Abstract
Small amounts of genetically foreign cells (microchimerism, Mc) traffic between a mother and fetus during pregnancy. Commonly, these grafts durably persist. For women, multiple naturally acquired Mc grafts can accrue, as they harbor Mc from their own mothers (maternal Mc, MMc) and subsequently acquire fetal Mc (FMc) through pregnancy. The nature of interactions between these naturally acquired grafts may inform, and be informed by, observations in transplantation, including the effect of noninherited maternal HLA antigens (NIMA) and double-unit cord blood transplantation (CBT). We asked whether FMc and MMc are impacted by the addition of new grafts as evaluated by increasing parity. Mc was identified by quantitative PCR for a nonshared polymorphism unique to the Mc source. Despite increasing sources of Mc, FMc did not increase with increasing parity. MMc concentration was significantly lower with increasing parity. The odds ratio for detection of MMc for 2 or more births compared with 1 birth was .11 (95% CI 0.03-0.42, P = .001). These observations suggest that interactions occur among naturally acquired grafts and are of interest in light of recent observations of graft-graft interaction resulting in predominance of 1 unit in double-unit CBT and the correlation of MMc with the NIMA effect.
Introduction
Individuals harbor small amounts of foreign cells or DNA, referred to as microchimerism (Mc). Acquisition of Mc naturally occurs primarily during pregnancy, through transplacental cell trafficking between mother and fetus. An adult woman acquired Mc from her own mother (maternal Mc, or MMc) when she herself was a fetus. This “graft,” acquired during fetal immune system development, can remain in her system into adulthood1 and represents a pre-existing inhabitant as she experiences pregnancy herself. During subsequent pregnancies, new fetal sources of microchimerism (fetal Mc, or FMc) are acquired. The interactions of each of these grafts with the host, and with pre-existing other inhabitants, may be beneficial or detrimental to an individual.
Data show that persistence of Mc is associated both positively and negatively with certain disease states, including autoimmune diseases2 and malignancy.3,4 In autoimmunity, higher detection rates and concentrations of Mc suggest a possible allo-autoimmune or auto-alloimmune functionality.5 In the case of malignancy, lower detection rates and concentrations of Mc in cancer cases suggest a possible graft-versus-tumor effect.6 Interestingly, some disease states that have been found to vary according to a woman's reproductive history (primarily parity, or number of deliveries) also are associated with altered Mc.7-23 Thus, there exist parallel associations between Mc and disease, and parity and disease. Given the concurrent associations, we sought to answer the question whether parity might alter Mc in such a way as to support the hypothesis of a functional connection between Mc and disease.
As a therapeutic parallel to this natural process, the arena of stem cell transplantation can provide some insights into the host response to Mc and the interaction between acquired grafts. In particular, double-unit cord blood transplantation (CBT) reflects a similar process, whereby interactions between more than 1 graft and the effects of such interactions on the host may be observed. Historically, investigations of CBT sought to overcome the relatively low cell dose available from a single unit of cord blood by using double-unit transplants.24,25 Since then, data suggest that even when cell dose is similar, double-donor transplant compared with single-donor results in improved outcomes, including better engraftment and survival.26,27 Long-term, it appears that 1 graft predominates.25-28 Overall, the evolving understanding of graft-graft interactions in CBT and of graft-graft interactions as naturally acquired through reproduction may be mutually informative.
We asked whether there is a relationship between increasing parity and the prevalence of Mc. Specifically, we hypothesized that prevalence and concentration of fetal Mc would increase with increasing parity and that maternal Mc might decrease with an increasing number of grafts from fetal Mc.
