Abstract
The risk of cardiac hospitalization (CH) in Hodgkin lymphoma (HL) patients with preexisting heart disease was evaluated. Patients with HL were identified from a population-based registry (N = 3964). Data were abstracted from records of a randomly selected subcohort (N = 1096). A population-based registry was used to identify CH. Factors associated with CH and the incidence of CH after HL were estimated with competing risk models. Preexisting heart disease was the strongest predictor of posttreatment CH (hazard ratio = 3.98, P < .001) and significantly modified (P = .01) the effect of treatment on the risk of CH. Among patients with preexisting heart disease, treatment with mediastinal radiation therapy plus doxorubicin-based chemotherapy was associated with a 10-year incidence of CH more than 20% higher than treatment with chemotherapy alone. There is a high risk of CH after mediastinal radiation therapy plus doxorubicin-based chemotherapy among patients with preexisting heart disease; this is an important consideration when weighing treatment options, and in the follow-up of these patients.
Introduction
Cardiac toxicity is an important consequence of Hodgkin lymphoma (HL) treatment. The reliance on cardiotoxic agents to cure HL makes treatment of patients with preexisting heart disease particularly challenging. To our knowledge, no prior study has explicitly evaluated the cardiac morbidity of doxorubicin-based HL therapy among patients with preexisting heart disease. Prior studies have either excluded patients with prior cardiac history1,2 or do not specifically report their outcomes.3-5 We conducted a population-based cohort study to assess the risk of post-HL cardiac hospitalization (CH) among HL patients with preexisting heart disease.
Methods
Patients with a new diagnosis of HL from April 1, 1988, to December 31, 2003, were identified in the Ontario Cancer Registry (N = 4660). A unique numeric identifier, based on (encrypted) Ontario Health Insurance Number, could be identified for 4448 patients (95.5%) and linked to a provincial hospitalization registry. Patients younger than 18 years at HL diagnosis (N = 484) were excluded. The remaining 3964 adult HL patients were the source cohort for this study.
From this cohort, we selected a 30% random sample (N = 1190) for medical record abstraction. If the HL diagnosis was incorrect (N = 16), the chart was not locatable (N = 125) or contained inadequate information (N = 47), a replacement chart from the same hospital was requested using the source cohort list. In some cases (N = 72), no replacement chart was available, or also had inadequate information (N = 22). The resulting subcohort contained 1096 patients. Elderly patients who died shortly after diagnosis were less likely to have a locatable/complete medical record. Consequently, subcohort patients were slightly younger at diagnosis than patients not included in the subcohort (median age, 34 years vs 36 years) and had a better 1-year survival (96% vs 92%).
For this subcohort, data regarding patient demographics, HL characteristics, cardiac history, cardiac risk factors at the time of HL diagnosis (hypertension [yes/no], smoking [ever/never], elevated cholesterol [yes/no], diabetes [yes/no], and body mass index [normal/abnormal]) and patient outcome were abstracted. Patient records were also linked to the Ontario Diabetes Registry to supplement chart-abstracted data in the identification of diabetes. Preexisting cardiac disease was coded as present when the administrative data indicated that a CH had occurred before the HL diagnosis, or when the clinical notes indicated. CH was identified by linking patients to a provincial hospitalization registry, using ICD-9 or ICD-10 codes to determine the primary cause of hospitalization. The end date for follow-up was March 31, 2007.
The hazard for the risk of CH accounting for the competing risk of death was modeled using the method of Fine and Gray.6 Covariates analyzed were age at HL diagnosis, sex, HL stage (I/II vs III/IV), cardiac risk factors at HL, the presence of preexisting cardiac disease (yes/no), and treatment factors. Hypertension, hypercholesterolemia, and diabetes were combined into a single “any risk factor” variable because of the low prevalence of each individually. Body mass index and smoking (ever/never) were not significant when they were added and therefore were not included in the final multivariate model. Age at HL diagnosis was modeled as a continuous linear variable, and the hazard associated with age was allowed to vary linearly over time (ie, allowed for nonproportional hazard over time). Initial HL treatment was categorized as doxorubicin-containing chemotherapy alone without mediastinal radiation therapy (MRT), MRT without chemotherapy, both MRT and doxorubicin, and other (nondoxorubicin-chemotherapy +/− RT, nonmediastinal RT without chemotherapy). Interaction was tested between treatment categories and preexisting cardiac disease, preexisting cardiac risk factors, age, and sex. Only the interaction between treatment and preexisting cardiac disease was significant (P = .01): the effect of MRT was significantly different between those with and without prior cardiac disease.
