for clearance of preexisting arterial thrombus in murine models of ischemic stroke.
Arterial thrombosis is initiated by platelets adhering to the damaged vessel wall. These adherent platelets become activated, leading to activation of the platelet integrin glycoprotein (GP) IIb-IIIa (αIIbβ3), which in binding fibrinogen and von Willebrand factor allows formation of a platelet aggregate or mural thrombus. At the same time, the damaged vessel wall and the activated platelet surface provide an acceleration of localized coagulation leading to thrombus stabilization by fibrin.2
Thrombosis precipitating ischemic stroke is a major cause of morbidity and death. The capacity to limit brain infarct formation and resultant permanent neurologic damage requires resolution of the arterial thrombus, ideally within a narrow therapeutic window of up to 4 hours postocclusion. Clinical studies with GPIIb-IIIa receptor antagonists3 or other profibrinolytic approaches4 are not optimal and are typically associated with a high incidence of bleeding. Current treatment of acute ischemic stroke with tissue plasminogen activator shows best utility within 4.5 hours of occlusion and can also be associated with clinically significant bleeding,5 highlighting the pressing need for better and safer therapeutic approaches.
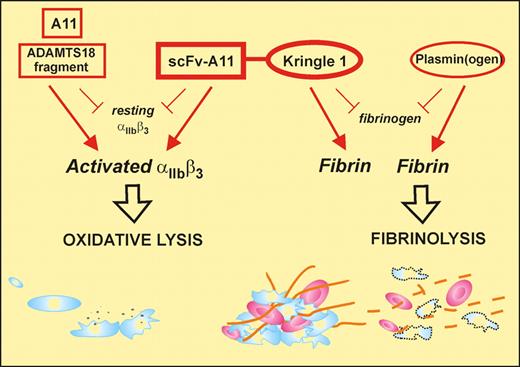
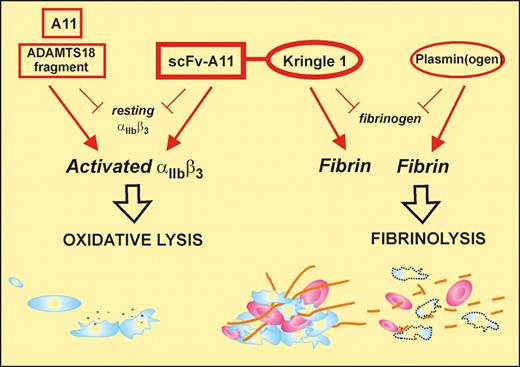
Zhang and colleagues have previously described a unique antiplatelet autoantibody in patients with HIV- or hepatitis C–related thrombocytopenia that recognizes the sequence GPIIIa49-66 and induces complement-independent platelet lysis by generation of reactive oxygen species and peroxide after platelet-reduced nicotinamide adenine dinucleotide phosphate oxidase activation.6,7 Subsequently, they identified a human single-chain fragment variable region (scFv) antibody that recognized GPIIIa49-66 (A11), with similar functional properties to the patient autoantibody in that it preferentially bound to activated platelets and could also lyse arterial thrombus in vitro. In the current study,1 Zhang et al have taken this translational medicine approach full circle by assessing the capacity of A11 to clear arterial thrombus in 2 murine stroke models. Although A11 showed considerable promise in these studies, restoring blood flow, for example, when administered 2 hours after carotid artery occlusion, the most dramatic and encouraging findings were obtained with a second-generation derivative of A11 (see figure).
The bifunctional SLK consisting of the scFv-A11, that binds to an epitope on activated αIIbβ3 (GPIIb-IIIa) and induces platelet lysis, fused to the kringle 1 domain that recognizes fibrin (not fibrinogen). This construct can target an active thrombus (via activated αIIbβ3/polymerized fibrin) providing both dual antithrombotic activity and a unique therapeutic approach. Monovalent antibody also induces oxidative lysis via αIIbβ3, while kringle-containing plasminogen interacts directly with fibrin.
The bifunctional SLK consisting of the scFv-A11, that binds to an epitope on activated αIIbβ3 (GPIIb-IIIa) and induces platelet lysis, fused to the kringle 1 domain that recognizes fibrin (not fibrinogen). This construct can target an active thrombus (via activated αIIbβ3/polymerized fibrin) providing both dual antithrombotic activity and a unique therapeutic approach. Monovalent antibody also induces oxidative lysis via αIIbβ3, while kringle-containing plasminogen interacts directly with fibrin.
This variant, termed SLK (single-chain linked first kringle), was produced by coupling the A11 scFv fragment through a linker peptide to the first kringle domain of plasminogen. The rationale, experimentally confirmed in this study, was that the first kringle domain of plasminogen would allow SLK to home to, and covalently bond with, terminal lysine residues within partially degraded fibrin associated with the platelet thrombus, similar to the approach recently used to create Delta-plasmin8 and serving to concentrate the A11 scFv component directly at the platelet thrombus where platelet lysis would then occur. Critically, SLK behaves in mice with many of the advantages that would be hoped for in an optimal therapeutic thrombolytic. First, SLK could reproducibly lyse thrombus and restore blood flow when administered 4 hours after cessation of blood flow or induction of stroke, whereas A11 was ineffective at this time period after arterial occlusion. SLK showed partial protection against infarct (34% reduction) and significantly preserved neurologic function (as measured by postural reflex), without evidence of cerebral bleeding. In contrast, in the same murine model, one-third of mice treated with tissue plasminogen activator died of intracranial hemorrhage.9 Second, SLK caused minimal thrombocytopenia (∼11% reduction in platelet count at the 4-hour postadministration nadir), with restoration of normal count at 24 hours. Third, SLK bound preferentially to activated platelets and lysed platelets by the same functional mechanism as A11. Fourth, SLK had no affect on platelet aggregation in vitro. Importantly, ex vivo analysis of blood platelets taken from mice 4 hours after SLK administration showed no evidence of platelet activation and did show normal collagen and thrombin agonist responses in terms of P-selectin expression and formation of platelet-monocyte aggregates. Fifth, SLK had no effect on bleeding time. Finally, as a human scFv antibody construct, SLK would be less likely to act as a foreign antigen and would be unlikely to activate complement or invoke an inflammatory phagocytic cytokine response because it lacks an Fc domain.
The current study did not address SLK's use as a thrombolytic in other vascular beds, for example, in coronary artery thrombosis, where it might act as an alternative in some circumstances to coronary artery stenting and other more interventional approaches. Any potential effect of A11 and/or SLK on the endothelial integrin αvβ3 sharing the A11 epitope-bearing β3 chain also remains to be elucidated. If these promising preclinical studies can be replicated in first studies in humans, they are likely to provide better and safer treatment options in ischemic stroke.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■