Abstract
Streptococcus pyogenes is a significant bacterial pathogen in humans. In this study, histidine-rich glycoprotein (HRG), an abundant plasma protein, was found to kill S pyogenes. Furthermore, S pyogenes grew more efficiently in HRG-deficient plasma, and clots formed in this plasma were significantly less effective at bacterial entrapment and killing. HRG-deficient mice were strikingly more susceptible to S pyogenes infection. These animals failed to control the infection at the local subcutaneous site, and abscess formation and inflammation were diminished compared with control animals. As a result, bacterial dissemination occurred more rapidly in HRG-deficient mice, and they died earlier and with a significantly higher mortality rate than control animals. HRG-deficient mice supplemented with purified HRG gave the same phenotype as control animals, demonstrating that the lack of HRG was responsible for the increased susceptibility. The results demonstrate a previously unappreciated role for HRG as a regulator of inflammation and in the defense at the local site of bacterial infection.
Introduction
Histidine-rich glycoprotein (HRG) is an abundant plasma protein synthesized in the liver and also stored in the α-granules of platelets, from which it is released on activation.1 The protein has a diverse array of ligands, including heparin, plasminogen, immunoglobulin, thrombospondin, and fibrinogen.2 The diversity of ligands probably contributes to the fact that new activities are regularly attributed to HRG in vitro, whereas a definitive biologic function in vivo has been difficult to identify. Based on in vitro studies, HRG has been reported to modulate angiogenesis,3 phagocytosis,4 and complement function5 and to be antibacterial.6 HRG is negatively charged at physiologic pH but becomes positively charged at low pH or in the presence of zinc. Several the reported biologic effects have also been shown to be dependent on pH or zinc, suggesting that HRG may be a sensitive adaptor molecule to changing environments.7
The generation of HRG-knockout mice has facilitated the search for the biologic function of HRG. These animals were viable with no apparent abnormalities, but ex vivo studies demonstrated a mild dysregulation of hemostasis with effects on plasma-clotting factors and platelets, and in vivo HRG seemed to have both anticoagulant and antifibrinolytic properties.8 Recently, it has been shown that HRG-deficient animals have diminished antifungal activity in vivo.9 In the present study, we have assessed the role of HRG in response to a pathologic situation (ie, bacterial infection). Streptococcus pyogenes is a significant human bacterial pathogen responsible for both superficial and invasive infection. The pathogenesis of S pyogenes infection is a complex multifactorial process, and diverse host systems are involved.10-12 For instance, S pyogenes can counteract phagocytosis, complement activity, antibody recognition, and antimicrobial peptides. S pyogenes infection also involves important interactions with the host hemostasis system, and the bacteria have been shown to bind to and modulate the function of several important factors involved in coagulation and fibrinolysis: fibrinogen,13,14 coagulation factors,15 plasminogen,16,17 and platelets.18 The role of HRG in hemostasis in combination with the recently reported antibacterial effects of HRG in vitro prompted us to investigate the importance of HRG during S pyogenes infection. The results reveal a novel and important protective role for HRG in the innate immune defense against S pyogenes infection.
Methods
Proteins, peptide, antiserum, and bacteria
Human and mouse HRG was purified from plasma as previously described.6 Streptococcal Inhibitor of Complement (SIC) was purified from Escherichia coli as previously described.19 The peptide GHH20 (GHHPHGHHPHGHHPHGHHPH) and rabbit antiserum against GHH20 were purchased from Innovagen. The AP1 strain of S pyogenes (40/58 strain from the WHO Collaborating Center for references and research on Streptococci, Institute of Hygiene and Epidemiology, Prague, Czech Republic) was used throughout the study. SIC from S pyogenes AP1 was purified as previously described.19
Animals
C57/BL6 HRG−/− mice were supplied by Dr W. Jahnen-Dechent, University Hospital, Aachen, Germany.8 These animals lack the translation start point of exon 1 of the hrg gene. The original knockout mice 129/B6-HRGtm1wja1 were crossed with C57/BL6 mice (Taconic Farms) for 14 generations to obtain a uniform genetic background. Wild-type C57/BL6 mice and HRG-deficient C57/BL6 HRG−/− mice were bred in the animal facility at Lund University. The animals were housed under standard conditions of light and temperature and fed laboratory chow and water ad libitum. Experiments were carried out when the mice were 9 to 12 weeks old, and all procedures were approved by the local ethics committee (Lund University, Lund, Sweden).
Antibacterial assays
S pyogenes was grown overnight in Todd Hewitt broth in the presence of 5% CO2 at 37°C. An aliquot of these cells was added to fresh media and grown up to the exponential phase of growth (OD620 = 0.4). The cells were washed twice with wash buffer (10mM Tris-HCl containing 5mM glucose, pH 7.4, or 10mM 2-(N-morpholino)ethanesulfonic acid [MES] containing 5mM glucose, pH 5.5) and diluted to 2 × 106/mL in wash buffer. Bacterial cells were incubated with wash buffer containing purified human HRG, mouse HRG, or GHH20 peptide for 1 hour at 37°C, 5% CO2. The reaction was stopped on addition of 450 μL of ice-cold wash buffer. Serial titrations of duplicate samples were inoculated onto Todd Hewitt agar plates and incubated overnight to determine the amount of viable bacteria in the supernatant. For inhibition studies, purified SIC (0-1.3μM) was added to the antibacterial assay at the same time as HRG or GHH20.
The antibacterial effect of plasma clots was determined ex vivo. Citrated blood samples were collected from wild-type or HRG-deficient mice, and plasma was prepared. Addition of thrombin (1 U/mL) resulted in stable clot formation. The clot was washed once with wash buffer (10mM MES, pH 5.5, containing 5mM glucose), and 0.05 g of clot was incubated with S pyogenes for 2 hours at 37°C, 5% CO2. The reaction was stopped on addition of 450 μL of ice-cold wash buffer. Serial titrations of duplicate samples were inoculated onto Todd Hewitt agar plates and incubated overnight to determine the amount of viable bacteria in the supernatant.
Bacterial growth in plasma was determined in wild-type, HRG-deficient plasma, or HRG-deficient plasma reconstituted with purified HRG (200 μg/mL). A total of 10 μL of S pyogenes from the exponential phase of growth (diluted in 10mM MES, pH 5.5, containing 5mM glucose) was added to 50 μL of buffer containing 1% to 100% plasma (the pH of the mixture was 6.5-7.0) and grown at 37°C, 5% CO2. An aliquot of each sample was removed after 0, 2, 4, and 6 hours of growth, and the bacterial growth was determined by serial titration onto blood agar plates. A multiplication factor was calculated for each time point by dividing the bacterial count after 2, 4, and 6 hours by the bacterial count at time 0.
