In this issue of Blood, Isakson et al identify autophagy as a new pathway for therapy-induced PML/RARA degradation, a critical determinant for leukemia eradication.
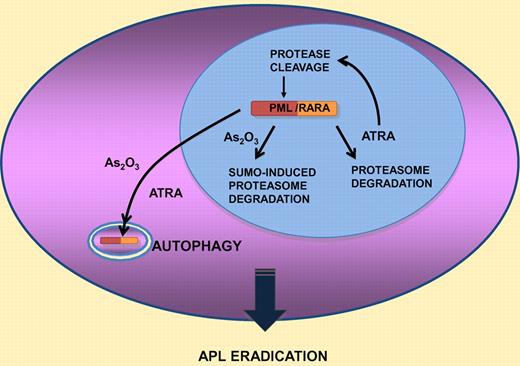
Acute promyelocytic leukemia (APL) is characterized by the t(15;17) chromosomal translocation that fuses the promyelocytic leukemia (PML) gene with that of retinoic acid receptor A (RARA). This rare acute myeloid leukemia exhibits a differentiation block at the promyelocytic stage of granulocytic maturation, hence its name. APL is a paradigm for oncoprotein-targeted therapy. Indeed, at the molecular level, the 2 clinically active therapies, all trans-retinoic acid (ATRA) and As2O3, both induce the degradation of the PML/RARA fusion protein. ATRA degrades both RARA and PML/RARA, while conversely As2O3 degrades both PML and PML/RARA. Thus, these 2 serendipitously discovered agents actually target each of the 2 constitutive moieties of the oncoprotein (see figure). Several lines of evidence, among which is the synergistic effect of the ATRA/As2O3 combination for APL eradication in mice and man, have strongly implicated PML/RARA degradation in disease clearance.1-3
Schematic representation of the 4 pathways implicated in PML/RARA degredation by retinoic acid or arsenic trioxide (see text for details).
Schematic representation of the 4 pathways implicated in PML/RARA degredation by retinoic acid or arsenic trioxide (see text for details).
PML/RARA is a transcriptional repressor that binds both RARA response elements and de novo DNA sequences, resulting in dominant-negative and gain-of-function properties. Pharmacologic ATRA concentrations release PML/RARA-mediated transcriptional repression and induce differentiation. PML/RARA also disrupts structures formed by the normal PML protein, PML nuclear bodies. These enigmatic domains have been repeatedly implicated in control of cell death or self-renewal of normal or leukemia stem cells.4 Treatment-induced PML/RARA degradation reverses these effects, allowing differentiation and loss of clonogenic activity, as shown by transplantation studies in mice, culminating in APL eradication.2,3,5
Previous studies had identified 3 distinct pathways for therapy-induced PML/RARA degradation. The first one is cleavage of the PML moiety of PML/RARA by proteases activated by ATRA-induced differentiation.6 A second ATRA-initiated catabolic pathway activates direct proteasome-dependent degradation of DNA-bound, hormone-activated RARA or PML/RARA,7 a feedback mechanism conserved for all nuclear receptors. Finally, As2O3 targets the PML part of the fusion protein and specifically induces a SUMO-dependent ubiquitin-mediated degradation process that was recently elucidated at the biochemical and cellular level.8
In the accompanying report,9 a fourth pathway, autophagy, was conclusively implicated in both ATRA- and As2O3-mediated PML/RARA degradation. The authors first demonstrate that PML/RARA is a highly insoluble protein. Misfolded proteins form aggregates that are often cleared by autophagy within the cytoplasm. The authors thus examine the consequence of activation or inhibition of autophagy on PML/RARA stability and observe that this process regulates basal PML/RARA protein levels. Importantly, both ATRA and As2O3 activate autophagy and PML/RARA is detected in cytoplasmic autophagic vesicles after treatment with these agents. Functionally, activation of autophagy by the mammalian target of rapamycin (mTOR) inhibitor triggers PML/RARA destabilization in the NB4 APL cell line, resulting in enhanced ATRA-induced differentiation. Conversely, the autophagy inhibitor BafA impedes treatment-induced PML/RARA degradation and biologic response.
These results are important in several respects. They identify a novel cytoplasmic PML/RARA catabolic pathway and explain why some studies had observed PML/RARA in the cytoplasm. Other oncogenic fusion proteins may share similar features of poor solubility and may be targets of autophagy. This study also raises interesting questions as to the possible links between autophagy and PML, which is highly stress-sensitive and may also assemble into cytoplasmic bodies.4 Finally, PML suppresses mTOR activity,10 a key inhibitor of the autophagic process. Then, release of PML from PML/RARA by ATRA or As2O3 treatment induces a self-perpetuating cycle accelerating PML/RARA degradation. The latter could be implicated in enhanced differentiation, as suggested in the Isakson study, but also in loss of self-renewal of APL clonogenic progenitors. Thus, modulation of autophagy in APL could have important therapeutic consequences.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal