Abstract
Limited number of hematopoietic stem cells in umbilical cord blood (UCB) presents a problem when using UCB for stem cell transplantation. Improving their homing capacity could reduce the need for high initial cell numbers during transplantation procedures. Although it is evident that protein kinase B (PKB/c-Akt) plays an important role in regulation of migration of various cell types, a role for PKB in regulation of migration and homing of human hematopoietic stem and progenitor cells remains to be determined. PKB activity was found to be required for induction of adhesion to bone marrow–derived stromal cells and detrimental for migration of UCB-derived CD34+ hematopoietic progenitors. In addition, PKB activity was found to positively regulate integrin expression. CD34+ hematopoietic progenitors, and their capacity to form colonies in vitro, were not affected by transient inhibition of PKB. Finally, transplantation of β2-microglobulin−/− nonobese diabetic/severe combined immunodeficient mice with CD34+ cells ectopically expressing constitutively active PKB resulted in reduced migration to the bone marrow, whereas inhibition of PKB activity resulted in an induction in bone marrow homing and engraftment. These results indicate that transient inhibition of PKB activity may provide a means for ex vivo stem cell manipulation to improve bone marrow transplantation regimes.
Introduction
Aberrant regulation of blood cell formation can result in the development of severe hematopoietic malignancies, including leukemia. Repopulation of the bone marrow with hematopoietic stem cells (HSCs) from a healthy donor after conditioning regimens is an important means of therapy. Although bone marrow is widely used as a source of stem cell transplantation, evidence supporting the efficacy of umbilical cord blood (UCB) for transplantation has increased over the past few years.1,2 In contrast to bone marrow transplantation in which human leukocyte antigen–matched donors are required, the use of UCB allows a more permissive human leukocyte antigen mismatching without enhanced risk of graft-versus-host disease while preserving the graft-versus-leukemia effect.3-6 The use of UCB for transplantation increases the number of potential donors considerably, especially for those patients lacking matched related or unrelated bone marrow donors. However, the relatively small volume of UCB consists of limited number of HSCs which can present a problem when using UCB for bone marrow transplantation. Improving the bone marrow homing capacity of UCB-derived HSCs could reduce the need for high levels of HSCs required during transplantation procedures and may result in acceleration of recovery, a process that is usually delayed compared with transplantation with bone marrow–derived HSCs.
Homing is a rapid, coordinated process in which circulating HSCs actively enter the bone marrow within a few hours after transplantation. Rolling and firm adhesion of HSCs to endothelial cells in small marrow sinusoids is followed by transendothelial migration across the endothelium/extracellular matrix barrier. Finally, HSCs anchor to their specialized niches within the bone marrow compartment near osteoblasts and initiate long-term repopulation. Adhesion molecules7-11 have been shown to be involved in homing of HSCs.
In addition, the chemokine stromal cell–derived factor-1 (SDF-1 or CXCL12), which is produced by osteoblasts in the bone marrow, and its receptor CXCR4 have been implicated in human HSC migration in vivo.10,12 The molecular mechanisms underlying SDF-1–mediated induction of homing are, thus far, incompletely understood. However, it has been shown that the phosphatidylinositol-3-kinase (PI3K) signaling module is activated on stimulation of hematopoietic progenitors13 and leukemic cell lines with SDF-1,14 suggesting that PI3K and its downstream effector protein kinase B (PKB/c-akt) could be involved in regulation of SDF-1–induced HSC homing.
Although it has been described that PKB plays a critical role in regulation of migration of various cell types,15-17 the role of PKB in regulation of homing of human HSCs to the bone marrow after transplantation remains to be investigated.
Here, we have investigated the role of PKB in regulating the migration of hematopoietic progenitors with the use of both ex vivo experiments as well as an in vivo mouse transplantation model. PKB activity was found to be involved in the induction of firm adhesion of hematopoietic progenitors to bone marrow–derived stromal cells and detrimental for migration of hematopoietic progenitors. In addition, manipulation of PKB activity showed that integrin expression is positively regulated by PKB. Our results show that transient inhibition of PKB can increase the homing capacity of hematopoietic progenitors to the bone marrow, resulting in enhanced engraftment levels.
Methods
Isolation and culture of human CD34+ and mesenchymal stromal cells
Mononuclear cells were isolated from UCB by density centrifugation over a Ficoll-Paque solution (density, 1.077 g/mL). Immunomagnetic cell separation (Miltenyi Biotec) with a CD34 antibody that was coupled to beads, was performed to isolate CD34+ cells. Cells were cultured in Iscove modified Dulbecco medium (Gibco) supplemented with 9% Hyclone, 50μM β-mercaptoethanol, 10 U/mL penicillin, 10 μg/mL streptomycin, and 2mM glutamine at a density of 0.3 × 106 cells/mL. Cells were cultured in the presence of stem cell factor (SCF; 50 ng/mL) and fms-like tyrosine kinase-3 (FLT-3) ligand (50 ng/mL). Cells were cultured either in the absence or presence of 10μM of the pharmacologic PKB inhibitor VIII (Calbiochem).18,19 To isolate mesenchymal stromal cells (MSCs), bone marrow–derived mononuclear cells were plated in α-minimum essential medium supplemented with 10% Hyclone serum, 10 U/mL penicillin, 10 μg/mL streptomycin, 2mM l-glutamine, 1 ng/mL basic fibroblast growth factor, and 0.2mM l-ascorbic acid-2-phosphate at a density of 250 000 cells/cm2. MSCs, selected by their capacity to adhere to culture flasks, were cultured at 37°C until a confluence of 80% to 90% was reached. UCB and bone marrow were collected after informed consent was provided according to the Declaration of Helsinki. Protocols were approved by the ethics committee of the University Medical Center in Utrecht.
Viral transduction of CD34+ cells
A bicistronic retroviral DNA construct was used, expressing myrPKB and an internal ribosomal entry site followed by the gene encoding for enhanced green fluorescent protein (eGFP), LZRS-eGFP, as previously described.20 LZRS-eGFP retrovirus was produced by stable transfection of the retroviral packaging cell line, Phoenix-ampho, by calcium phosphate coprecipitation. Viral supernatants were collected and filtered through a 0.2-μm filter. CD34+ cells were transduced in 24-well dishes precoated with 1.25 μg/cm2 recombinant human fibronectin fragment CH-296 (RetroNectin; Takara) for 2 hours. Transduction was performed by adding 0.5 mL of viral supernatant to 0.5 mL of medium containing 0.5 × 106 cells.