Negative staining and transmission electron microscopy of bacteria
S pyogenes cells were washed twice with wash buffer (10mM Tris-HCl containing 5mM glucose, pH 7.4, or 10mM MES containing 5mM glucose, pH 5.5) and diluted to 2 × 109/mL in wash buffer. A total of 10 μL of bacterial cells was incubated with wash buffer or purified human HRG (5μM) for 1 hour at 37°C, 5% CO2. Samples were absorbed onto carbon-coated copper grids, negatively stained with 0.75% uranyl formate, and analyzed in a Jeol JEM 1230 electron microscope operated at 80 kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 charge-coupled device (CCD) camera (Gatan Inc).
Electron microscopy of plasma clots
Plasma clot morphology in the absence or presence of S pyogenes was determined using scanning electron microscopy. A total of 50 μL of plasma from wild-type, HRG-deficient, HRG-deficient plus purified human HRG (200 μg/mL), or wild-type plus SIC (3.3μM) was incubated for 1 minute with S pyogenes AP1 (4 × 109/mL) in 13mM sodium citrate, pH 7.4, or buffer alone. Clot formation was initiated on addition of 50 μL Thrombomax reagent (Trinity Biotech). The final clot was immersed in 1 mL of fixation fluid (2.5% glutaraldehyde in 0.15M sodium cacodylate, pH 7.4) and incubated overnight. The samples were washed with cacodylate buffer, dehydrated, critical point dried, sputtered with 30-nm gold, and analyzed using a Jeol J-330 scanning electron microscope operated at an accelerating voltage of 5 kV, working distance of 10 minutes, and a magnification of 300× to 2500×. Images were acquired using a Gatan Multiscan 791 CCD camera.
The clot formed by wild-type plasma in the presence of S pyogenes AP1 (4 × 109/mL) was thin sectioned, placed on grids, and incubated with primary antibodies against HRG, followed by secondary antibodies against rabbit IgG labeled with 5-nm gold beads. Samples were stained with uranyl acetate and lead citrate and analyzed using a Jeol JEM 1230 electron microscope operated at 80 kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 CCD.
Animal model of S pyogenes infection
Wild-type and HRG-deficient mice were divided into age- and sex-matched groups and subjected to a subcutaneous infection model. S pyogenes was grown overnight in Todd Hewitt broth at 37°C in the presence of 5% CO2. An aliquot of these cells was added to fresh media and grown up to OD620 = 0.5. Bacteria (1 × 107) were administered by subcutaneous injection into the scruff of the neck. The animals were closely monitored for signs and symptoms of infection and weighed on a daily basis.
To determine bacterial dissemination from the local site of administration, 2 groups of animals were killed 48 hours after initiation of infection. The spleen, kidney, and a citrated blood sample were removed, and the bacterial load was determined by viable count determination on blood agar plates incubated overnight. The plasma interleukin-6 (IL-6) and IL-10 levels in plasma were determined using an ELISA kit (Invitrogen) according to the manufacturer's instructions.
Histopathology of mouse tissue samples
The local tissue sites of bacterial injection were excised and fixed in formalin 2 days after infection. The tissue samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin using standard methods. Immunocytochemistry was used to localize S pyogenes bacteria, neutrophils, and macrophages using the following primary antisera in respective optimal working dilutions: goat anti–streptococcus A for S pyogenes (code 8435-0004, AbD Serotec, 1:4000), rat anti–mouse Ly6 for neutrophils (code 16-5931, eBioscience, 1:3000), and goat anti–mouse CD14 for macrophages (code GAY016071, R&D Systems, 1:6000), goat anti–MIP-2 (code UR04, R&D Systems, 1:6000), and goat anti-KC (code VF04, R&D Systems, 1:200). Paraffin sections were deparaffinized and dehydrated and further processed for visualization of the immunoreactive products. Vectastain ABC-kits (Vector, ImmunKemi) were used for rat anti–mouse Ly6 and goat anti–mouse CD14, KC, and MIP-2 antisera, and EnVision Kit (Dako) was used for goat anti–streptococcus A antiserum. In control experiments, no specific immunoreactivities were obtained when the respective primary antiserum was used. Slides were viewed with a Nikon ECLIPSE 80 microscope using a Plan APO 10×/0.75 DIC N2 lens. Images were acquired using NIS Elements BR imaging software Version 2.33 (Nikon).
Ex vivo chemotaxis assay
A dorsal portion of mouse skin was removed from wild-type or HRG-deficient animals and cut into small pieces to induce an injury response. These epidermal keratinocytes were cultured for 4 days as previously described.20 The release of chemotactic factors from the cultured epidermal keratinocytes was assessed in a neutrophil chemotaxis assay.20 Purified neutrophils were placed in a microchemotaxis chamber at 37°C, and directed migration toward the keratinocyte media was measured. All assays were performed in duplicate for a total of 3 animals per group.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 4 for Macintosh, Version 4.0C software.