The impact of treatment modality was modeled separately for patients with preexisting cardiac disease, and the impact of treatment on the risk of CH in this group is the focus of this study. Because of the older age and prevalence of cardiac risk factors among patients with preexisting cardiac disease, modeled estimates of the cumulative incidence of CH in this group are presented for modeled age = 60 years at HL diagnosis, and with “any cardiac risk factor” = yes.
The study was approved by the Research Ethics Boards of all hospitals where record abstraction occurred.
Results and discussion
The median age at HL diagnosis of the subcohort was 34 years (range, 18-86 years), with a median follow-up duration of 10 years (range, 1 month to 19 years).
Ninety-six patients (9%) had a history of preexisting cardiac disease. The preexisting cardiac conditions were ischemic heart disease (48 of 96), valvular disease (23 of 96), congestive heart failure (7 of 96), and other noncongenital cardiac diagnoses (arrhythmias, pericardial diseases, 18 of 96). Patients with preexisting cardiac disease were significantly older than those without (median age, 63 vs 33 years, P < .001). Initial treatment is described in Table 1.
The median length of time from HL diagnosis to cardiac admission was 3.5 years (range, 1 month to 16.9 years). In a multivariable model of the 1096-person subcohort, preexisting cardiac disease was the most significant predictor of CH (hazard ratio [HR] = 3.98, P < .001). Other significant risk factors were age at diagnosis (HR = 1.03/years, P < .001) and male sex (HR = 1.67, P = .019). Combined doxorubicin chemotherapy plus MRT was associated with a significantly higher risk than doxorubicin chemotherapy alone (HR = 1.76, P = .02; Table 1).
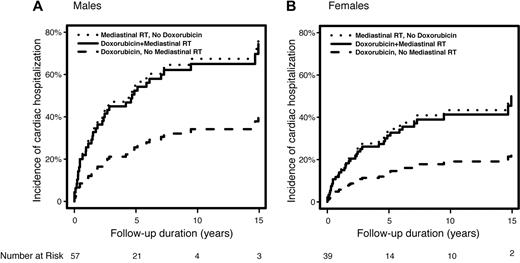
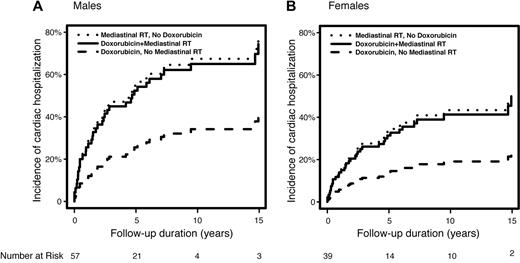
Among those with prior cardiac disease, the median time from HL diagnosis to cardiac admission was only 1.8 years (range, 1 month to 14.9 years). MRT conferred a significantly higher risk of CH among those with preexisting cardiac disease than those without. Among patients with preexisting cardiac disease, MRT plus doxorubicin was associated with a significantly higher risk of CH compared with doxorubicin-based chemotherapy alone (HR = 2.51, P = .036); in addition, MRT without chemotherapy was associated with a significantly higher risk than doxorubicin-based chemotherapy alone (HR = 2.70, P = .025). The predicted 10-year cumulative incidence of CH among patients with preexisting cardiac disease (modeled age 60 years at HL) for males receiving doxorubicin-based chemotherapy without MRT, MRT alone, or both, are 34%, 67%, and 65%, respectively (Figure 1A). For females age 60 years at diagnosis, these rates are 19%, 43%, and 41%, respectively (Figure 1B). Adjusting for age (ie, modeled age 60 years) and cardiac risk factors (present), the predicted 10-year cumulative incidence of CH for males without preexisting cardiac disease were: doxorubicin, no MRT = 24.3%, MRT alone = 13.5%, and both = 33.1%. For females, these estimates were 16.8%, 9.2%, and 23.4%, respectively.
