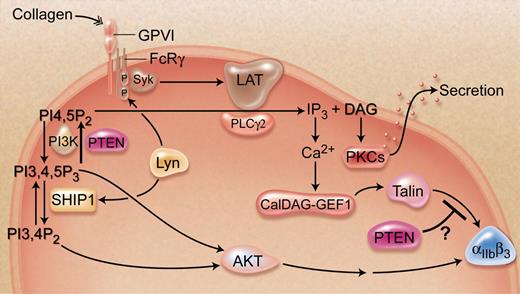
In this issue of Blood, Weng and colleagues provide genetic evidence for the PI3K (phosphatidylinositol-3-OH kinase)–dependent and –independent regulatory role of PTEN (phosphatase and tensin homologue deleted from chromosome 10) during collagen-induced platelet activation.1
During vascular injury, the monolayer of endothelial cells that line blood vessel walls becomes disrupted, thereby exposing subendothelial adhesive proteins such as collagen. Circulating platelets attach to exposed collagen through integrin α2β1 and glycoprotein VI (GPVI).2,3 Collagen binding to the receptor GPVI induces a complex signaling cascade within the platelet that leads to a rise in intracellular calcium levels, cytoskeletal reorganization, and activation of integrin αIIbβ3, the platelet fibrinogen receptor.4 One of the early events of this signaling pathway involves generation of inositol-1,4,5-trisphosphate (IP3).5 Phosphatidylinositol (PI) is a membrane phospholipid that can be phosphorylated at the 3, 4, and 5 position of the inositol ring to produce PI-3-phosphate, PI-4-phosphate, PI-5-phosphate, PI-3,4-biphosphate, PI-3,5-biphosphate, PI-4,5-biphosphate (PIP2), and PI-3,4,5-triphosphate (PIP3).3 Lipid kinases andphosphatases can rapidly interconvert phosphoinositide species to dynamically regulate their levels. Importantly, PI3K phosphorylates PIP2 to produce PIP3, whereas PTEN converts it back to PIP2.3 In addition, phospholipase C (PLC) hydrolyzes PIP2 to generate second messengers IP3 and diacylglycerol.3 PIP3 recruits serine/threonine kinase AKT to the plasma membrane where it is activated by a variety of activators such as the mammalian target of rapamycin, integrin-linked kinase, and 3-phosphoinositide–dependent protein kinase 1.6 PI3K activity in AKT activation is counterbalanced by PTEN. Although the roles of PI3K and AKT are well recognized in regulating platelet activation, the function of PTEN has not been studied. Weng and colleagues, for the first time, provide convincing evidence about the negative regulatory role of PTEN in collagen-induced platelet activation.1 They show that PTEN regulates collagen signaling in both a PI3K/AKT-dependent and -independent manner.
Because nonspecific ablation of PTEN is embryonically lethal, Weng and colleagues used inducible and hematopoietic tissue–specific deleted mice and found that PTEN suppress collagen-induced platelet activation.1 PTEN deficiency significantly reduced bleeding time and increased responsiveness of platelets to collagen. Deficiency of PTEN enhanced collagen-induced activation of AKT. Surprisingly, inhibition of PI3K prevented the aggregation of wild-type platelets, but not PTEN-deficient platelets, suggesting that PTEN may regulate collagen-induced signaling through PI3K/AKT in both a dependent and independent manner.1
As depicted in the figure, PI3K converts PIP2 to PIP3, which activates AKT, whereas PTEN opposes this process. One would therefore predict that PTEN deletion would increase AKT activation. As expected, PTEN-deficient platelets show enhanced AKT activation. Furthermore, PTEN helps replenish the pool of PIP2, which is important for granular secretion. Thus, decreased secretion due to reduced PIP2 availability was expected in PTEN-deficient platelets. Contrary to the prediction, collagen-induced granular secretion is increased in the absence of PTEN. Furthermore, inhibition of PI3K in wild-type platelets abrogates collagen-induced AKT activation and platelet aggregation, suggesting that collagen-induced platelet activation is entirely PI3K/AKT-dependent.
Schematic representation of the signaling pathway induced by collagen, emphasizing the regulatory role of PTEN. Professional illustration by A. Y. Chen.
Schematic representation of the signaling pathway induced by collagen, emphasizing the regulatory role of PTEN. Professional illustration by A. Y. Chen.
Surprisingly, in PTEN-deficient platelets, inhibition of PI3K failed to inhibit collagen-induced aggregation. These results suggest that, in addition to dephosphorylation of PIP3, PTEN exerts an inhibitory effect on granular secretion and/or integrin activation, which is PI3K/AKT independent. One possibility is that PTEN may dephosphorylate signaling proteins downstream of protein kinase C (PKC) during granular secretion and integrin activation. This is conceivable, considering the protein phosphatase activity of PTEN. In addition to being a phosphatidylinositol-3-phosphatase, PTEN is also a dual specificity protein phosphatase.7 It is also well established that PTEN is a tumor suppressor.8 PTEN's role in tumor suppression has been extended beyond simply down-regulation of the PI3K/AKT pathway. It has been shown to regulate phospholipase D and PLC.9 Furthermore, genetically modified mice confirm that mutation in PTEN results in tumorigenesis; however, activated AKT transgenic lines do not develop tumors, suggesting that AKT-independent mechanisms contribute to PTEN tumorigenesis.10 Another exciting possibility is that deletion of PTEN together with inhibition of PI3K may alter PIP2 levels or its interaction with downstream signaling proteins. For example, PIP2 has been shown to regulate talin, a key activator of integrin αIIbβ3.11,12
Further experimentation will be needed to identify the PI3K/AKT-independent inhibitory mechanism of collagen-induced platelet activation by PTEN. Nevertheless, the finding reported by Weng and colleagues not only identifies PTEN as a novel regulator of collagen signaling, but also opens new avenues for investigation of its role beyond PIP2 production in platelets.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■