Western blot analysis
Western blot analysis was performed with the use of standard techniques. Hematopoietic progenitors were lysed in Laemmli buffer (0.12M Tris [tris(hydroxymethyl)aminomethane] HCl pH 6.8, 4% sodium dodecylsulfate, 20% glycerol, 0.05 μg/μL bromophenol blue, and 35mM β-mercaptoethanol) and boiled for 5 minutes. Equal amounts of total lysate were analyzed by 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis. Proteins were transferred to Immobilon-P and incubated with Tris-buffered saline/Tween20 containing 5% low-fat milk for 16 hours at 4°C before incubating with antibodies against phosphorylated PKB (Cell Signaling Technology) or β-actin (Santa Cruz Biotechnology Inc) overnight. Subsequently, blots were incubated with peroxidase-conjugated secondary antibodies for 1 hour. Enhanced chemical luminescence was used as a detection method according to the manufacturers' protocol (Amersham Pharmacia).
Transwell migration assay
A transwell migration assay was performed in 96-well plates (Corning; pore size 8 μm). SDF-1 (20nM) was added to the lower chamber. CD34+ hematopoietic progenitors were added to the upper compartment, and the assays were performed for 3 hours at 37°C. Both the absolute number of viable, migrated cells and input were determined by flow cytometric analysis (Becton Dickinson) with the use of 7-amino-actinomycin D (Becton Dickinson) and a known number of flow count beads (Beckman Coulter). The percentage of migrated cells was subsequently calculated by dividing the absolute number of viable, migrated cells through the absolute number of input cells. All experiments were performed in triplicate.
Adhesion assay
MSCs (30 × 105) were plated in 96-well plates, resulting in a confluent layer. Hematopoietic progenitors were subsequently plated on the MSCs. After 2 hours, the nonadherent hematopoietic cells were washed 4 times with medium and once with phosphate-buffered saline (PBS). To detach both hematopoietic progenitors and MSCs, cells were incubated with PBS with 25mM EDTA (ethylenediaminetetraacetic acid). Flow cytometric analysis (Becton Dickinson), using a known number of flow count beads (Beckman Coulter) and 7-amino-actinomycin D (Becton Dickinson), was performed to determine both the absolute number of viable adherent cells and input. The percentage of adherent cells was subsequently calculated. CD34+ cells and MSCs could be distinguished by either eGFP expression or differences in forward and sideward scatter.
Immunohistochemical staining of hematopoietic cells
Integrin expression on hematopoietic progenitors was determined with the use of antibodies against CD18, CD49d, CD34 (all from Becton Dickinson), lineage markers (lineage mix), CD19 and CD235a (all from eBioscience). Cells were incubated for 30 minutes on ice with different antibodies. After incubation, cells were washed and resuspended in PBS/5% fetal calf serum (FCS), and flow cytometric analysis was performed.
Integrin expression was also analyzed by intracellular staining. Cells were first washed in PBS/5% FCS and resuspended in BD Cytofix/Cytoperm (Becton Dickinson). After 10 minutes of incubation at 4°C, cells were washed once with BD Perm/Wash buffer (Becton Dickinson). Cells were subsequently resuspended in BD Perm/Wash buffer on which the different antibodies were added to the cells. After 30 minutes of incubation on ice, cells were again washed with BD Perm/Wash buffer and resuspended in PBS/5% FCS, and flow cytometric analysis was performed.
Analysis of hematopoietic progenitors
CD34+ cells were washed and resuspended in PBS/5% FCS and incubated for 30 minutes on ice with a mixture of antibodies (all from Becton Dickinson). Lineage markers included CD2, CD3, CD4, CD7, CD8, CD14, and CD235a. The lineage negative (Lin−), CD34+, and CD38− populations consist of HSCs, whereas Lin−, CD34+, CD38+ cells are more committed progenitors. Analysis was performed with a FACSCanto II (from Becton Dickinson). Isotype antibody staining was used to ensure gating of the correct population.
Colony-forming unit assay
CD34+ cells were plated in Iscove-modified Dulbecco medium (Life Technologies) supplemented with 35.3% FCS (Hyclone), 44.4% methylcellulose-based medium called Methocult (StemCell Technologies), 11.1μM β-mercaptoethanol, 2.2 U/mL penicillin, 2.2 μg/mL streptomycin, and 0.44mM glutamine at a density of 500 cells/well. Colony-forming unit assays were done in the presence of SCF (50 ng/mL), FLT-3 ligand (50 ng/mL), granulocyte-macrophage colony-stimulating factor (0.1 nmol/L), interleukin-3 (0.1 nmol/L), granulocyte colony-stimulating factor (60 ng/mL), interleukin-5 (0.2 nmol/L), and erythropoietin (6 U/mL). Colonies were scored after 10 days of culture.
Histochemical staining of hematopoietic cells
May-Grünwald-Giemsa staining was used to analyze myeloid differentiation. Cytospins were prepared from 5 × 104 cells and were fixed in methanol for 3 minutes. After fixation, cytospins were stained in a 50% Eosin Methylene Blue solution according to May-Grünwald (Sigma-Aldrich GmbH,) for 20 minutes and rinsed in water for 5 seconds, and the nuclei were counterstained with 10% Giemsa solution (Merck kGaA) for 15 minutes.
Hoechst staining
CD34+ cells were resuspended in cytofix/cytoperm (Becton Dickinson) and incubated for 10 minutes on ice. Cells were subsequently washed and resuspended in perm/wash buffer (Becton Dickinson) that contained 10μM Hoechst 33342. After 30 minutes of incubation on ice, cells were washed in perm/wash buffer, and the percentage of Hoechst-positive cells was determined by flow cytometric analysis
Transplantation of human CD34+ cells into β2-microglobulin−/− nonobese diabetic/severe combined immune deficient mice
The β2-microglobulin−/− nonobese diabetic/severe combined immune deficient (NOD/SCID) mice were bred and maintained under sterile conditions in microisolator cages and provided with autoclaved food and acidified water containing 111 mg/L ciprofloxacin (Ciproxin). Eight- to 12-week-old mice, sublethally irradiated with 300 cGy, received a transplant by vein injections with hematopoietic progenitors along with 1.106 irradiated (1500 cGy) CD34-depleted UCB-derived accessory cells. For homing experiments, cells were stained with CellTracker Red (Invitrogen) before transplantation. After 4, 22, or 44 hours the mice were killed, and both tibiae and femora were flushed or crushed. The percentage of EGFP-positive or CellTracker-positive bone marrow cells was analyzed on a FACSCanto II. Flow count beads (Beckman Coulter) were used to calculate the absolute number of cells in bone marrow and spleen and the percentage of cells migrated to bone marrow and spleen. For longer experiments (3 and 6 weeks), the percentage of human cells was determined with the use of an antibody directed against human CD45 (eBioscience). Experiments were performed after protocols were approved by the ethics committee of the University Medical Center Utrecht.
