Abstract
Phosphatidylinositol 3-kinase (PI3K) has been shown to play an important role in collagen-induced platelet activation, but the role(s) of PTEN, a major regulator of the PI3K/Akt signaling pathway, has not been examined in platelets. Here, we report that Pten−/− mouse blood contains 25% more platelets than Pten+/+ blood and that PTEN deficiency significantly shortened the bleeding time, increased the sensitivity of platelets to collagen-induced activation and aggregation, and enhanced phosphorylation of Akt at Ser473 in response to collagen. Furthermore, we found that PP2, and the combination of apyrase, indomethacin + 1B5, respectively, inhibited collagen-induced aggregation in both PTEN+/+ and PTEN−/− platelets. In contrast, LY294002 (a PI3K inhibitor) prevented the aggregation of PTEN+/+, but not PTEN−/−, platelets. Therefore, PTEN apparently regulates collagen-induced platelet activation through PI3K/Akt-dependent and -independent signaling pathways.
Introduction
The role of collagen/platelet interactions in hemostasis has been studied extensively. Collagen, acting on the glycoprotein VI (GPVI)/Fc receptor γ chain (FcR-γ chain) complex, sequentially activates Src and Syk family tyrosine kinases that propagate potent signaling via phosphatidylinositol 3-kinase (PI3K) that culminates in αIIbβ3 activation, and platelet aggregation.1-3
PTEN (phosphatase and tensin homologue deleted on chromosome 10) is a dual-specificity protein phosphatase and an inositol phospholipid phosphatase. The role of PTEN as a tumor suppressor is well established.4 PTEN dephosphorylates phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) producing phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), thereby negatively regulating oncogenic and nononcogenic PI3K/Akt signaling.5 PTEN/PI3K/Akt constitutes an important pathway regulating the signaling of multiple biologic processes, including apoptosis, cell growth, and proliferation. PI3K plays an important role in GPVI-mediated platelet activation, yet the role of PTEN, the major regulator of PI3K/Akt signaling, has not been characterized in GPVI-mediated platelet activation. Here, using inducible and hematopoietic tissue-specific deletion of Pten in mice, we demonstrate that PTEN negatively regulates PI3K-dependent and -independent collagen-induced platelet activation.
Methods
Materials
Collagen was from Nycomed Arzneimittel. PP2 and LY294002 were from Calbiochem. Apyrase and prostaglandin E1 were from Sigma-Aldrich. Hamster anti–mouse αIIbβ3 antibody (IB5) was a generous gift from Dr Barry Coller (Rockefeller University). Anti–phospho-Akt (Ser473), anti-PTEN, and anti–β-actin antibodies were from Cell Signaling Technology. Secondary antibodies were from Santa Cruz Biotechnology.
Animals
Mx1-Cre+ C57BL/6J mice were from Model Animal Research Center of Nanjing University (Nanjing, China). Ptenloxp-loxp mice from The Jackson Laboratory were backcrossed onto the C57BL/6J background for 6 generations. The Ptenloxp-loxp/Mx1-Cre+ mice were produced as described.6 Pten gene deletion was induced by intraperitoneal injection of polyriboinosinic acid/polyribocytidylic acid [poly(I:C)] as described.7 Ten days after the last injection, washed platelets were prepared from the treated Ptenloxp-loxp/Mx1-Cre+ mice as described.8 Deletion of PTEN in the washed platelets was confirmed using Western blots. FcR-γ−/− and control mice were from Taconic Farms. All animal research was approved by the Shanghai Jiao Tong University School of Medicine Animal Care and Use Committee.
Platelet preparation and aggregation
Blood was collected, and washed platelets prepared as described8 from mice anesthetized by intraperitoneal injection of pentobarbital. Inhibitors were incubated with the platelets for 3 minutes before stimulation. Human platelets were prepared as described.9 Institutional Review Board approval was obtained from the Shanghai Jiao Tong University School of Medicine, and informed consent was obtained from volunteers in accordance with the Declaration of Helsinki.