Results
HRG and HRG-containing clots exhibit a pH-dependent antibacterial effect on S pyogenes
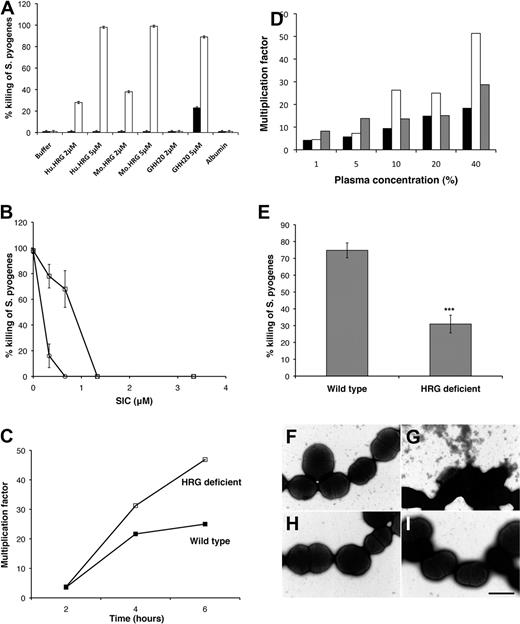
The antibacterial effect of HRG on S pyogenes (the AP1 strain of the M1 serotype was used throughout this study) was determined in a fluid phase assay. The purity of the isolated HRG was confirmed by silver staining, which generated a dominant band at 70 kDa corresponding to the predicted molecular mass of HRG (data not shown). S pyogenes was not affected by human or mouse HRG when the assay was performed at pH 7.4 (Figure 1A). When the pH was lowered to pH 5.5, S pyogenes was rapidly and effectively killed by purified human or mouse HRG (Figure 1A). Incubations at pH 5.5 alone did not affect bacterial viability. The histidine-rich region of HRG contains tandem repeats of the sequence GHHPH, and the antibacterial effect of HRG has previously been mapped to this region.6 The GHH20 peptide, containing 5 GHHPH repeats, was found to have an equally potent antibacterial effect on S pyogenes as the whole native protein at low pH (Figure 1A). The dominant plasma protein, albumin (10 mg/mL), did not affect bacterial survival (Figure 1A). S pyogenes produces an array of virulence factors, including SIC, which has previously been reported to bind to plasma HRG19 and to neutralize killing of S pyogenes by several human antimicrobial peptides and proteins.21,22 We demonstrate here that SIC also inhibits killing of S pyogenes by HRG and GHH20 in a dose-dependent manner (Figure 1B).
HRG kills S pyogenes and killing occurs in HRG-containing plasma and HRG-containing clots. (A) S pyogenes in Tris buffer, pH 7.4 (black bars) or MES buffer, pH 5.5 (white bars), was incubated with purified human HRG (Hu.HRG), mouse HRG (Mo.HRG), the HRG-derived peptide GHH20, or albumin, and the percentage killing was calculated. Data are mean ± SEM; n = 3. (B) S pyogenes in MES buffer, pH 5.5, was incubated with 5μM human HRG (squares) or GHH20 (circles) in the presence of an increasing concentration of purified SIC. Data are mean ± SEM; n = 3. (C) S pyogenes in MES buffer, pH 5.5, was added to wild-type or HRG-deficient plasma (final pH 6.5-7.0), and growth was determined over time. The data shown are representative of 5 experiments, which all gave the same profile of results. (D) S pyogenes in MES buffer, pH 5.5, was added to increasing concentrations of plasma diluted in MES buffer: wild-type plasma (black bars), HRG-deficient plasma (white bars), or HRG-deficient plasma reconstituted with purified HRG (gray bars). Growth was determined after 6 hours. Data are representative of 3 experiments, which all gave the same profile of results. (E) Thrombin (1 U/mL) was used to initiate clot formation in wild-type and HRG-deficient plasma. The washed clots were incubated with S pyogenes bacteria in MES buffer, pH 5.5, and the percentage killing was calculated. Data are mean ± SEM; n = 8. ***P < .001 (Student t test). (F-I) S pyogenes in MES buffer, pH 5.5 (F-G) or Tris buffer, pH 7.4 (H-I) was incubated with purified human HRG (5μM) and subjected to negative staining and transmission electron microscopy.
HRG kills S pyogenes and killing occurs in HRG-containing plasma and HRG-containing clots. (A) S pyogenes in Tris buffer, pH 7.4 (black bars) or MES buffer, pH 5.5 (white bars), was incubated with purified human HRG (Hu.HRG), mouse HRG (Mo.HRG), the HRG-derived peptide GHH20, or albumin, and the percentage killing was calculated. Data are mean ± SEM; n = 3. (B) S pyogenes in MES buffer, pH 5.5, was incubated with 5μM human HRG (squares) or GHH20 (circles) in the presence of an increasing concentration of purified SIC. Data are mean ± SEM; n = 3. (C) S pyogenes in MES buffer, pH 5.5, was added to wild-type or HRG-deficient plasma (final pH 6.5-7.0), and growth was determined over time. The data shown are representative of 5 experiments, which all gave the same profile of results. (D) S pyogenes in MES buffer, pH 5.5, was added to increasing concentrations of plasma diluted in MES buffer: wild-type plasma (black bars), HRG-deficient plasma (white bars), or HRG-deficient plasma reconstituted with purified HRG (gray bars). Growth was determined after 6 hours. Data are representative of 3 experiments, which all gave the same profile of results. (E) Thrombin (1 U/mL) was used to initiate clot formation in wild-type and HRG-deficient plasma. The washed clots were incubated with S pyogenes bacteria in MES buffer, pH 5.5, and the percentage killing was calculated. Data are mean ± SEM; n = 8. ***P < .001 (Student t test). (F-I) S pyogenes in MES buffer, pH 5.5 (F-G) or Tris buffer, pH 7.4 (H-I) was incubated with purified human HRG (5μM) and subjected to negative staining and transmission electron microscopy.
The antibacterial effect of HRG in a plasma environment was demonstrated by the ability of S pyogenes to grow more efficiently in HRG-deficient plasma (Figure 1C). At an acute inflammatory site, plasma leakage occurs and plasma will be present at various concentrations; therefore, a dose-response experiment was carried out at plasma concentrations from 1% to 40%. Increasing concentrations of plasma-enhanced bacterial growth in buffer, and this is not surprising because plasma is rich in nutrients. At low plasma concentrations, S pyogenes grew equally well in wild-type and HRG-deficient plasma (Figure 1D). When plasma was present at levels of 10% to 40%, bacteria grew more rapidly in HRG-deficient plasma (Figure 1D). The contribution of HRG to this growth advantage was confirmed by the finding that reconstitution of purified HRG to HRG-deficient plasma, significantly inhibited bacterial growth (Figure 1D).
At a site of acute inflammation and bacterial infection, the coagulation system will be activated and clot formation will occur.23 HRG has previously been reported to be accumulated at the surface of plasma clots,24 and the antibacterial effect of HRG in plasma clots was therefore investigated. Washed, thrombin-induced clots derived from HRG-deficient plasma were significantly less effective at killing S pyogenes compared with clots from normal plasma (Figure 1E).
To confirm that HRG mediates bacterial killing, bacterial morphology was analyzed using negative staining and transmission electron microscopy. S pyogenes AP1 appear as intact cocci in wash buffer at pH 7.4 (Figure 1H), and addition of purified human HRG (5μM) has no effect at this pH (Figure 1I). In the presence of wash buffer at pH 5.5, the bacteria maintain a normal appearance (Figure 1F). However, on addition of purified human HRG (5μM) at pH 5.5, the bacterial morphology is significantly compromised (Figure 1G). The membrane is no longer intact, the cells appear shrunken, and cytoplasmic content has leaked out of the cells.