Isolation of human CD45+ cells from mouse bone marrow
Cells were incubated for 15 minutes on ice with an anti–mouse CD16/32 antibody to block Fc binding. Immunomagnetic cell separation (Miltenyi Biotec) with the use of a CD45 antibody, which was coupled to beads, was performed to isolate CD45+ cells.
Statistics
A Levene test for equality of variances was performed in all experiments. Subsequently, an independent sample t test was performed to compare the differences between the control cells and transduced cells or cells pretreated with the PKB inhibitor. A Mann-Whitney U test was performed to compare the differences in fold induction. A P value of .05 or less was considered significant.
Results
PKB regulates migration and adhesion of hematopoietic progenitors
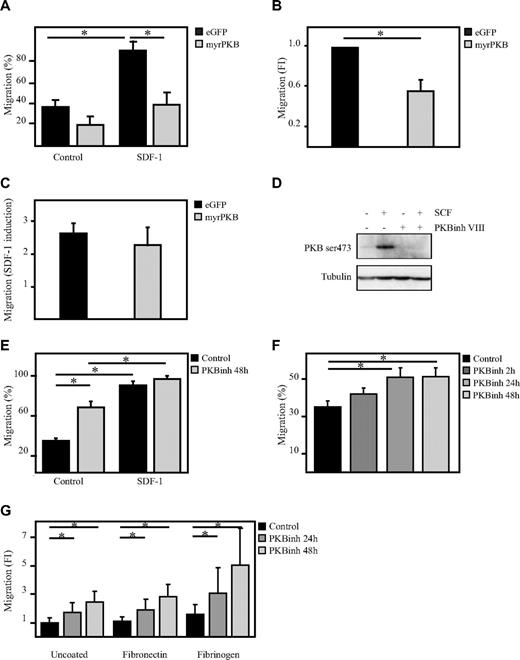
To determine whether PKB can regulate hematopoietic progenitor cell migration, a transwell migration system was used. The capacity of human CD34+ hematopoietic progenitor cells, ectopically expressing eGFP alone or a constitutively active form of PKBα (myrPKB),20 to migrate toward the chemoattractant SDF-1 was analyzed. Activation of PKB not only reduced the percentage of migrated cells on SDF-1 stimulation but also reduced the basal level of migration (Figure 1A-C). To investigate whether inhibition of PKB activity would then be sufficient to enhance the migratory capacity of hematopoietic progenitors, CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 48 hours. Incubation of CD34+ cells with the PKB inhibitor VIII was sufficient to abrogate SCF-mediated induction of PKB activity (Figure 1D), showing the potency of the compound. Inhibition of PKB activity significantly induced migration of hematopoietic progenitors even in the absence of SDF-1 (Figure 1E). Short-term inhibition of PKB activity for only 2 hours did not significantly induce migration in the absence of SDF-1 (Figure 1F), suggesting that PKB indirectly regulates the migration of human hematopoietic progenitors. The positive effect of PKB inhibition on the migration of hematopoietic progenitors was not affected by preincubation of the transwell inserts with the well-known integrin substrates fibronectin or fibrinogen (Figure 1G). These results suggest that PKB plays an important inhibitory role in the regulation of the migratory capacity of hematopoietic progenitors.
PKB inhibits migration of hematopoietic progenitors. CD34+ cells, cultured in presence of SCF and FLT-3L, were retrovirally transduced with myrPKB or eGFP alone. Three days after transduction, a transwell migration assay was performed. (A) The percentage of migrated cells was determined by flow cytometric analysis. Data were also depicted as fold induction compared with controls both in the (B) absence and (C) presence of SDF-1. (D) Hematopoietic progenitors were starved overnight in the absence of cytokines and in the presence of 0.5% FCS. Cells were left untreated (lanes 1 and 2) or incubated with 10μM PKB inhibitor VIII for 1 hour (lanes 3 and 4), before cells were stimulated with 0.5 μg/mL SCF for 15 minutes (lane 2 and 4). Protein lysates were made, and Western blot analysis was performed with an antibody against phosphorylated PKB and as a control for equal loading an antibody against tubulin. (E) Cells were cultured either in the absence or presence of the PKB inhibitor VIII for 48 hours, after which time a transwell migration assay was performed. Data were depicted as the percentage of migrated cells (n = 6). (F) Cells were cultured in the presence of the PKB inhibitor VIII for 2, 24, and 48 hours, after which time a transwell migration assay was performed. Data were depicted as the percentage of migrated cells. (G) Cells were cultured in the presence of the PKB inhibitor VIII for 24 and 48 hours, after which time a transwell migration assay was performed. The transwell inserts were preincubated for 1 hour with either fibronectin or fibrinogen. Data were depicted as the fold induction compared with untreated cells and uncoated inserts (n = 3). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
PKB inhibits migration of hematopoietic progenitors. CD34+ cells, cultured in presence of SCF and FLT-3L, were retrovirally transduced with myrPKB or eGFP alone. Three days after transduction, a transwell migration assay was performed. (A) The percentage of migrated cells was determined by flow cytometric analysis. Data were also depicted as fold induction compared with controls both in the (B) absence and (C) presence of SDF-1. (D) Hematopoietic progenitors were starved overnight in the absence of cytokines and in the presence of 0.5% FCS. Cells were left untreated (lanes 1 and 2) or incubated with 10μM PKB inhibitor VIII for 1 hour (lanes 3 and 4), before cells were stimulated with 0.5 μg/mL SCF for 15 minutes (lane 2 and 4). Protein lysates were made, and Western blot analysis was performed with an antibody against phosphorylated PKB and as a control for equal loading an antibody against tubulin. (E) Cells were cultured either in the absence or presence of the PKB inhibitor VIII for 48 hours, after which time a transwell migration assay was performed. Data were depicted as the percentage of migrated cells (n = 6). (F) Cells were cultured in the presence of the PKB inhibitor VIII for 2, 24, and 48 hours, after which time a transwell migration assay was performed. Data were depicted as the percentage of migrated cells. (G) Cells were cultured in the presence of the PKB inhibitor VIII for 24 and 48 hours, after which time a transwell migration assay was performed. The transwell inserts were preincubated for 1 hour with either fibronectin or fibrinogen. Data were depicted as the fold induction compared with untreated cells and uncoated inserts (n = 3). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
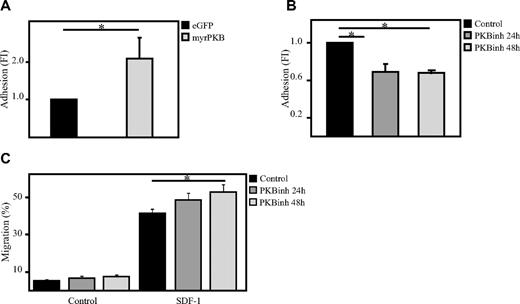
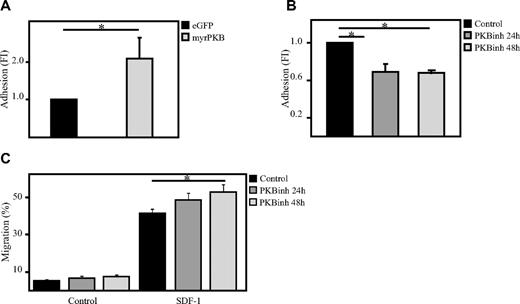
To investigate whether PKB could also be involved in the regulation of adhesion of hematopoietic progenitors, an ex vivo adhesion assay was performed with bone marrow–derived stromal cells (MSCs). Here, hematopoietic progenitors, either retrovirally transduced or cultured in the presence or absence of the PKB inhibitor VIII, were plated on a confluent layer of MSCs for 2 hours on which multiple washings steps were performed to remove the nonadherent cells. Flow cytometric analysis showed that activation of PKB activity induces the percentage of cells adhering to MSCs (Figure 2A), whereas inhibition of PKB activity significantly reduces the percentage of adherent cells (Figure 2B). Because short-term inhibition of PKB activity resulted in a reduced adherence of hematopoietic progenitors to bone marrow–derived MSCs, the question was raised whether inhibition of PKB activity would also affect the capacity of hematopoietic progenitors to migrate through endothelial cell layers. To investigate this, a confluent layer of human umbilical vein endothelial cells were cultured on transwell inserts. SDF-1 was again added to the lower compartment to induce migration and again short-term inhibition of PKB activity resulted in a significant increase in SDF-1–induced migration through the endothelial cell layer (Figure 2C).