Bleeding time analysis
Tails of anesthetized mice were cut 0.5 cm from the tip and immediately immersed in 0.9% NaCl at 37°C. Bleeding time was measured as described.10
Measurement of ATP secretion
Adenosine triphosphate (ATP) secretion was measured using CHORONO-LUME reagent (Chrono-Log) according to the manufacturer's protocol.
Western blotting
For detection of PTEN and phospho-Akt, samples of platelets were processed and developed as described.8 After detection of PTEN and phospho-Akt, the membranes were stripped and incubated with antiactin antibodies to demonstrate the amount of protein present in each lane.
Statistical analysis
Means were compared using the Student t test.
Results and discussion
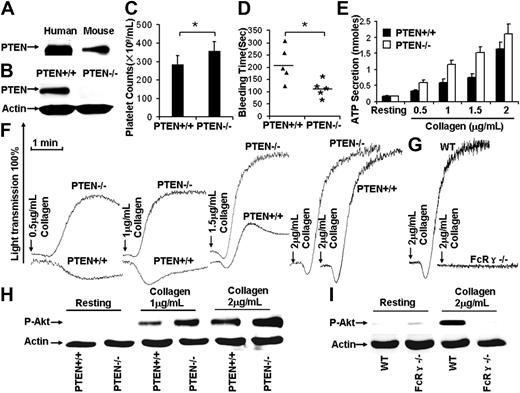
Despite the requirement for PI3K in GPVI-mediated signaling,2,3 the role(s) of PTEN, as a regulator of PI3K/Akt signaling in collagen-stimulated platelets, has not been reported. The presence of PTEN in human and mouse platelets was confirmed (Figure 1A). Reverse-transcribed polymerase chain reaction results demonstrated that mouse platelets and spleen expressed PTEN (data not shown).
PTEN deficiency facilitates collagen/GPVI-induced platelet activation. (A) Western blot results show that both human and mouse platelets express PTEN. (B) After poly(I:C) treatment for 10 days, washed platelets were prepared from Ptenloxp-loxp/Mx1-Cre− (designated PTEN+/+) and Ptenloxp-loxp/Mx1-Cre+ (designated PTEN−/−), Western blot results showed that PTEN was depleted in PTEN−/− platelets. (C) Hematopoietic depletion of PTEN caused thrombocytosis (Pten+/+ mouse platelet counts 284.8 ± 47.7 × 109/mL, Pten−/− mouse platelet counts 356.8 ± 51.4 × 109/mL). *P < .05. n = 20. (D) The bleeding time in the Pten+/+ mice (206 ± 71 seconds) was statistically different from Pten−/− mice (113 ± 35 seconds). *P < .05. n = 10. (E) PTEN deficiency facilitated collagen-induced platelet ATP secretion. Data were obtained from at least 3 tests. Bars represent the mean plus or minus SD. (F) PTEN deficiency facilitated collagen-induced platelet aggregation. (G) A total of 2 μg/mL collagen-induced platelet aggregation was dependent on GPVI/FcR-γ chain function. (H) Collagen-induced Akt phosphorylation was enhanced in PTEN-deficient platelets. (I) A total of 2 μg/mL collagen-induced Akt phosphorylation was dependent on GPVI/FcR-γ chain function.
PTEN deficiency facilitates collagen/GPVI-induced platelet activation. (A) Western blot results show that both human and mouse platelets express PTEN. (B) After poly(I:C) treatment for 10 days, washed platelets were prepared from Ptenloxp-loxp/Mx1-Cre− (designated PTEN+/+) and Ptenloxp-loxp/Mx1-Cre+ (designated PTEN−/−), Western blot results showed that PTEN was depleted in PTEN−/− platelets. (C) Hematopoietic depletion of PTEN caused thrombocytosis (Pten+/+ mouse platelet counts 284.8 ± 47.7 × 109/mL, Pten−/− mouse platelet counts 356.8 ± 51.4 × 109/mL). *P < .05. n = 20. (D) The bleeding time in the Pten+/+ mice (206 ± 71 seconds) was statistically different from Pten−/− mice (113 ± 35 seconds). *P < .05. n = 10. (E) PTEN deficiency facilitated collagen-induced platelet ATP secretion. Data were obtained from at least 3 tests. Bars represent the mean plus or minus SD. (F) PTEN deficiency facilitated collagen-induced platelet aggregation. (G) A total of 2 μg/mL collagen-induced platelet aggregation was dependent on GPVI/FcR-γ chain function. (H) Collagen-induced Akt phosphorylation was enhanced in PTEN-deficient platelets. (I) A total of 2 μg/mL collagen-induced Akt phosphorylation was dependent on GPVI/FcR-γ chain function.