Clot architecture in normal or HRG-deficient plasma in the presence of S pyogenes
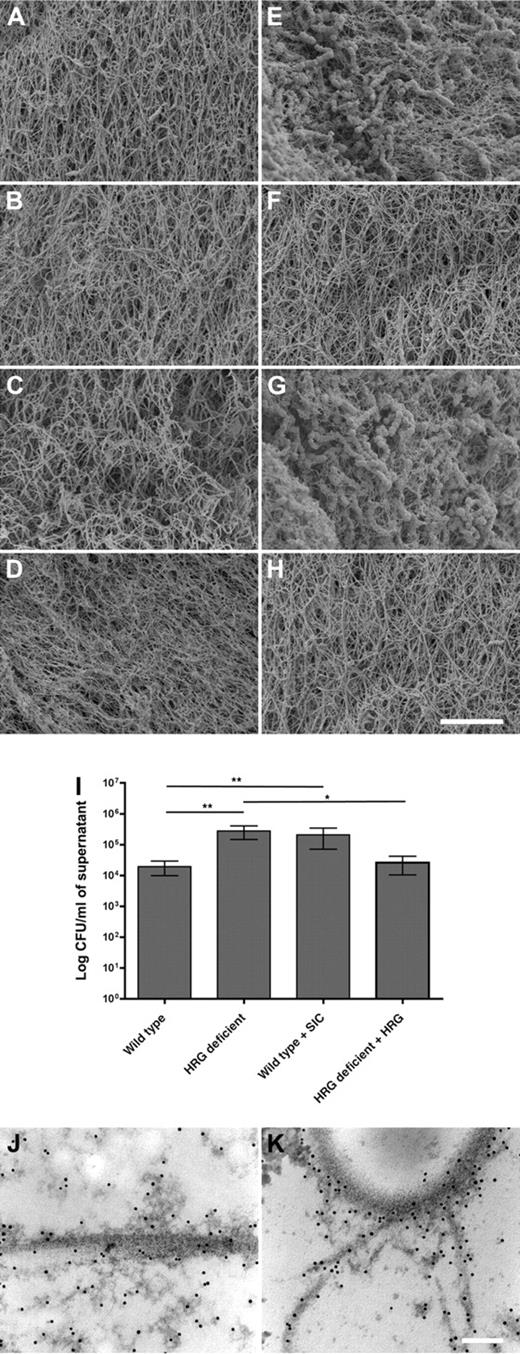
Plasma samples from wild-type or HRG-deficient animals were clotted with thrombin in either the presence or absence of S pyogenes, and scanning electron microscopy was used to study the clot morphology. A normal fibrin network was formed in plasma derived from both wild-type and HRG-deficient animals (Figure 2A), and there was no obvious difference in the fibrin network in the HRG-deficient plasma with or without HRG reconstitution (Figure 2B and Figure 2C, respectively), or in normal plasma on addition of the HRG-binding SIC protein (Figure 2D). It has previously been reported that S pyogenes infiltrates the clot and dysregulates the fibrin network,15 and this was confirmed here (Figure 2E). There is substantial bacterial infiltration of the clot, and the fibrin network is massively disrupted. This is in sharp contrast to the bacterial interaction with plasma clots from HRG-deficient animals or clots from normal plasma treated with SIC. In both cases, there is dramatically less bacterial infiltration (Figure 2F and Figure 2H, respectively). Importantly, reconstitution of HRG-deficient plasma with purified HRG restored the wild-type phenotype (Figure 2G). In this reconstituted plasma, the bacteria have once again infiltrated the clot and are highly associated with the clot surface. We conclude that the bacteria fail to interact with HRG-deficient clots. To confirm the absence of bacteria from the clots, we performed viable count assays from the supernatant of the clotted samples. The supernatant from HRG-deficient clots and SIC-treated wild-type clots contains significantly more viable bacteria than wild-type clots (Figure 2I, P = .002 and P = .001, respectively). The relative importance of HRG for entrapment of bacteria in the clot was assessed in wild-type plasma in the presence of S pyogenes. The clot formed was cut into thin sections and probed with antibodies against HRG, followed by gold-labeled secondary antibodies and analysis by transmission electron microscopy. Typical electron dense fibrin threads and fibrin clusters are evident, and the black dots represent sites where HRG is localized (Figure 2J). One large fibrin fibril is shown in Figure 2J, and HRG is closely associated with this fibrin thread. HRG is also associated with the electron-dense fibrin clusters that surround the fibrin thread. In Figure 2K, the cell wall of a bacterium is shown in the top of the picture. Fibrin threads and HRG are colocalized at the bacterial surface (Figure 2K). Importantly, HRG is present on the bacterial surface and at the interface between bacteria and fibrin. It is noteworthy that HRG is only identified in association with fibrin or bacteria. These results demonstrate that HRG is anchored on the fibrin network and mediates entrapment of bacteria in the fibrin clot.
Clots formed in HRG-deficient or SIC-treated plasma fail to contain S pyogenes bacteria. Washed S pyogenes (E-H) or phosphate-buffered saline (A-D) was added to plasma, and clot formation was initiated by addition of Thrombomax reagent to wild-type (A,E), HRG-deficient (B,F), HRG-deficient plus purified HRG (C,G), or SIC-treated wild-type plasma (D,H). The fibrin network formed in the absence or presence of bacteria was analyzed using scanning electron microscopy. Scale bar represents 5 μm. Representative images of 4 independent experiments are shown. (I) The amount of bacteria present in the supernatant of clots A to H was quantified. Data are mean ± SEM; n = 4. **P = .001; *P = .02 (Mann-Whitney test). (J-K) The clot formed in wild-type plasma in the presence of S pyogenes was thin-sectioned, immunolabeled with anti-HRG, gold-labeled secondary antibodies (black dots), and subjected to transmission electron microscopy.
Clots formed in HRG-deficient or SIC-treated plasma fail to contain S pyogenes bacteria. Washed S pyogenes (E-H) or phosphate-buffered saline (A-D) was added to plasma, and clot formation was initiated by addition of Thrombomax reagent to wild-type (A,E), HRG-deficient (B,F), HRG-deficient plus purified HRG (C,G), or SIC-treated wild-type plasma (D,H). The fibrin network formed in the absence or presence of bacteria was analyzed using scanning electron microscopy. Scale bar represents 5 μm. Representative images of 4 independent experiments are shown. (I) The amount of bacteria present in the supernatant of clots A to H was quantified. Data are mean ± SEM; n = 4. **P = .001; *P = .02 (Mann-Whitney test). (J-K) The clot formed in wild-type plasma in the presence of S pyogenes was thin-sectioned, immunolabeled with anti-HRG, gold-labeled secondary antibodies (black dots), and subjected to transmission electron microscopy.