Predicted cumulative incidence of post-Hodgkin lymphoma cardiac hospitalization according to treatment type. (A) Men diagnosed at age 60 years and with preexisting heart disease. (B) Women diagnosed at age 60 years and with preexisting heart disease.
Predicted cumulative incidence of post-Hodgkin lymphoma cardiac hospitalization according to treatment type. (A) Men diagnosed at age 60 years and with preexisting heart disease. (B) Women diagnosed at age 60 years and with preexisting heart disease.
Improvements in the disease-free and overall survival of HL patients are in part the result of the adoption of doxorubicin-based chemotherapy in the treatment of the disease.7-9 However, doxorubicin is associated with dose-dependent cardiotoxicity.10-13 MRT is also associated with cardiac toxicity,14-19 and the combination of these treatments may produce additive or supra-additive cardiac effects.1,5,20 This creates significant challenges when treating patients with preexisting cardiac disease because reduction in treatment intensity may reduce the chance of cure.
To our knowledge, no prior study has described the incidence of late cardiac morbidity associated with doxorubicin-based treatment among HL patients with preexisting cardiac disease. We found that these patients experienced a very high risk of subsequent CH after HL treatment, particularly after MRT either alone or in combination with doxorubicin-containing chemotherapy.
Although our findings suggest that avoidance of MRT, rather than reduction of doxorubicin exposure, may provide the least risk of significant cardiotoxicity among patients with heart disease, this conclusion must be taken with caution. Treatments are nonrandomly assigned with respect to HL prognosis, and are significantly associated with the risk of competing causes of death and therefore the probability of living to develop a cardiac complication. For example, among patients with preexisting cardiac disease, those treated with doxorubicin without MRT were significantly more likely to have stage III/IV disease than those treated with MRT (37% vs 6%; P = .002) and were also significantly (P = .032) more likely to experience noncardiac death. Consequently, the lower risk of CH in this group represents in part the greater influence of competing causes of death, rather than the lesser cardiotoxicity of doxorubicin per se. Even so, our findings are consistent with in vivo experiments showing that radiation causes the influx of inflammatory cells into atherosclerotic plaques, and can precipitate intraplaque hemorrhage,21-23 possibly explaining in part the rapid increase in the risk of CH after MRT among patients with preexisting cardiac disease in this study.
The sample size did not permit meaningful analysis of dose-response effects in patients with preexisting cardiac disease, and we did not have data to evaluate the cardiac ejection fractions before treatment. However, given these limitations, our findings show that patients with prior cardiac disease had a very high risk of CH after treatment for HL. The impact of different treatments was significantly different for these patients than for the majority of patients who had no preexisting cardiac disease, and was increased to a greater extent with the use of MRT than the use of doxorubicin-based chemotherapy. Our results suggest that efforts to reduce the cardiac exposure to radiation in patients with preexisting cardiac disease are warranted and that they should have careful monitoring and adequate treatment of their cardiac risk factors and disease after HL treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the collaborators at the participating institutions for their support and Drs Richard Hill, Bradley Wouters, and Fei-Fei Liu for their helpful suggestions in the preparation of the manuscript.
This work was supported by the Canadian Institutes of Health Research (grant MOP 77515). D.C.H. is supported by a Research Chair from Cancer Care Ontario.
Authorship
Contribution: S.M., M.P., and D.C.H. performed study design and data analysis and interpretation; L.Y. generated datasets; M.P. provided statistical analysis and generated figures; S.M. and D.C.H. prepared the manuscript; and M.P., L.Y., M.C., R.W.T., R.M.M., J.S., and E.Y. provided data analysis and manuscript review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David C. Hodgson, Princess Margaret Hospital, 610 University Ave, Toronto, ON M5G 2M9, Canada; e-mail: david.hodgson@rmp.uhn.on.ca.