Pten−/− homozygotes undergo early embryonic lethality, so conditional, Pten-deficient mice were studied. Mice carrying the Pten gene flanked by loxP recognition sites (Ptenloxp-loxp)6 were crossed with transgenic mice carrying Cre recombinase under the control of the Mx1 promoter (Mx1-Cre) to generate mice with conditional hematopoietic tissue-specific Pten deficiency. The Mx1 promoter activity was induced by synthetic double-stranded RNA, [poly(I:C)] to initiate Cre recombinase expression. Induction of Cre recombinase caused removal of the loxP-flanked Pten gene. After poly(I:C) treatment,7 washed platelets were prepared from Ptenloxp-loxp/Mx1-Cre− (PTEN+/+) and Ptenloxp-loxp/Mx1-Cre+ (PTEN−/−) mice. PTEN was undetectable in PTEN−/− platelet Western blots (Figure 1B).
Although increased megakaryopoisis is characteristic of Pten-deficient mice,11 the role of PTEN in thrombopoiesis has not been reported. Counting revealed that Pten+/+ mice had 284.8 plus or minus 47.7 × 109 platelets/mL, and Pten−/− mice had 356.8 plus or minus 51.4 × 109 platelets/mL (N = 20 mice/group). PTEN−/− blood contained 25% more platelets than controls (P < .05; Figure 1C), demonstrating that hematopoietic depletion of PTEN causes thrombocytosis. The role of PTEN in hemostasis was evaluated by measuring bleeding times. The average bleeding time of Pten−/− mice was 113 plus or minus 35 seconds, significantly shorter than that (206 ± 71 seconds) of the Pten+/+ mice (P < .05, n = 10). Thus, PTEN deficiency decreased the bleeding time (Figure 1D).
PTEN-deficient platelets were stimulated with 0.5, 1, 1.5, and 2 μg/mL collagen to investigate the role of PTEN in collagen-induced platelet activation. Collagen-induced aggregation is GPVI-dependent,12,13 as confirmed by the lack of aggregation (Figure 1G) and Akt phosphorylation (Figure 1I) by the GPVI/FcR-γ chain-deficient platelets treated with 2 μg/mL collagen. PTEN-deficient platelets were induced to aggregate by a much lower level of collagen than was required to induce the same response by PTEN+/+ platelets (Figure 1F), ATP secretion was also enhanced (Figure 1E). Western blot analyses revealed that PTEN deficiency enhanced collagen-induced phosphorylation of Akt at Ser473 (Figure 1H). Thus, PTEN deficiency apparently enhances collagen-induced platelet activation by enabling low concentrations of collagen to elicit more extensive Akt activation than is elicited in PTEN+/+ platelets.
Akt phosphorylation was evaluated in PTEN+/+ and PTEN−/− platelets stimulated with 2 μg/mL collagen plus or minus specific inhibitors to elucidate the role of PTEN in the PI3K/Akt pathway. The Src family kinase inhibitor PP2, the PI3K inhibitor LY294002, or the combination of the adenosine diphosphate scavenger apyrase, the Cox nonspecific inhibitor indomethacin (prevents TXA2 production), and the aggregation inhibitory antibody 1B5 were used for these studies. PP2, and apyrase + indomethacin + 1B5 inhibited 2 μg/mL collagen induced PTEN+/+ and PTEN−/− platelet aggregation (Figure 2A). PP2 totally inhibited collagen-induced ATP secretion in both strains, whereas LY294002 inhibited all the ATP secretion by the PTEN+/+, but only approximately 75% of the ATP secretion by the PTEN−/− strain (Figure 2B). Phosphorylation of Akt Ser473 was inhibited by PP2 and LY294002, but not by apyrase + indomethacin + 1B5 in PTEN+/+ and PTEN−/− platelets (Figure 2C). These latter results demonstrate that collagen can induce PI3K/Akt activation without adenosine diphosphate-, TxA2-, and αIIbβ3-mediated amplification of the signaling in both strains.