HRG-deficient mice are more susceptible to S pyogenes infection
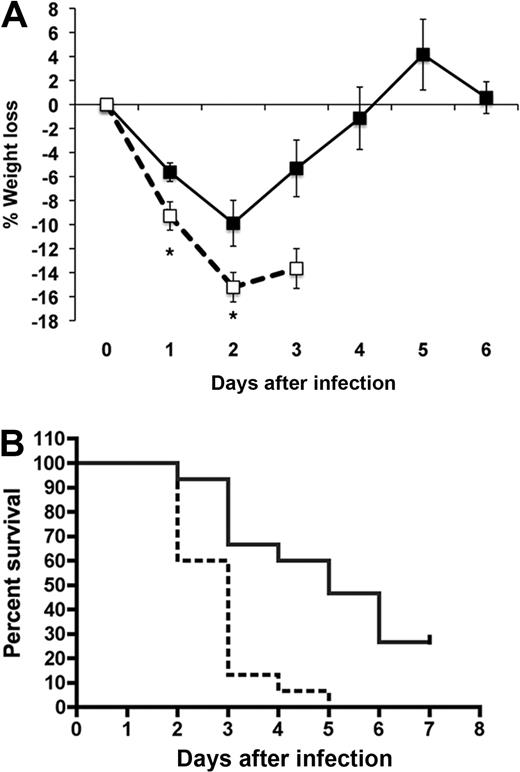
Wild-type and HRG-deficient mice (age- and sex-matched) were inoculated with S pyogenes by subcutaneous injection. Four individual experiments were performed, and the data were pooled. The survival of the mice in the 2 groups (n = 15 per group) over time is illustrated in Figure 3B. The HRG-deficient animals exhibited significantly increased mortality in response to S pyogenes infection (Figure 3B; P < .001). The median survival time for wild-type animals was 5 days, whereas the HRG-deficient animals had a median survival time of 3 days. On day 2 after infection, 67% of wild-type animals had survived, whereas only 13% of HRG-deficient animals had survived at this time point. On day 1 and 2 after infection, the HRG-deficient animals exhibited significantly increased weight loss compared with wild-type animals (P = .015 and P = .03, respectively; Figure 3A). On day 3, the difference in weight loss between the 2 groups of animals did not achieve statistical significance, explained by the fact that only 3 HRG-deficient animals survived to this time point. Moreover, 27% (4 of 15) of the wild-type animals survived infection and recovered, whereas none of the HRG-deficient animals survived infection. We conclude that the inability to produce HRG resulted in a more rapid bacterial dissemination and increased overall mortality.
HRG-deficient animals have significantly increased mortality in response to S pyogenes infection. S pyogenes (1 × 107 CFU) was administered by subcutaneous injection to wild-type (full lines) or HRG-deficient animals (dashed lines) (n = 15 per group). (A) The surviving animals were weighed on a daily basis, and the percentage weight loss was calculated for each animal. Data are mean ± SEM; n = 14: * (Left) P = .01 and * (Right) P = .03, respectively. (B) The percentage survival per group was determined on a daily basis and is represented in a Kaplan-Meier survival curve.
HRG-deficient animals have significantly increased mortality in response to S pyogenes infection. S pyogenes (1 × 107 CFU) was administered by subcutaneous injection to wild-type (full lines) or HRG-deficient animals (dashed lines) (n = 15 per group). (A) The surviving animals were weighed on a daily basis, and the percentage weight loss was calculated for each animal. Data are mean ± SEM; n = 14: * (Left) P = .01 and * (Right) P = .03, respectively. (B) The percentage survival per group was determined on a daily basis and is represented in a Kaplan-Meier survival curve.
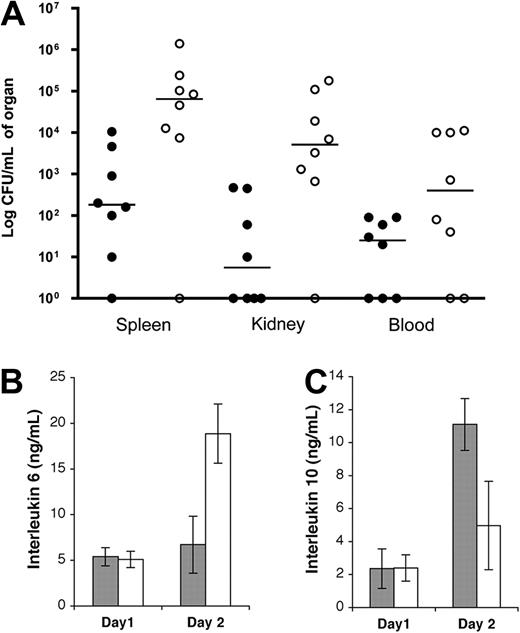
To determine the cause of death in these animals, dissemination of infection was determined 2 days after initiation of infection. Two individual experiments were performed, and the data were pooled. Mice alive at this time point formed the 2 groups, and the HRG-deficient animals had significantly higher bacterial load in the spleen, kidneys, and blood, compared with the wild-type animals (Figure 4A; P = .01, .005, and .05, respectively), demonstrating that bacteria spread more rapidly from the local site in HRG-deficient mice. The systemic immune response was investigated by determining plasma levels of the proinflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10. On day 2, the HRG-deficient animals had a massive proinflammatory response (IL-6) and a low anti-inflammatory response (IL-10) (Figure 4B-C). This was in stark contrast to the wild-type animals, which had relatively low IL-6 and slightly elevated IL-10 compared with HRG-deficient animals. The massive proinflammatory response seen in HRG-deficient animals reflects the high bacteria load seen at the same time point in these animals (Figure 4A). Samples taken from wild-type animals, which died of infection, also exhibited elevated IL-6 and diminished IL-10 (data not shown). We can therefore conclude that both groups of animals mount a systemic immune response to the bacteria; however, this response occurs more rapidly in HRG-deficient animals.