PKB induces adhesion of hematopoietic progenitors to bone marrow–derived stromal cells. (A) CD34+ cells, cultured in the presence of SCF and FLT-3L, were retrovirally transduced with myrPKB or eGFP alone. Four days after transduction, hematopoietic progenitors were plated on a confluent layer of bone marrow–derived stromal cells, and an adhesion assay was performed. The percentage of adherent cells was determined by flow cytometric analysis (n = 3). (B) CD34+ cells were cultured either in the absence or presence of the PKB inhibitor VIII for 24 or 48 hours, after which time hematopoietic progenitors were plated on a confluent layer of bone marrow–derived stromal cells, and an adhesion assay was performed (n = 3). The percentage of adherent cells was determined by flow cytometric analysis. (C) CD34+ cells were cultured either in the absence or presence of the PKB inhibitor VIII for 24 or 48 hours, after which time a transwell migration assay was performed. Human umbilical vein endothelial cells (HUVECs) were plated before the migration experiment on the transwell inserts to obtain a confluent layer of HUVECs. The percentage of migrated cells was determined by flow cytometric analysis (n = 6). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
PKB induces adhesion of hematopoietic progenitors to bone marrow–derived stromal cells. (A) CD34+ cells, cultured in the presence of SCF and FLT-3L, were retrovirally transduced with myrPKB or eGFP alone. Four days after transduction, hematopoietic progenitors were plated on a confluent layer of bone marrow–derived stromal cells, and an adhesion assay was performed. The percentage of adherent cells was determined by flow cytometric analysis (n = 3). (B) CD34+ cells were cultured either in the absence or presence of the PKB inhibitor VIII for 24 or 48 hours, after which time hematopoietic progenitors were plated on a confluent layer of bone marrow–derived stromal cells, and an adhesion assay was performed (n = 3). The percentage of adherent cells was determined by flow cytometric analysis. (C) CD34+ cells were cultured either in the absence or presence of the PKB inhibitor VIII for 24 or 48 hours, after which time a transwell migration assay was performed. Human umbilical vein endothelial cells (HUVECs) were plated before the migration experiment on the transwell inserts to obtain a confluent layer of HUVECs. The percentage of migrated cells was determined by flow cytometric analysis (n = 6). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
These results suggest that PKB negatively regulates migration of human hematopoietic progenitors at least in part by induction of firm adhesion.
PKB regulates integrin expression in hematopoietic progenitors
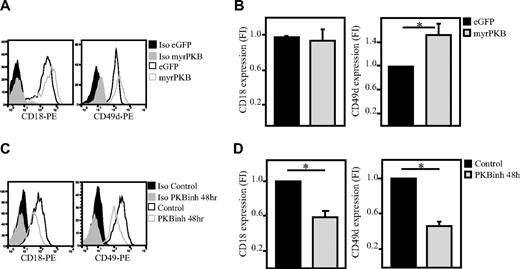
Because we observed that PKB plays an important role in regulation of adhesion and migration of hematopoietic progenitors and because integrins are known to play an important role in regulating the adhesion of various cell types, we wanted to determine whether PKB regulates integrin expression in hematopoietic progenitors. To investigate this, CD34+ cells were either transduced to express constitutively active PKB or cultured in the presence of the PKB inhibitor VIII. Flow cytometric analysis showed that CD18 and CD49d expression are regulated by PKB. Activation of PKB increased expression of CD49d (Figure 3A-B), whereas CD18 was unaffected (Figure 3A-B). Inhibition of PKB activity resulted in reduced levels of CD18 and CD49d (Figure 3C-D) in a time-dependent manner (Figure 4A-B). Analysis of the level of CD18 and CD49d both immediately after isolation and after a 2-day culture period showed that ex vivo culture results in an up-regulation of these integrins, which is reduced on inhibition of PKB activity (Figure 4C-D). To investigate whether PKB regulates translocation of integrins to the membrane or total integrin expression, the intracellular level of these integrins was also analyzed (Figure 4E-F). These experiments showed that not only the expression level of CD18 and CD49 on the cell surface but also the total level of CD18 and CD49d was reduced in a time-dependent manner on inhibition of PKB activity.