Methods
Healthy women of reproductive age (considered to be 14 years old or more) and their families were recruited for participation in a multigenerational study of Mc between 1995 and 2008. Reproductive history was assessed through administration of a comprehensive health history questionnaire. Women (referred to as probands), their mothers, and their children were enrolled in the study. The study was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board; informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Peripheral blood samples were obtained from all probands (primary participants). Family members provided peripheral blood, mouthwash, or buccal swab samples. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood collected in acid citrate dextrose solution A vacutainer tubes, by Ficoll Hypaque (Pharmacia Biotech) with density gradient centrifugation at 1.077 g/mL. Mouthwash samples were processed with Roche Extraction Kit, and buccal swab samples were processed with BuccalAmp DNA Extraction Kit following the BuccalAmp Extraction Protocol (Epicentre Biotechnologies). DNA was extracted from PBMCs or whole blood using the Promega Wizard Genomic DNA Purification Kit.
HLA genotyping was conducted using Dynal linestrips (prior to January 2008) or Luminex-based (One Lambda after January 2008) polymerase chain reaction–sequence specific oligonucleotide probe (PCR-SSOP)–based techniques. DNA was extracted from whole blood or PBMC samples for probands and from buccal swabs for family members. All probands and family members were HLA-genotyped for the class II loci DRB1 and DQB1, and many were also typed for the HLA class I locus HLA-B. Familial HLA relationships were examined to identify nonshared HLA polymorphisms that could be used to identify Mc from the proband's mother or her children.
To quantify Mc from a proband's mother required that the mother of the proband had an HLA allele that was not shared with the proband and also not shared with any of the proband's children. Conversely, to quantify Mc from a child of the proband (FMc) required that the child have an HLA allele that was not shared with the proband and also not shared with the mother of the proband. FMc was considered if it could have been acquired from any of the proband's children. Specific differentiation of each unique source of Mc for probands with more than 1 child further required that each child inherited different paternal HLA alleles and that both of the paternal alleles also differed from both the proband and the mother of the proband. For all of these criteria to be met within 1 family is uncommon; however, all of these criteria were met by 4 of the families in our study for which specific results are provided in Table 4.
MMc and FMc were then quantified within DNA extracted from the proband's PBMCs, employing a panel of quantitative (q) PCR assays targeting nonshared HLA polymorphisms. The development and validation of this panel of qPCR assays has previously been reported.29 Reaction volumes were 50 uL, with 5 uL of extracted DNA. For each sample, the assay was tested 6 times with a maximum of 25 000 genome equivalents per well. A standard curve for the specific assay was included to quantify the amount of Mc and validate the assay on each plate. Every sample was also tested for a housekeeping gene, betaglobin. A betaglobin standard curve was concurrently evaluated on each plate to quantify the number of genome equivalents (gEq) of DNA tested in each reaction. The results were expressed as the genome equivalent number of microchimeric cells per 1 million cells of the proband (gEq/mil).
Overall prevalence of Mc positivity in relation to parity was evaluated using logistic regression analysis. In addition to comparing the absolute detection rates (positive or negative) for Mc, we also compared the quantitative concentrations of Mc detected. Because of the highly skewed distribution of the results, we analyzed the ranks of the Mc concentrations as the outcome in linear regression models.
More than 1 sample was obtained from many subjects, providing valuable information about within-patient variability over time; 39% of subjects had results which changed from positive to negative, or negative to positive, across samples. For a few subjects, a change in positive status corresponded to a change in parity between samples. However, because parity did not generally vary across samples within a subject, the study was essentially cross-sectional instead of longitudinal. Adjustment for possible correlation between repeated measures from the same subject was conducted in the regression analyses via generalized estimating equations (GEE).
Results
The focus of our investigation was 121 subjects (probands) who were parous at the time of blood draw and had 1 or more samples tested over time. Among the 121 parous subjects, after complete family genotyping, unique polymorphisms for both maternal and fetal Mc were identified and testable for 41 subjects employing our panel of HLA polymorphism specific assays. Another 70 subjects were tested for fetal Mc but not for maternal Mc. The final 10 subjects were tested for maternal Mc but not for fetal Mc. Some women contributed multiple samples over a time period in which their parity changed (ie, they had an additional delivery). For MMc studies, we also tested 25 women who were nulliparous at the time of the blood draw. The overall number of study subjects was 138 (slightly less than the sum of subjects tested when parous and when nulliparous due to some women who contributed samples both before and after their first birth). Table 1 describes demographic data for all 138 probands studied. The total number of samples evaluated for fetal Mc was 215; for maternal Mc, 136 samples were tested including 100 among parous women and 36 among nulliparous women.