Bacterial dissemination and plasma IL-6 levels are significantly increased in HRG-deficient animals early during S pyogenes infection. Wild-type (●) or HRG-deficient animals (○) were infected with S pyogenes AP1 (1 × 107 CFU) by subcutaneous injection. (A) Two days after infection, surviving animals were killed, and the bacterial load in the spleen, kidney, and blood was determined. Data are expressed as CFU/mL of organ from each animal with lines drawn at the median for each group (n = 8). (B-C) IL-6 and IL-10 levels were determined in plasma samples from wild-type (gray bars) or HRG-deficient animals (white bars) taken one and 2 days after infection. Animals alive at these time points were included from both groups. Data are mean ± SEM; n = 7.
Bacterial dissemination and plasma IL-6 levels are significantly increased in HRG-deficient animals early during S pyogenes infection. Wild-type (●) or HRG-deficient animals (○) were infected with S pyogenes AP1 (1 × 107 CFU) by subcutaneous injection. (A) Two days after infection, surviving animals were killed, and the bacterial load in the spleen, kidney, and blood was determined. Data are expressed as CFU/mL of organ from each animal with lines drawn at the median for each group (n = 8). (B-C) IL-6 and IL-10 levels were determined in plasma samples from wild-type (gray bars) or HRG-deficient animals (white bars) taken one and 2 days after infection. Animals alive at these time points were included from both groups. Data are mean ± SEM; n = 7.
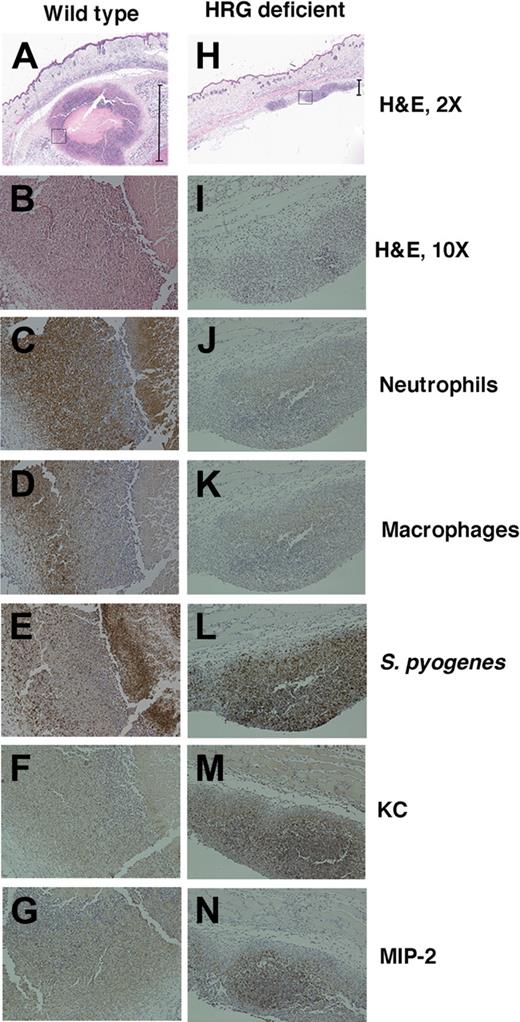
To investigate the local site of infection further, tissue samples were harvested from this area 2 days after administration of bacteria. At this time point, the wild-type animals showed a large abscess at the local site of infection. Microscopically, these abscesses contained a central area of necrosis clearly walled off from the remaining tissue (Figure 5A). HRG-deficient animals did not develop a visible abscess, and no abscess formation was observed in the infected area when analyzed by light microscopy (Figure 5H). Both groups of mice showed signs of a proinflammatory response with leukocyte infiltration. However, this was significantly less pronounced and more diffuse in HRG-deficient animals (Figure 5I). The size of the inflammatory foci was measured using NIS Elements BR Version 2.33 (Nikon) software for a total of 4 animals in each group. The average size of the inflammatory foci was 1125 plus or minus 240 μm in wild-type animals and 218 plus or minus 39 μm in HRG-deficient animals (P = .009). S pyogenes could be demonstrated in the infected tissue using a specific antibody for the bacteria. In wild-type animals, bacteria were localized to the center and the wall of the abscess and were contained in this area (Figure 5E). Fewer bacteria were present in the HRG-deficient animals, and these bacteria were dispersed throughout the inflamed tissue (Figure 5L). The infiltrating leukocytes were differentiated using immunostaining. The dominating cell type was neutrophils, and a massive influx of these cells occurred in wild-type animals, together with a smaller number of macrophages (Figure 5C-D). The HRG-deficient animals had significantly diminished neutrophil infiltration (Figure 5J), and macrophages were also diminished (Figure 5K). The local chemotaxis response was assessed by immunostaining for the chemokines KC and MIP-2. Both chemokines were present at the site of acute inflammation in wild-type (Figure 5F-G) and HRG-deficient animals (Figure 5M-N). HRG-deficient animals have a diminished chemokine response, correlated to the lower overall size of the inflammatory foci.
HRG-deficient animals fail to contain S pyogenes at the local site of infection. Tissue samples from the local site of infection from wild-type animals (A-G) and HRG-deficient animals (H-N) were hemotoxylin and eosin-stained and immunostained for inflammatory markers. Representative images are shown at 2× magnification (A,H), and the black bars define the size of the inflammatory focus that was quantified as 1375 μm in the wild-type animal and 217 μm in the HRG-deficient animal. (A,H) The black box represents the section of the inflammatory focus that is shown at 10 × magnification in all subsequent images stained for hemotoxylin and eosin (B,I), neutrophils (C,J), macrophages (D,K), S pyogenes (E,L), and the chemokines KC (F,M) and MIP-2 (G,N).
HRG-deficient animals fail to contain S pyogenes at the local site of infection. Tissue samples from the local site of infection from wild-type animals (A-G) and HRG-deficient animals (H-N) were hemotoxylin and eosin-stained and immunostained for inflammatory markers. Representative images are shown at 2× magnification (A,H), and the black bars define the size of the inflammatory focus that was quantified as 1375 μm in the wild-type animal and 217 μm in the HRG-deficient animal. (A,H) The black box represents the section of the inflammatory focus that is shown at 10 × magnification in all subsequent images stained for hemotoxylin and eosin (B,I), neutrophils (C,J), macrophages (D,K), S pyogenes (E,L), and the chemokines KC (F,M) and MIP-2 (G,N).