PKB induces membrane expression of integrins on hematopoietic progenitors. CD34+ cells were either retrovirally transduced with myrPKB or eGFP alone (A-B; n = 10 and 8, respectively) or cultured in presence of the PKB inhibitor VIII for 48 hours (C-D); n = 5 and 7, respectively). The expression levels of CD49d and CD18 were determined by flow cytometric analysis. (B,D) Data were depicted as fold induction compared with untransduced or untreated cells. PE indicates phycoerythrin. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
PKB induces membrane expression of integrins on hematopoietic progenitors. CD34+ cells were either retrovirally transduced with myrPKB or eGFP alone (A-B; n = 10 and 8, respectively) or cultured in presence of the PKB inhibitor VIII for 48 hours (C-D); n = 5 and 7, respectively). The expression levels of CD49d and CD18 were determined by flow cytometric analysis. (B,D) Data were depicted as fold induction compared with untransduced or untreated cells. PE indicates phycoerythrin. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
PKB induces expression of integrins. CD34+ cells were cultured in presence of the PKB inhibitor VIII for 4, 24, or 48 hours. Flow cytometric analysis was performed to determine the level of (A) CD18 (n = 5) and CD49d (n = 5). (B) Data were depicted as fold induction compared with untreated cells. (C) CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 24 or 48 hours. Flow cytometric analysis was performed to determine the expression level of CD18 (n = 3) and CD49d (n = 3) directly after isolation and after a 48-hour culture period. (D) Data were depicted as fold induction compared with cells analyzed immediately after isolation. (E) CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 2, 24, or 48 hours. Flow cytometric analysis was performed to determine the intracellular level of CD18 (n = 5) and (CD49d (n = 5). (F) Data were depicted as fold induction compared with untreated cells. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
PKB induces expression of integrins. CD34+ cells were cultured in presence of the PKB inhibitor VIII for 4, 24, or 48 hours. Flow cytometric analysis was performed to determine the level of (A) CD18 (n = 5) and CD49d (n = 5). (B) Data were depicted as fold induction compared with untreated cells. (C) CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 24 or 48 hours. Flow cytometric analysis was performed to determine the expression level of CD18 (n = 3) and CD49d (n = 3) directly after isolation and after a 48-hour culture period. (D) Data were depicted as fold induction compared with cells analyzed immediately after isolation. (E) CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 2, 24, or 48 hours. Flow cytometric analysis was performed to determine the intracellular level of CD18 (n = 5) and (CD49d (n = 5). (F) Data were depicted as fold induction compared with untreated cells. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
Colony forming capacity is not affected by transient inhibition of PKB activity
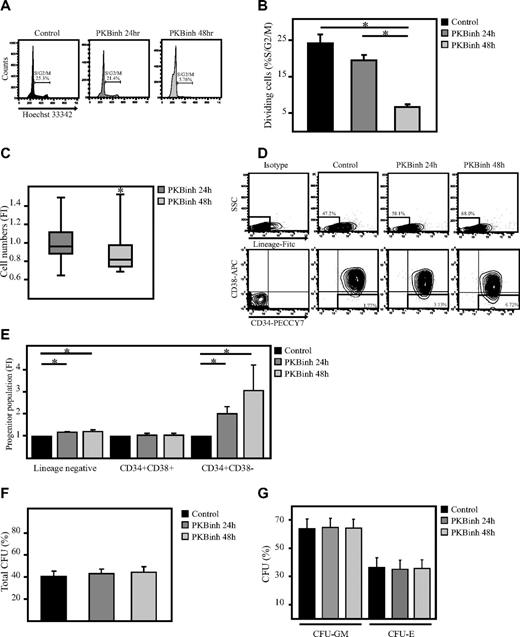
Because it has been shown that quiescent cells exhibit an improved migratory capacity compared with proliferating cells,21 it was of interest to determine whether transient inhibition of PKB activity could also affect the cell cycle status of hematopoietic progenitors. CD34+ cells were therefore cultured in the presence or absence of a PKB inhibitor for 24 or 48 hours after which time cells were stained with Hoechst 33342 to determine the percentage of dividing and nondividing cells on the basis of their DNA content. Flow cytometric analysis showed that the percentage of dividing cells was indeed reduced on inhibition of PKB activity (Figure 5A-B). In addition, although, in control samples, SCF and FLT-3L induces proliferation of hematopoietic progenitors, this induction in cell numbers was reduced after 48, but not 24, hours of inhibition of PKB activity (Figure 5C). However, cell numbers were not decreased consistently in all experiments, suggesting donor-dependent effects.
Transient inhibition of PKB does not alter the colony-forming capacity of hematopoietic progenitors. CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours, on which (A) cells were stained with Hoechst33342 to determine the percentage of dividing cells (n = 4). (B) Data were depicted as the percentage of cells in S/G2/M phase. (C) In addition, the number of cells in culture was depicted as the fold induction compared with controls in a box plot (n = 12). (D) The percentage of Lin− cells, Lin−CD34+CD38+ cells, and Lin−CD34+CD38− cells was determined by flow cytometric analysis. (E) Data were depicted as fold induction compared with untreated cells (n = 3). (F-G) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours on which the cells were plated into methylcellulose. Colony formation was analyzed after another 12 days of culture. The total number of colonies was scored. Data were depicted as (F) the percentage of cells developed into colonies (n = 7) and (G) the percentage of granulocyte macrophage colony-forming unit (CFU-GM) and erythroid colony-forming unit (CFU-E). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
Transient inhibition of PKB does not alter the colony-forming capacity of hematopoietic progenitors. CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours, on which (A) cells were stained with Hoechst33342 to determine the percentage of dividing cells (n = 4). (B) Data were depicted as the percentage of cells in S/G2/M phase. (C) In addition, the number of cells in culture was depicted as the fold induction compared with controls in a box plot (n = 12). (D) The percentage of Lin− cells, Lin−CD34+CD38+ cells, and Lin−CD34+CD38− cells was determined by flow cytometric analysis. (E) Data were depicted as fold induction compared with untreated cells (n = 3). (F-G) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours on which the cells were plated into methylcellulose. Colony formation was analyzed after another 12 days of culture. The total number of colonies was scored. Data were depicted as (F) the percentage of cells developed into colonies (n = 7) and (G) the percentage of granulocyte macrophage colony-forming unit (CFU-GM) and erythroid colony-forming unit (CFU-E). Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
Because we have previously shown that continuous inhibition of PKB activity regulates lineage development,20 the question was raised as to whether transient inhibition of PKB activity would be able to induce migration of hematopoietic progenitors to the bone marrow niche without affecting lineage development. To investigate whether short-term inhibition of PKB activity would affect differentiation, CD34+ cells were cultured in the presence of the PKB inhibitor VIII for 24 or 48 hours. Inhibition of PKB activity resulted in an induction in the percentage of Lin− progenitors and Lin−CD34+CD38− HSCs, suggesting that cells remain immature (Figure 5D-E). In addition, comparison of the average cell size before and after migration showed that both small and larger cells migrate equally through the transwell inserts, indicating that the induction in migration of hematopoietic progenitors on inhibition of PKB activity is not due to a decrease in cell size (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To further investigate whether transient inhibition of PKB activity affects the colony-forming capacity of hematopoietic progenitors, cells were plated in semisolid medium, in the presence of a cytokine cocktail sufficient to induce myeloid development, after a 48-hour culture period in the absence or presence of a PKB inhibitor. Transient inhibition of PKB activity did not affect colony formation (Figure 5F-G). These results indicate that transient inhibition of PKB activity preserves immature hematopoietic progenitors and their capacity to form colonies.