Fetal microchimerism
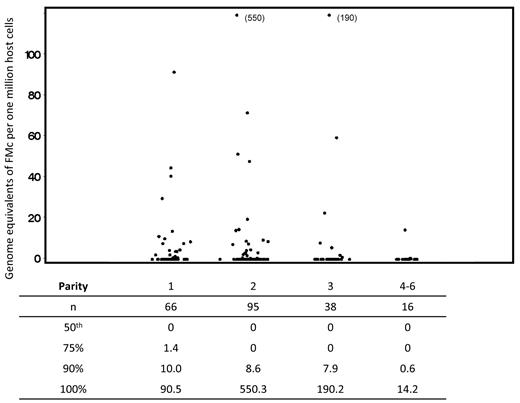
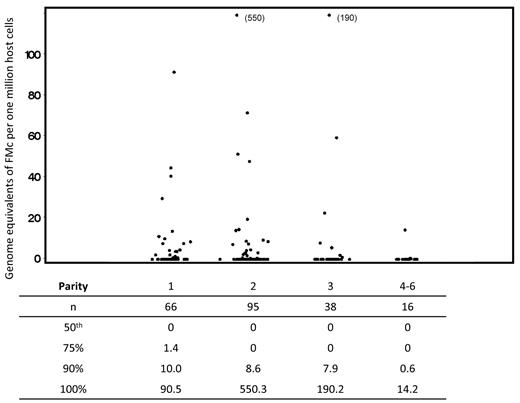
A total of 111 subjects were tested for FMc, with 215 separate observations. Sixty subjects (54%) contributed 1 measurement; the remainder contributed 2 or more measurements, with a maximum of 6. Despite the addition of sources of FMc with each pregnancy, the association of FMc prevalence with parity suggested a slight decreasing trend across level, but a test for linear trend was not statistically significant (P = .27; see Table 2). Quantitative results were similar to the differences seen in detection prevalence for FMc. From the linear regression of ranks model, we found no significant associations between FMc concentrations and parity (Figure 1).
Concentration of fetal microchimerism by parity. Scatterplots indicate all values included in the complete analysis. Repeated measures from some subjects are included; linear regression of ranks analysis adjusted for possible correlation between values within a subject via generalized estimating equations. For the comparison between parity of 2-6 compared with 1, also adjusting for age at draw date, P = .42. Below the scatterplot, in tabular form, we have included data reflecting the distribution of fetal Mc quantities detected. For each level of parity, the underlying column indicates the number of samples evaluated, the median concentration of fetal Mc, and the concentrations at the 75th, 90th, and 100th percentiles for the data from all tests at each level of parity.
Concentration of fetal microchimerism by parity. Scatterplots indicate all values included in the complete analysis. Repeated measures from some subjects are included; linear regression of ranks analysis adjusted for possible correlation between values within a subject via generalized estimating equations. For the comparison between parity of 2-6 compared with 1, also adjusting for age at draw date, P = .42. Below the scatterplot, in tabular form, we have included data reflecting the distribution of fetal Mc quantities detected. For each level of parity, the underlying column indicates the number of samples evaluated, the median concentration of fetal Mc, and the concentrations at the 75th, 90th, and 100th percentiles for the data from all tests at each level of parity.
To ensure that the results were not altered by time from recent delivery, we performed a stratified analysis by time less than or greater than 12 months since the subject's most recent delivery. We found that the proportions of subjects with FMc by parity were similar in the subgroups to those seen in the complete cohort (Table 2).