Treatment of HRG-deficient animals with purified HRG restores the wild-type phenotype
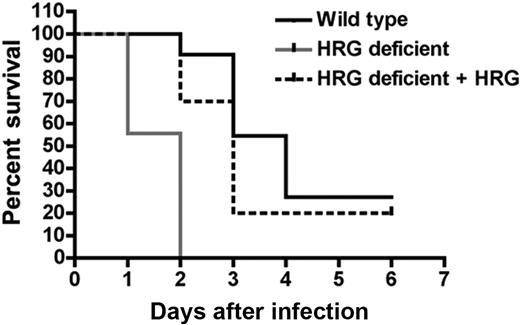
To confirm that the increased susceptibility of the HRG-deficient animals to S pyogenes infection was because of the absence of HRG, we reconstituted plasma HRG during infection. Two individual experiments were performed and the data were pooled. One group of HRG-deficient animals (n = 10) received purified human HRG (500 μg per animal) by intraperitoneal injection followed by a subcutaneous injection of S pyogenes. At 24 hours after infection, the mice received a second intraperitoneal injection of HRG (500 μg per animal). All of the untreated, HRG-deficient animals (n = 9) died of infection, and this group had a median survival of 2 days. The HRG-deficient animals that received HRG survived longer and had a median survival of 3 days. The wild-type animals (n = 11) had a median survival of 4 days (Figure 6). The difference in mortality was highly significant between HRG treated and untreated mice (P < .001). Twenty-seven percent of the wild-type and 20% of the HRG-treated mice survived the infection, whereas none of the untreated animals survived the infection.
Reconstitution of plasma HRG improves survival after bacterial challenge of HRG-deficient animals. Wild-type (n = 11) or HRG-deficient animals (n = 9) were infected with S pyogenes (1 × 107 CFU/animal) by subcutaneous injection. One group of HRG-deficient animals (n = 10) received an intraperitoneal injection of purified HRG (500 μg/animal) at the same time point as bacteria and again at 24 hours after infection. The percentage survival per group is represented in a Kaplan-Meier survival curve.
Reconstitution of plasma HRG improves survival after bacterial challenge of HRG-deficient animals. Wild-type (n = 11) or HRG-deficient animals (n = 9) were infected with S pyogenes (1 × 107 CFU/animal) by subcutaneous injection. One group of HRG-deficient animals (n = 10) received an intraperitoneal injection of purified HRG (500 μg/animal) at the same time point as bacteria and again at 24 hours after infection. The percentage survival per group is represented in a Kaplan-Meier survival curve.
The increased susceptibility of HRG-deficient animals to S pyogenes infection is determined at the local site of infection
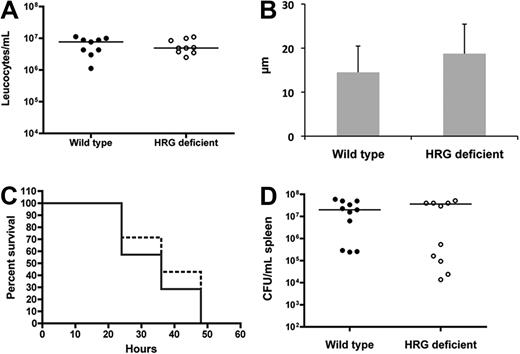
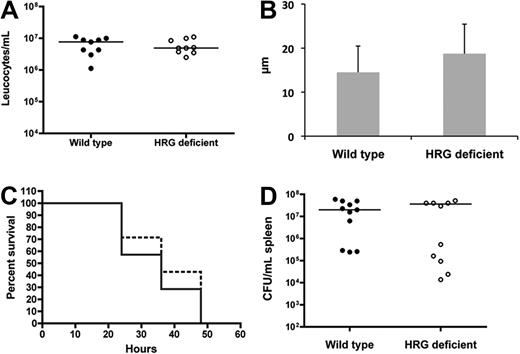
Histopathologic examination of the local site of infection revealed decreased inflammation, chemokine levels, and neutrophil recruitment in the HRG-deficient animals (Figure 5), which could be explained by defective chemotaxis. We therefore investigated the presence of a global chemotaxis defect in the HRG-deficient animals. S pyogenes (1 × 107 colony-forming units (CFU)/mL) was administered by intraperitoneal injection, and after 18 hours the peritoneum was washed and the leukocyte population was counted. There was a massive abdominal influx of leukocytes, and no difference was observed between wild-type and HRG-deficient animals (Figure 7A), indicating functional chemotaxis in both groups. We further assessed the local chemotaxis response in HRG-deficient animals by culturing keratinocytes from mice and performing a chemotaxis assay with the media from these cells. On injury, keratinocytes from both groups of animals produced equivalent levels of chemoattractants for neutrophils (Figure 7B, P = .25).
HRG-deficient animals do not have a systemic chemotaxis defect and are not more susceptible to systemic S pyogenes infection. (A) Wild-type or HRG-deficient animals (n = 9 per group) received an intraperitoneal injection with S pyogenes (1 × 107 CFU/animal). The animals were killed 18 hours later and leucocytes were counted in the peritoneal fluid. (B) Epidermal keratinocytes were isolated and cultured from wild-type and HRG-deficient animals. The media from these cells was tested in a neutrophil chemotaxis assay, and the migration was calculated. Data are representative of 3 experiments, which all gave the same profile of results. (C) Wild-type (unbroken lines) or HRG-deficient (broken lines) animals (n = 11 per group) received an intraperitoneal injection with S pyogenes (1 × 107 CFU/animal). Health status and survival were monitored and are represented in a Kaplan-Meier survival curve. (D) At the time of death the spleen was harvested, and bacterial load was determined for all animals.
HRG-deficient animals do not have a systemic chemotaxis defect and are not more susceptible to systemic S pyogenes infection. (A) Wild-type or HRG-deficient animals (n = 9 per group) received an intraperitoneal injection with S pyogenes (1 × 107 CFU/animal). The animals were killed 18 hours later and leucocytes were counted in the peritoneal fluid. (B) Epidermal keratinocytes were isolated and cultured from wild-type and HRG-deficient animals. The media from these cells was tested in a neutrophil chemotaxis assay, and the migration was calculated. Data are representative of 3 experiments, which all gave the same profile of results. (C) Wild-type (unbroken lines) or HRG-deficient (broken lines) animals (n = 11 per group) received an intraperitoneal injection with S pyogenes (1 × 107 CFU/animal). Health status and survival were monitored and are represented in a Kaplan-Meier survival curve. (D) At the time of death the spleen was harvested, and bacterial load was determined for all animals.
To investigate the importance of the subcutaneous local site for HRG function, we performed a systemic infection model. After intraperitoneal infection with S pyogenes, the bacteria use the rich vascular bed to rapidly enter the bloodstream; and in contrast to the subcutaneous model, a local infection focus is not formed. We monitored susceptibility to infection in these animals, and no difference in survival (Figure 7C) or bacterial dissemination (Figure 7D) was recorded between wild-type and HRG-deficient animals.
Discussion
The definitive role of HRG in human or animal biology has yet to be clearly defined. In the present study, we identify an important function for HRG in the host defense against S pyogenes infection. HRG-deficient animals failed to control streptococcal infection at the local site and died more rapidly of an overwhelming systemic infection. Our in vitro studies demonstrate that S pyogenes bacteria are susceptible to killing by HRG. Killing was optimal at low pH, which is in concordance with previously published results on the antibacterial effect of human HRG on Enterococcus spp.6 The inflammatory phase of wound healing is associated with ischemia and acidosis,25 and infected wounds have been shown to be even more acidic than noninfected wounds.26 Low pH and Zn enhance the antibacterial effect of HRG, which is relevant in relation to the present study. The microcirculation is severely disturbed in tissues invaded by S pyogenes resulting from thrombi formed by activated platelets.18 This will further lower the pH, and the activated platelets will release their zinc-containing α-granules27 together with HRG. Finally, we have previously shown that HRG is present in wound exudates at concentrations comparable with plasma.9 These different data underline that the conditions at the local site of infection will favor HRG-mediated bacterial killing.
In response to a subcutaneous injection of S pyogenes, a local inflammatory reaction will be initiated and abscess formation will contain the bacteria and protect against systemic infection.28 The HRG-deficient animals rapidly die of systemic infection (Figure 3) and reconstitution of HRG decreases the mortality in this group (Figure 6), therefore identifying HRG as a host defense factor. Histopathology revealed that the HRG-deficient animals initiate a diminished local inflammatory response with reduced neutrophil recruitment and local chemokine production. Significantly fewer bacteria remain at the local site, and the remaining bacteria escape the local site to disseminate to the blood and organs. Notably, these findings were specific to the subcutaneous infection model, and there was no effect for HRG when bacteria were administered intraperitoneally. This is not surprising because in the intraperitoneal model the bacteria use the rich vascular bed to rapidly enter the bloodstream and local abscess formation does not occur. The importance of the local subcutaneous site for the immunomodulatory effects of HRG was further confirmed when we investigated the systemic immune response after subcutaneous injection of S pyogenes. IL-6 is an important proinflammatory cytokine that has previously been reported to be elevated in murine29 and human30,31 invasive S pyogenes infection, whereas IL-10 has pleiotropic anti-inflammatory effects. On day 1 after infection, both groups of animals had low levels of IL-10 and IL-6, indicating that no significant immune response was initiated. This is not surprising because the bacteria do not emerge in the bloodstream until day 2. On day 2, the HRG-deficient animals exhibit massively elevated IL-6 and diminished IL-10. The wild-type animals die more slowly of infection, but at the time of death these animals have the same cytokine imbalance. Taken together, the data suggest that a systemic immune response is elicited in both groups of animals, and it is the kinetics of this response that differentiates the HRG-deficient animals. We conclude that the immunomodulatory effects of HRG are confined to the inflamed subcutaneous wound site.
HRG-deficient animals exhibit increased plasminogen activation and subsequent fibrinolysis.8 Therefore, the clots formed in these animals are subject to more rapid lysis than in wild-type animals. The importance of clot formation for defense against bacteria is supported by our ex vivo studies, where HRG-deficient animals failed to contain S pyogenes at the clot surface and the bacteria escaped into the supernatant (Figure 2). Furthermore, HRG binds to the fibrin threads in the clot and to the surface of the bacteria, thus directly trapping the bacteria (Figure 2J-K). Those bacteria that are not associated with the clot at the local site will also fail to be killed by HRG and will disseminate. Fibrin clot formation has previously been shown to play a critical role during S pyogenes infection in the same mouse model of subcutaneous infection used in this study.32,33 Significantly, Sun et al32 also showed that fibrin clot formation did not provide protection when the bacteria were administered directly into the bloodstream. This is analogous to the results observed in our intraperitoneal model. When we administered bacteria intraperitoneally, allowing a rapid vascular invasion, there was no difference in susceptibility between wild-type and HRG-deficient animals (Figure 7). This further emphasizes that the protective role of HRG is restricted to the local and confined subcutaneous site of infection.
It is well appreciated that S pyogenes uses a large number of molecular mechanisms to manipulate human host defenses, and we were surprised that the removal of a single plasma protein could have such striking consequences for pathogenesis. An important role for HRG in host defense against S pyogenes infection is also implied by the fact that S pyogenes has developed a virulence factor that binds and neutralizes HRG. SIC efficiently inhibited the effects of HRG in vitro. The importance of SIC for bacterial virulence is underlined by the finding that a SIC-negative strain of S pyogenes has reduced virulence in a mouse model of infection22 and that the SIC gene is found in all S pyogenes isolates of the M1 serotype, a prominent serotype in invasive infection.34
HRG has previously been shown to have antibacterial activity in vitro against several different bacterial pathogens, including both Gram-positive and Gram-negative species.6 Here we find that also S pyogenes is susceptible to HRG, both in vitro and in vivo, in a pH environment highly relevant to deep skin infections caused by this important human pathogen. In addition, the present data demonstrate that clot formation is crucial for bacterial entrapment and killing and that HRG plays an essential role in this defense mechanism.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ingbritt Gustafsson and Maria Baumgarten for excellent technical assistance.
This work was supported in part by the foundations of Ragnar and Torsten Söderberg, Crafoord, Greta and Johan Kock, Alfred Österlund, and Thelma Zoéga, the Royal Physiographic Society in Lund, Hansa Medical AB, and the Swedish Research Council (projects 7480, 12610, and 21112).
Authorship
Contribution: O.S. designed and performed experiments, analyzed the data, and wrote the manuscript; V.R., M.M., and O.E.S. designed and performed experiments and analyzed the data; A.S. and P.A. designed experiments and analyzed the data; and L.B. designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oonagh Shannon, Department of Clinical Sciences, Biomedical Centre, B14, Lund University, SE-22184 Lund, Sweden; e-mail: oonagh.shannon@med.lu.se.