Inhibition of PKB induces homing of human hematopoietic progenitors
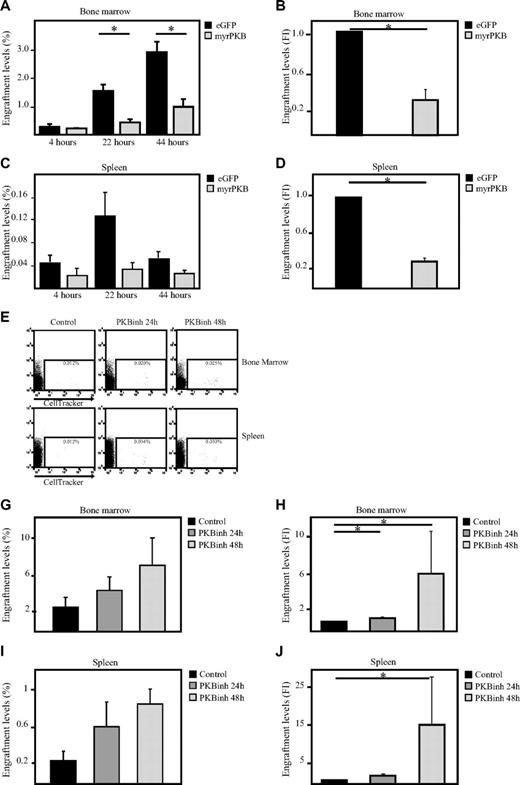
Because our data show that PKB can regulate both migration and adhesion of hematopoietic progenitors, the question arose as to whether PKB could also affect homing of hematopoietic progenitors to the bone marrow niche on transplantation. To address this question, sublethally irradiated β2 microglobulin−/− NOD/SCID mice received a transplant with retrovirally transduced human CD34+ progenitors expressing either constitutively active PKB or eGFP alone. At multiple time points after transplantation, bone marrow cells were harvested from both femurs and tibiae. The percentage of eGFP+ cells that had migrated to the bone marrow was determined by flow cytometric analysis with the use of flow count beads. Activation of PKB resulted in a significant inhibition of the percentage of cells that migrate to the bone marrow (Figure 6A-B) and spleen (Figure 6C-D). To investigate whether transient inhibition of PKB activity would thus be sufficient to induce bone marrow homing, hematopoietic progenitors were injected into the tail vein of β2 microglobulin−/− NOD/SCID mice after a 24- or 48-hour culture period in presence or absence of a PKB inhibitor (Figure 6E). Inhibition of PKB activity resulted in an enhanced level of cells that migrated to both bone marrow (Figure 6E-G) and spleen (Figure 6E,H-I) 24 hours after transplantation. These results show that PKB can regulate of in vivo homing of hematopoietic progenitors, suggesting that pharmacologic inhibition of PKB activity in HSCs may accelerate recovery after transplantation.
Inhibition of PKB induces homing of human hematopoietic progenitors in β2-microglobulin−/− NOD/SCID mice. CD34+ cells, cultured in the presence of SCF and FLT-3L, were transduced with empty vector alone or myrPKB. Three days after transduction, cells were injected into β2-microglobulin−/− NOD/SCID mice. Four, 22, and 44 hours after injection, mice were killed, and the percentages of eGFP+ cells migrated to the (A) bone marrow and (C) spleen were determined by flow cytometric analysis and flow count beads (n = 3). (B,D) Data obtained 22 hours after injection was also depicted as fold induction compared with controls (n = 5) (E,I) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours, on which cells were stained with cell tracker and injected into β2-microglobulin−/− NOD/SCID mice. Twenty-two hours after injection, mice were killed, and the percentage of cell tracker–positive cells in the (E) bone marrow and spleen was determined by flow cytometric analysis and flow count beads (n = 5). (F,H) The percentage of injected cells migrated to the bone marrow and spleen was calculated. (G) Data were depicted as fold induction compared with cells cultured in absence of the PKB inhibitor. Error bars represent SEM. Samples significantly different are indicated with horizontal lines and asterisks.
Inhibition of PKB induces homing of human hematopoietic progenitors in β2-microglobulin−/− NOD/SCID mice. CD34+ cells, cultured in the presence of SCF and FLT-3L, were transduced with empty vector alone or myrPKB. Three days after transduction, cells were injected into β2-microglobulin−/− NOD/SCID mice. Four, 22, and 44 hours after injection, mice were killed, and the percentages of eGFP+ cells migrated to the (A) bone marrow and (C) spleen were determined by flow cytometric analysis and flow count beads (n = 3). (B,D) Data obtained 22 hours after injection was also depicted as fold induction compared with controls (n = 5) (E,I) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 24 and 48 hours, on which cells were stained with cell tracker and injected into β2-microglobulin−/− NOD/SCID mice. Twenty-two hours after injection, mice were killed, and the percentage of cell tracker–positive cells in the (E) bone marrow and spleen was determined by flow cytometric analysis and flow count beads (n = 5). (F,H) The percentage of injected cells migrated to the bone marrow and spleen was calculated. (G) Data were depicted as fold induction compared with cells cultured in absence of the PKB inhibitor. Error bars represent SEM. Samples significantly different are indicated with horizontal lines and asterisks.
