In this issue of Blood, Wall and colleagues report the use of radiolabeled 11-1F4 to visualize amyloid deposits.1 This biodistribution study in humans demonstrates the ability of an antibody to bind to and identify amyloid deposits in vivo.
Liver uptake was seen in a high proportion of patients, although only 3 actually were felt to have clinically important hepatic amyloidosis. Unfortunately, 10 of the 18 patients with renal involvement and 3 of the 18 patients who had cardiac involvement without renal involvement had no uptake of the radiolabeled antibody in the kidney and heart, respectively. The fact that renal amyloid deposits in tissue sections (in vitro) immunostained weakly suggests that the antibody itself may not be able to bind to amyloid deposits in the kidney or heart and not because of antibody dosage. Despite these limitations, this report represents an exciting proof of principle. The imaging paper builds on previous research from this group where human light-chain–associated tumors in mice underwent marked regression, without toxicity, after the administration of the same antibody.2
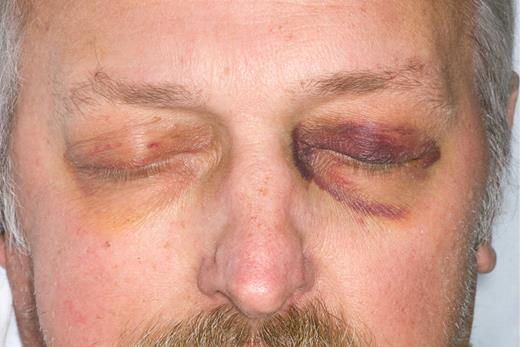
If there is to be any hope in improving the outcomes in patients with amyloidosis, there has to be a better mechanism for earlier detection of this disease. Not all patients will present with characteristic physical findings (see figure).
Diagnostic physical findings of purpura or glossomegaly are found in only 15% of patients. Clinicians seek the availability of in vivo imaging that would specifically recognize amyloid for their patients, when the clinician suspects the diagnosis but is unwilling to engage in a series of uncomfortable biopsies that entail a risk of bleeding. Attempts to improve on the diagnosis of cardiac amyloidosis using strain rate echocardiography3 as well as cardiac magnetic resonance imaging4 have recently been introduced in an attempt to identify cardiac amyloid deposits before significant functional compromise occurs. These are the patients most likely to benefit from chemotherapy or stem cell transplantation. The existence of a radio-immunoantibody capable of imaging amyloid deposits may prove to be of value in future monitoring of the disorder after therapy. Current techniques rely heavily on measurements of changes in the precursor protein, either the monoclonal protein in the serum or urine5 or the immunoglobulin-free light chain ratio in serum.6 Although these measures have been shown to correlate with outcomes, they do not directly assess what has happened with the actual amyloid deposits in the tissues. Imaging the amyloid burden at diagnosis, using the antibody, may have important prognostic value. This is reminiscent of the serum amyloid P (SAP) scan available in Europe, which can determine organ involvement and indicate the body amyloid load.7 SAP scintigraphy, which identifies all forms of amyloid deposits including hereditary and secondary forms, often reveals more widespread organ involvement than is identified clinically and represents useful additional information. Unfortunately, the SAP scan is not available in the United States, and investigators have had to rely on indirect evidence of organ involvement such as the serum alkaline phosphatase for liver amyloid, brain natriuretic peptide and troponin levels for cardiac amyloid, and the level of urinary protein loss for renal amyloid to assess organ involvement response and progression.
The therapeutic potential of 11-1F4 cannot be overlooked. Once small tracer doses identify the deposits, investigation of the antibody's ability to cause dissolution of the amyloid would be a natural next step. Similar therapeutic approaches that involved depleting the SAP component have recently been published.8 Even if the current antibody does not bind to the heart or the liver, limited organ regression using an agent with a high therapeutic profile can set the stage for important immunotherapeutic approaches to the management of this devastating disease with successive generations of antiamyloid antibodies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal