In this issue of Blood, Moyer and colleagues show that deficiency of tropomodulin, an actin filament capping protein in red cell membranes, leads to mild spherocytic anemia, thus identifying yet another molecular defect that can lead to inherited red cell membrane disorders.1
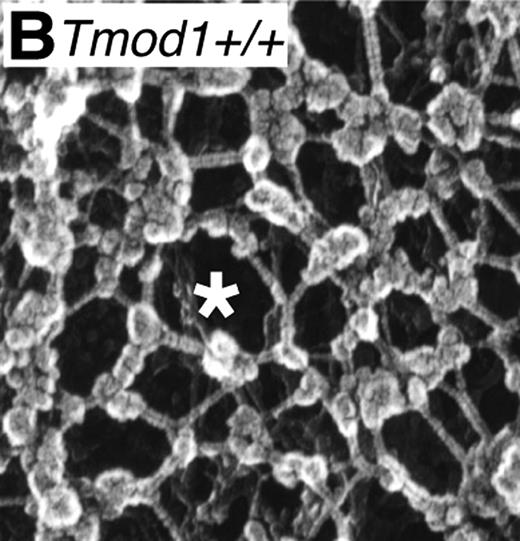
Whereas nucleated cells have an actin-based cytoskeleton, non-nucleated mammalian red cells have a unique membrane-associated spectrin-actin–based skeletal network.2 The 2-dimensional skeletal network (see figure) is quasi-hexagonal with long, flexible spectrin connecting strands and short actin filaments at vertices serving as cores for assembly of junctional complexes composed of protein 4.1R, adducin, dematin, tropomyosin, and tropomodulin.2,3 This network, in conjunction with its coupling to the membrane by linking proteins, ankyrin and protein 4.1R,4 is responsible for the remarkable flexibility, mechanical stability, and cohesion of the normal red cell membrane.2 Various inherited red cell membrane disorders, such as hereditary elliptocytosis, ovalocytosis, and spherocytosis, result from either a misassembled skeletal network and/or defects in linkage of the network to the membrane.5-8 Over the past 3 decades, identification of mutations in genes encoding α- and β-spectrin, protein 4.1R, ankyrin, adducin, dematin, and band 3 in various red cell membrane disorders of both human and mouse,5-10 in conjunction with detailed biochemical and structural characterization of the various protein components, has greatly expanded our understanding of the molecular and structural basis of red cell membrane disorders.
Ordered spectrin-actin–based membrane skeletal network in normal red cells. (Image is Figure 4B from Moyer et al.1 )
Ordered spectrin-actin–based membrane skeletal network in normal red cells. (Image is Figure 4B from Moyer et al.1 )
Despite significant progress, several questions remain unanswered. Why and how is the actin filament length tightly regulated to be 37 nm in the red cell membrane skeleton? What are the contributions of tropomyosin, tropomodulin, dematin, and adducin in regulating actin filament length? Can deficiency of these proteins account for the 10% to 15% of cases of human hereditary spherocytosis and elliptocytosis in which the underlying molecular defect has yet to be defined? The study of Moyer and colleagues1 begins to provide some answers to these questions by showing that tropomodulin does play a role in regulating actin filament length and that absence of tropomodulin leads to assembly of a disordered membrane skeleton.
What are the implications of these findings? One is that tropomodulin—like other protein components of the red cell membrane skeleton that interact with actin such as adducin, dematin, and protein 4.1R—is a key regulator of red cell membrane stability. Hence, deficiency of tropomodulin in mouse red cells results in spherocytic elliptocytosis. These findings, along with similar findings from adducin- and dematin-deficient mouse red cells,9,10 also raise the possibility that mutations in genes encoding the actin-capping proteins may account for the 10% to 15% of cases of human hereditary spherocytosis and elliptocytosis in which the underlying molecular defect has yet to be defined. But in the broader context, the reported findings could lead to obtaining new insights into the assembly during terminal erythroid differentiation of the unique and highly ordered spectrin-actin–based red cell membrane skeleton, which is critical to ensure the remarkable durability of the mature erythrocyte during its 120-day life span in circulation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■