Transient inhibition of PKB results in enhanced engraftment levels
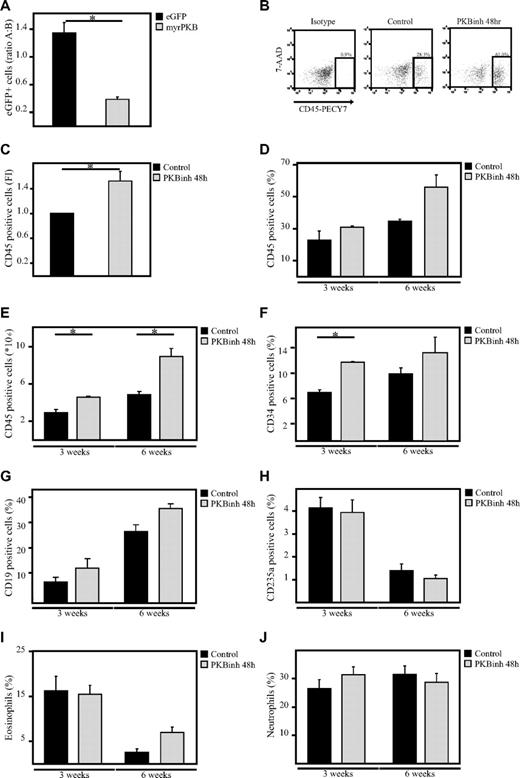
To investigate whether manipulation of PKB activity before transplantation would be sufficient to affect engraftment, sublethally irradiated β2 microglobulin−/− NOD/SCID mice received a transplant with a mixed population of untransduced cells and transduced human CD34+ progenitors expressing either eGFP alone or cells expressing both constitutively active PKB and eGFP. Six weeks after transplantation, bone marrow cells were harvested from both femurs and tibiae. The percentage of eGFP+ cells in the human CD45+ population in the bone marrow was determined. Comparison of the percentages of eGFP+ cells before and after transplantation showed that the percentage of control, eGFP-transduced cells in the human population was slightly increased after transplantation (ratio after/before > 1), suggesting that transduced cells have an advantage compared with nontransduced cells. In contrast, the percentage of myrPKB-expressing cells in the human population was dramatically decreased 6 weeks after transplantation (ratio after/before < 1), suggesting that cells expressing myrPKB have a disadvantage compared with nontransduced cells (Figure 7A). To investigate whether transient inhibition of PKB activity would then be sufficient to improve engraftment, hematopoietic progenitors were injected into the tail vein of β2 microglobulin−/− NOD/SCID mice after a 48-hour culture period in the presence or absence of a PKB inhibitor. The same number of cells was injected for both conditions. Because our results show that transient inhibition of PKB activity differentially affect proliferation of CD34+ cells from different donors and result in reduced cell numbers in some experiments (Figure 5C), transplantation experiments were performed with donors that were either unaffected in proliferation and donors that exhibited reduced cell numbers. No increase in engraftment levels was found if donors were used that exhibited reduced cell numbers (approximately 30% reduced; supplemental Figure 1C). However, experiments performed with donors that were either unaffected or mildly affected by the PKB inhibitor in terms of cell numbers resulted in an induction in the percentage of human CD45+ cells in the mouse bone marrow levels, 3 and 6 weeks after transplantation (Figure 7B-D). In addition, calculation of the absolute numbers in both femurs and tibiae showed that the number of human cells in the bone marrow was also increased at both time points (Figure 7E). Lineage development was subsequently analyzed by flow cytometry and histochemistry within the human, CD45+ cell fraction, showing the presence of immature progenitors and diverse hematopoietic lineages in vivo (Figure 7F-J). Taken together, these results show that transient inhibition of PKB activity before transplantation not only results in enhanced bone marrow homing but also results in increased engraftment levels 3 and 6 weeks after transplantation.
Inhibition of PKB improves engraftment levels in β2-microglobulin−/− NOD/SCID mice. (A) CD34+ cells, cultured in the presence of SCF and FLT-3L, were transduced with empty vector alone or myrPKB. One day after transduction, cells were injected into β2-microglobulin−/− NOD/SCID mice. Six weeks after injection, mice were killed, and the percentage of eGFP+ cells in the human CD45 population in the bone marrow was determined. Data were depicted as the ratio between the percentage of eGFP+ cells in the human population before and after migration. A indicates after; and B, before. (B) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 48 hours, on which cells were injected into β2-microglobulin−/− NOD/SCID mice from 3 different cages (1, 2, and 3). For 3-week experiments, 348 000 cells were injected, whereas for the 6-week experiments 36 166 cells were injected. Six weeks after injection, mice were killed, and the percentage of human CD45+ cells in the bone marrow was determined by flow cytometric analysis. (C) Data were depicted as the fold induction of the percentage of human CD45+ cells in the bone marrow of mice that received a with cells pretreated with the PKB inhibitor compared with controls as a combination of results from mice killed 3 and 6 weeks after transplantation (n = 4). (D) Data were depicted as the percentage of human CD45+ cells in the bone marrow. (E) Flow count beads were used to calculate the absolute numbers of human cells in the bone marrow. (F-J) Magnetic separation was performed to separate the human CD45+ population from the mouse bone marrow. (F-H) Flow cytometric analysis was subsequently performed to determine the percentage of (F) CD34+, (G) CD19+, and (H) CD235a+ cells in the human population. (I-J) In addition, cytospins were made to determine the percentage of eosinophils and neutrophils in the human population. 7-AAD indicates 7-amino-actinomycin D. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
Inhibition of PKB improves engraftment levels in β2-microglobulin−/− NOD/SCID mice. (A) CD34+ cells, cultured in the presence of SCF and FLT-3L, were transduced with empty vector alone or myrPKB. One day after transduction, cells were injected into β2-microglobulin−/− NOD/SCID mice. Six weeks after injection, mice were killed, and the percentage of eGFP+ cells in the human CD45 population in the bone marrow was determined. Data were depicted as the ratio between the percentage of eGFP+ cells in the human population before and after migration. A indicates after; and B, before. (B) CD34+ cells were cultured in the absence or presence of the PKB inhibitor VIII for 48 hours, on which cells were injected into β2-microglobulin−/− NOD/SCID mice from 3 different cages (1, 2, and 3). For 3-week experiments, 348 000 cells were injected, whereas for the 6-week experiments 36 166 cells were injected. Six weeks after injection, mice were killed, and the percentage of human CD45+ cells in the bone marrow was determined by flow cytometric analysis. (C) Data were depicted as the fold induction of the percentage of human CD45+ cells in the bone marrow of mice that received a with cells pretreated with the PKB inhibitor compared with controls as a combination of results from mice killed 3 and 6 weeks after transplantation (n = 4). (D) Data were depicted as the percentage of human CD45+ cells in the bone marrow. (E) Flow count beads were used to calculate the absolute numbers of human cells in the bone marrow. (F-J) Magnetic separation was performed to separate the human CD45+ population from the mouse bone marrow. (F-H) Flow cytometric analysis was subsequently performed to determine the percentage of (F) CD34+, (G) CD19+, and (H) CD235a+ cells in the human population. (I-J) In addition, cytospins were made to determine the percentage of eosinophils and neutrophils in the human population. 7-AAD indicates 7-amino-actinomycin D. Error bars represent SEM. Samples significantly different (P < .05) are indicated with horizontal lines and asterisks.
Discussion
In this study, we have investigated the mechanisms underlying migration of human UCB-derived hematopoietic progenitors. Our data show that inhibition of PKB activity in hematopoietic progenitors results in a reduction in firm adhesion to bone marrow–derived stromal cells, whereas migration is induced. In addition, inhibition of PKB activity is sufficient to increase homing of human hematopoietic progenitors to the bone marrow, resulting in higher engraftment levels.
PKB is known to play an important role in the migration of a variety of cell types. For example, it has been shown that activation of PKB induces migration of fibroblasts15 and mammary epithelial cells.22 In addition, the motility of various tumor cells, including fibrosarcoma cells,16 and human pancreatic cancer cells17 is positively regulated by PKB. In contrast, our results show that PKB negatively regulates migration of human hematopoietic progenitors (Figure 1A). Similarly, it has been shown that the motility of several breast carcinoma cell lines23,24 and breast epithelial cells24 is negatively regulated by PKB. Although the effect of PKB on migration and adhesion appears to be cell specific, the molecular mechanisms underlying this specificity are still incompletely understood.
SDF-1 has been implicated in playing an important role in homing of human HSCs in NOD/SCID mice.10 Although it has been shown that PI3K is activated on stimulation of leukemic cell lines with SDF-1,14 suggesting that PI3K could be positively involved in regulation of SDF-1–induced homing of HSCs, our results show that activation of PKB inhibits the basal migratory capacity of human hematopoietic progenitors (Figure 1A-B) and results in reduced bone marrow homing (Figure 6A-B). Similarly, Basu et al13 have recently shown that protein phosphatase 2A plays an important role in the regulation of SDF-1–mediated migration of CD34+ cells in vitro by inhibition of PKB activity. In addition, in vivo homing studies performed with the use of mouse HSCs showed that cells deficient for either SH2 domain–containing inositol 5′-phosphatase-1, both negative regulators of the PI3K/PKB signaling module, migrate to the bone marrow with a decreased efficiency compared with wild-type cells in irradiated and unconditioned mice, respectively25,26 Although transient activation of PKB activity on SDF-1 stimulation may play a positive role in induction of migration (Figure 1E-F), we show that prolonged activation of PKB activity results in a decrease in migration (Figure 1A) and bone marrow homing (Figure 6A), suggesting that correct regulation of both the level and duration of PKB activation is essential for optimal homing.
The engraftment potential of HSCs not only depends on chemokines but also on the expression level of integrins and selectins and the degree of mitotic quiescence. Our data show that PKB positively regulates the expression of CD18 and CD49d integrins (Figure 3). It has previously been shown that PKB and its downstream effector glycogen synthase kinase 3 plays an important role in recycling of the CD49e/CD29(α5β1) and CD51/CD61 (αvβ3) integrins to the membrane, resulting in enhanced cell spreading of NIH 3T3 fibroblasts.27 Although, we cannot exclude that the reduction in adhesion of hematopoietic progenitors to MSCs on inhibition of PKB activity is in part due to inhibition of integrin recycling or abrogated inside out signaling, intracellular flow cytometric experiments showed that the inhibition of PKB activity reduced the total level of CD18 and CD49d (Figure 4E-F), suggesting that PKB regulates integrin expression. Similarly, it has also been shown that PKBβ induces expression of CD29 in breast cancer cells.28
It has been shown that integrins play an important, although differential, role in the regulation of homing. Although experiments with blocking antibodies showed that CD49d is involved in bone marrow homing of short-term repopulating mouse HSCs, CD49F (α6) integrins are important for homing of both short-term and long-term HSCs.29 In contrast, experiments with CD18-deficient HSCs showed that CD18 is not involved in bone marrow homing.8 Our experiments show that inhibition of PKB activity results in a reduction of CD49d and CD18 (Figures 3–4), which correlates with an enhanced homing capacity (Figure 6E-I). In addition, experiments with blocking antibodies, performed by Voermans et al,30 showed that both CD29 and CD18 play an important role in transendothelial migration of human HSCs, indicating that correct integrin expression is essential for homing to the bone marrow compartment. Although deletion of integrin expression will result in the abrogation of adhesion to endothelial cells, a significant up-regulation of integrins will conversely prevent HSCs from detaching from endothelial cells. Both deletion and constitutive up-regulation of integrins will therefore result in a reduction in extravasation to the bone marrow. Although inhibition of PKB activity significantly reduced integrin expression (Figure 3), the levels were comparable with those on CD34+ cells analyzed immediately after isolation (Figure 4C-D). Although it has been shown that ex vivo culture of UCB-derived hematopoietic progenitors with SCF results in enhanced engraftment levels compared with untreated progenitors,31-33 our results suggest that abrogation of culture induced up-regulation of integrin expression by inhibition of PKB activity could further enhance the homing capacity.
Long-term HSC function is associated with quiescence.34,35 It has, for example, been shown that the long-term engraftment of cells residing in G0/G1 at the time of transplantation is increased compared with cycling cells.35 A correlation between cell cycle and integrin expression has also been suggested. Expression of CD49d/CD2936 and CD49e/CD2937 has, for example, been shown to be increased in cycling cells compared with quiescent cells, resulting in enhanced adhesion.36 Our results also show that transient inhibition of PKB activity not only decreases CD49d expression (Figures 3–4) but also increases the level of cells in G0/G1 (Figure 5A-B).
Our results show that transient inhibition of PKB activity differentially affect proliferation of CD34+ cells from different donors and result in reduced cell numbers in some experiments (Figure 5C), Although the homing capacity was not affected in donors exhibiting reduced cell number after pretreatment with the PKB inhibitor (Figure 6E-I; data not shown), the engraftment levels, 6 weeks after transplantation, appear to be affected. No improvement in engraftment levels were found if donors were used that exhibited reduced cell numbers (supplemental Figure 1C). Because homing is a rapid process that does not require cell division, whereas engraftment does, it could be hypothesized that in these cells the induction of proliferation, after migration to the bone marrow, is delayed. Importantly, experiments performed with donors unaffected by the PKB inhibitor in terms of cell numbers resulted in an induction in the percentage of human CD45+ cells in the bone marrow (Figure 7B-D) without significantly affecting lineage development (Figure 7G-J). Further research should be done to explore the possibilities of using lower concentrations of the PKB inhibitor, while preserving the homing effect, resulting in increased engraftment levels after transplantation of cells from all donors. Initial results indicate that pretreatment of cells with lower concentrations of the PKB inhibitors preserves the positive effect on migration in vitro (supplemental Figure 1D)
Taken together, this is the first study that clearly implicates the PI3K/PKB signaling module in playing a critical role in regulation of bone marrow homing. Transient inhibition of PKB activity is sufficient to induce homing of hematopoietic progenitors to bone marrow, while preserving immature hematopoietic progenitors and their capacity to form mature cells, suggesting that pharmacologic modulation of this signaling molecule may provide a clinical means of improving engraftment levels and accelerate recovery on bone marrow transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Dutch Cancer Society (research grant NKB UU 2005-3659; M.B.), by ZonMW (Veni 916.76.137; M.B.), and by the Dutch Cancer Society (NKB UU 2005-3659; E.L.).
Authorship
Contribution: M.B. designed experiments, performed experiments, analyzed data, and wrote the paper; E.v.d.L. and F.M.H. performed experiments; L.H.U. and M.B.B. designed experiments and analyzed data; and P.J.C. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.B. is Department of Hematology, Erasmus MC, Rotterdam, The Netherlands.
Correspondence: Miranda Buitenhuis, Department of Hematology, Erasmus MC, Dr. Molewaterplein 50, Faculty Bldg Office H-Ee1330F, 3015 GE Rotterdam, The Netherlands; e-mail: m.buitenhuis@erasmusmc.nl