Maternal microchimerism
A total of 51 parous probands were tested for MMc, with a total of 100 observations. Overall, 31 subjects (61%) contributed 1 measurement; the remainder contributed 2 or more measurements, with a maximum of 6. Table 3 shows the presence of MMc by parity from the primary analysis. The last column of Table 3 describes the estimated associations between the presence of MMc and each level of parity compared with 1. MMc detection in subjects with parity of 2 or more was significantly less common than in those with a single delivery. We also tested samples from nulliparous women (36 observations from 25 subjects); 7 of these subjects also contributed samples after becoming parous. MMc among the nulliparous group was not significantly different from MMc in parous women.
Because dynamic changes could occur in the postpartum period we conducted a further stratified analysis according to probands who were less than 12 months versus greater than or equal to 12 months since their most recent delivery. We found that the proportions of subjects with MMc by parity were similar in these subgroups to those seen in the complete cohort (Table 3). To further assess a possible effect of time from delivery on MMc detection, models were considered with adjustment for time from last delivery. In these models, the comparisons of MMc detection and concentration were similar.
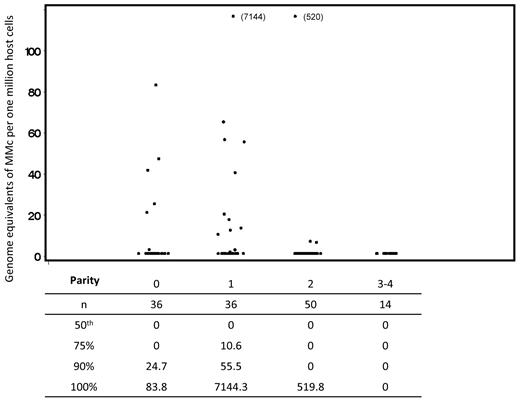
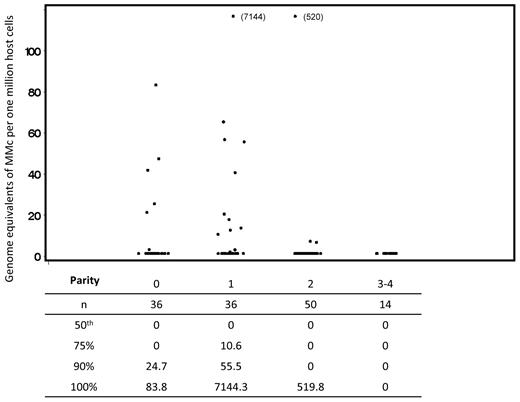
Quantitative results were similar to the differences seen in detection of prevalence for MMc. From the linear regression of ranks model, we found that MMc concentrations in subjects with parity of 2 or more were significantly lower than those with a single delivery (Figure 2).
Concentration of maternal microchimerism by parity. Scatterplots indicate all values included in the complete analysis. Repeated measures from some subjects are included; linear regression of ranks analysis adjusted for possible correlation between values within a subject via generalized estimating equations. For the comparison between parity of 2-4 compared with 1, also adjusting for age at draw date and time from last delivery, P = .02. Below the scatterplot, in tabular form, we have included data reflecting the distribution of maternal Mc quantities detected. For each level of parity, the underlying column indicates the number of samples evaluated, the median concentration of maternal Mc, and the concentrations at the 75th, 90th, and 100th percentiles for the data from all tests at each level of parity.
Concentration of maternal microchimerism by parity. Scatterplots indicate all values included in the complete analysis. Repeated measures from some subjects are included; linear regression of ranks analysis adjusted for possible correlation between values within a subject via generalized estimating equations. For the comparison between parity of 2-4 compared with 1, also adjusting for age at draw date and time from last delivery, P = .02. Below the scatterplot, in tabular form, we have included data reflecting the distribution of maternal Mc quantities detected. For each level of parity, the underlying column indicates the number of samples evaluated, the median concentration of maternal Mc, and the concentrations at the 75th, 90th, and 100th percentiles for the data from all tests at each level of parity.