PTEN regulated collagen-induced platelet activation through PI3K/Akt-dependent and -independent signaling pathways. (A) PP2 (10μM) or the combination of apyrase (Apy; 10 U/mL), indomethacin (Indo) (50μM), and 1B5 (10 μg/mL) inhibited 2 μg/mL collagen-induced aggregation of PTEN+/+ and PTEN−/− platelets. PI3K inhibitor LY294002 (25μM) inhibited 2 μg/mL collagen-induced aggregation of PTEN+/+, but not PTEN−/− platelets. (B) PP2 (10μM) inhibited 2 μg/mL collagen-induced secretion of PTEN+/+ and PTEN−/− platelets. LY294002 (25μM) completely inhibited 2 μg/mL collagen-induced secretion by PTEN+/+, but not by PTEN−/− platelets. (C) Akt phosphorylation was inhibited by PP2 (10μM) and LY2940022 (25μM), but not by Apy + Indo + 1B5 in both PTEN+/+ and PTEN−/− platelets stimulated with 2 μg/mL collagen. (D) The role of PTEN in regulation of GPVI-mediated signaling pathway.
PTEN regulated collagen-induced platelet activation through PI3K/Akt-dependent and -independent signaling pathways. (A) PP2 (10μM) or the combination of apyrase (Apy; 10 U/mL), indomethacin (Indo) (50μM), and 1B5 (10 μg/mL) inhibited 2 μg/mL collagen-induced aggregation of PTEN+/+ and PTEN−/− platelets. PI3K inhibitor LY294002 (25μM) inhibited 2 μg/mL collagen-induced aggregation of PTEN+/+, but not PTEN−/− platelets. (B) PP2 (10μM) inhibited 2 μg/mL collagen-induced secretion of PTEN+/+ and PTEN−/− platelets. LY294002 (25μM) completely inhibited 2 μg/mL collagen-induced secretion by PTEN+/+, but not by PTEN−/− platelets. (C) Akt phosphorylation was inhibited by PP2 (10μM) and LY2940022 (25μM), but not by Apy + Indo + 1B5 in both PTEN+/+ and PTEN−/− platelets stimulated with 2 μg/mL collagen. (D) The role of PTEN in regulation of GPVI-mediated signaling pathway.
Surprisingly, LY294002 eliminated the aggregation of PTEN+/+ platelets, but not the aggregation of PTEN−/− platelets (Figure 2A and supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, PTEN may regulate GPVI-mediated platelet activation through PI3K/Akt-dependent and -independent signaling. In this regard, PTEN, as a protein phosphatase, is known to dephosphorylate several signaling proteins.14 Therefore, PTEN may use its protein phosphatase function to interrupt the hypothetical signaling pathway that apparently elicits collagen-induced platelet activation in the absence of PI3K activity. This hypothetical pathway appears to be negatively regulated by PTEN and an Src family kinase(s) because PP2 inhibits the aggregation PTEN−/− platelets by apparently inactivating that pathway (Figure 2A,D). Overall, these results demonstrate that PTEN plays a dual regulatory role in platelet activation elicited by GPVI-mediated signaling.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Shanghai Pujiang Program (09PJ1406800), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the Program for New Century Excellent Talents in University (NCET-08-0351), Key Laboratory of Thrombosis and Hemostasis (KLTH-2009-01), and the Ministry of Health, China.
Authorship
Contribution: Z.W., T.K.G., and J.L. designed the experiments, analyzed data, and wrote the paper; Z.W., D.L., and L.Z. performed the experiments; and J.C., C.R., and G.C. helped with the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junling Liu, Institute of Medical Science, Shanghai Jiao Tong University School of Medicine, 280 South Chongqing Rd, Shanghai, China 200025; e-mail: liujl@shsmu.edu.cn.