Though genetic similarity within families limits the ability to test for all potential sources of Mc at a single time point, there were 4 families in this study population for whom such testing could be conducted. For these 4 subjects, evaluation of FMc from 2 children and MMc was completed at a single time point. For 2 probands, testing was positive for 1 source of FMc, negative for the other source of FMc, and negative for MMc. For the other 2 probands, all results were negative (see Table 4). In addition, there were 41 subjects tested at least once for both FMc and MMc. Twenty of these subjects (49%) were negative at all time points for both fetal and maternal Mc, 2 (5%) had both positive MMc and FMc, 6 (15%) were positive at least once for MMc but negative for FMc, and 13 (32%) were negative for MMc but positive at least once for FMc. Because parity varied across samples, those subjects tested for FMc and MMc were not necessarily tested at the same time point.
Discussion
To our knowledge, this is the first study to evaluate the relationship between Mc and parity. We sought to explore potentially parallel associations between reproductive history and Mc, and reproductive history and disease. We were able to quantitatively measure Mc from both fetal and maternal sources among women with well-characterized obstetric histories to evaluate these relationships.
Our data show that despite the addition of new sources of FMc, there was not a significant association in FMc prevalence or concentration with parity. In contrast, higher parity was significantly associated with a lower prevalence and concentration of MMc. While the explanation for our observations is unknown, the lack of an increase in FMc may represent competition between grafts whereby 1 source predominates and therefore the overall prevalence of any detectable FMc is not altered. On the other hand, the decrement in MMc with increasing parity could represent replacement of MMc with FMc.
Combined, the lack of increase in FMc and the decrease in MMc that we observed suggest that the addition of grafts to 1 host does not in fact result in an additive number of detectable grafts. This would suggest dynamic interaction of both FMc and MMc grafts. As with the therapeutic situation that parallels these natural interactions, double-unit CBT, competition between grafts may benefit the host. The nature of naturally acquired graft-graft interactions is unknown. However, in CBT, the predominance of 1 graft occurs due to activation of naive T cells in 1 cord blood unit in response to antigen expression in the second cord blood unit, leading to rejection of the second unit by the first.30 Prior studies of Mc have identified FMc and MMc within CD3+ T cells, with a greater prevalence of the former than the latter (58% vs 25%).31 A graft with particular characteristics or higher fitness may ultimately predominate and offer a protective, graft-versus-tumor effect for the host thus, for example, decreasing cancer risk. It may also be that a particularly aggressive or similar graft may predispose to long-term alloimmune reactions manifesting as autoimmune disease. Prediction of which Mc graft may provide better fitness for the host is confounded by the complexity of the system, acquisition at distinct time points and circumstances, and the differential age of the grafts.
In addition to general graft-graft interactions, the replacement of 1 type of graft (MMc) with another type (FMc) may differentially impact the health of the host. Because MMc is acquired by the developing fetal immune system, and FMc is acquired by the fully mature maternal immune system, we expect the relationship of these grafts to their host to differ. In addition to T cells as mentioned in the preceding paragraph, both MMc and FMc have been detected in a number of cell subsets, including B cells (CD19+), monocyte/macrophages (CD14+), NK cells (CD56+/CD16+), and CD34+ progenitor populations.2,31-35 Both MMc and FMc have also been found in tissues,36-40 demonstrating local tissue cell phenotypes.36,37,40-43 Whether the Mc that was initially exchanged by mother and fetus demonstrated such variability or rather, that transfer of pluripotent populations led to subsequent differentiation of these subsets is unknown. A fundamental difference in the 2 sources of Mc is suggested by the way in which tolerance to MMc develops in the fetus. When acquired by the nascent fetal immune system, MMc occupies fetal lymph nodes and influences the development of fetal regulatory T cells.44 The well-established effect of tolerance to the noninherited maternal HLA antigen (NIMA)45,46 could in part depend on enduring MMc.47 The shift from MMc to FMc that is indicated by our data may have direct relevance to the NIMA effect. While a relationship between the NIMA effect and parity has not been specifically evaluated, there is a gender difference in the effect whereby males show a greater NIMA tolerance response than females.48 In addition, the NIMA effect appears to wane with age.49 Both of these relationships may in fact depend upon parity resulting in a shift away from MMc.
Despite the rare occurrence of Mc, functional consequences of these changes in Mc are supported by 3 considerations. First, as mentioned, Mc is detectable within CD34+ progenitor populations, implicating a role in cell regeneration. Second, Mc concentrations in this study generally ranged from 1 to 50 genome equivalents per 1 million host cells, with some samples containing as many as 0.05%-0.7% Mc. In comparison, peptide-specific T cells have been reported at 30-50 per 1 million cells.50 Thus, the quantitative estimation of Mc concentration indicates its presence at levels with the potential for immunologic effects. Third, tissue concentrations of Mc may be higher than concentrations in peripheral blood, supporting concentrated local activity.29
Our study has a number of limitations. Because siblings often share 1 or both HLA-haplotypes, we were in general not able to test probands for FMc from each different child, which would have allowed us to directly assess whether 1 graft was predominant. Of all subjects included in this study, there were 4 families as described in Table 4 above with unique polymorphisms for all family members, allowing for evaluation of Mc from all fetal and maternal sources at a single time point. Although this limited number of subjects cannot provide conclusive results, the data are consistent with the hypothesis that a parous woman is more likely to harbor microchimerism from a single source rather than several concurrently, and that the more likely source is fetal rather than maternal.
It would also be of interest to ask whether FMc prevalence is affected by the HLA relationships between the proband and each source of FMc as well as the HLA-relationships among the FMc sources. To address these questions would require a much larger number of subjects and the development of other techniques for identification and quantification of FMc such as a large panel of qPCR assays based on nonallelic polymorphisms on multiple different chromosomes.
The amount of time elapsed since birth could be an especially important variable for samples obtained in the postpartum time period. Our stratified analysis considering the year postpartum separately found no significant difference from more than 1 year from delivery. However, we cannot rule out postpartum changes as important to our overall observations especially since half of all samples studied for MMc derived from the postpartum period. Immunologically, the postpartum year differs from other time periods in reproductive life. Clinically, the example of rheumatoid arthritis (RA) illustrates the uniqueness of the postpartum period, in that there is amelioration of RA during pregnancy and an increased risk of flare postpartum. In addition, the reduction in RA risk that is associated with increasing parity is only seen after the first year postpartum.23
In conclusion, despite a higher number of FMc sources, no significant change in FMc was observed with increasing parity, and increasing parity was associated with a significantly lower concentration of MMc. These observations raise interesting questions about the interaction of acquired grafts within a host, including whether such interactions may ultimately lead to the emergence and persistence of 1 dominant source of Mc, as is seen in dual CBT and which may impact the endurance of the NIMA effect. Such interactions, or the pressure for a particularly advantageous graft to predominate, may play an important role in subsequent health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank D. Stief for assistance with subject recruitment.
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI41721 and R01 AI45659, J.L.N.) and National Institute of Child Health and Human Development (HD-01 264-06, H.S.G.).
National Institutes of Health
Authorship
Contribution: H.S.G., K.M.A.W., and J.L.N. generated the hypothesis; H.S.G, T.M.A., and J.L.N. designed the research; H.S.G. and T.M.A. performed experiments; H.S.G. and K.A.G. conducted the data analyses and prepared the figures; and H.S.G., K.A.G., T.M.A., K.M.A.W., and J.L.N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Hilary Seglin Gammill, University of Washington, Box 356460, Seattle, WA 98195-6460; e-mail: hgammill@u.washington.edu.