Abstract
Mechanisms of action and resistance of histone deacetylase inhibitors (HDACIs) are not well understood. A gene expression analysis performed in a phase 1 trial of vorinostat in leukemia indicated that overexpression of genes involved in antioxidant defense was associated with clinical resistance. We hypothesized that nonepigenetic mechanisms may be involved in resistance to HDACI therapy in leukemia. Here we confirmed up-regulation of a series of antioxidants in a pan-HDACI–resistant leukemia cell line HL60/LR. Vorinostat induced reactive oxygen species (ROS) through nicotinamide adenine dinucleotide phosphate oxidase in leukemia cells. An increase in ROS resulted in translocation of nuclear factor E2-related factor 2 from cytosol to nucleus, leading to up-regulation of antioxidant genes, including a majority of glutathione-associated enzymes as a cellular protective mechanism. Addition of β-phenylethyl isothiocyanate, a natural compound capable of depleting cellular glutathione, significantly enhanced the cytotoxicity of vorinostat in leukemia cell lines and primary leukemia cells by inhibiting the cytoprotective antioxidant response. These results suggest that ROS plays an important role in action of vorinostat and that combination with a redox-modulating compound increases sensitivity to HDACIs and also overcomes vorinostat resistance. Such a combination strategy may be an effective therapeutic regimen and have potential clinical application in leukemia.
Introduction
Histone deacetylase inhibitors (HDACIs) are a class of agents with the capacity to induce acetylation of histone and nonhistone proteins.1 HDACIs have been intensively investigated in preclinical models as well as in clinical trials for a variety of malignancies. Various mechanisms of action have been proposed for the anticancer activity of HDACIs. Early work has focused on their effect on gene transcription by inducing permissive histone marks. Other pharmacologic actions include activation of extrinsic and intrinsic apoptotic pathways,2-4 induction of cell-cycle arrest,5 autophagic cell death,6 and senescence.7 Despite these well-characterized properties of HDACIs, the precise mechanism of their in vivo activity still remains to be elucidated.
Suberoylanilide hydroxamic acid (vorinostat) is a small-molecule inhibitor of class I and II HDACIs.1 Vorinostat has significant activity in cutaneous T-cell lymphoma.8,9 Previous studies have also demonstrated that vorinostat has antileukemia activity in vitro and in rodent models.5,10-12 In a phase 1 clinical trial, vorinostat was shown to have modest clinical activity in patients with advanced leukemia.13 A cDNA microarray analysis performed in that trial suggested that a gene signature composed mainly of antioxidants was associated with clinical resistance to vorinostat. Thus, induction of reactive oxygen species (ROS) could be a potential mechanism of vorinostat action, whereas increased antioxidant expression may contribute to vorinostat resistance.
It is known that excessive production of ROS can cause cellular damage, which ultimately leads to cell death.14 Therefore, cells have developed a highly regulated antioxidant defense system to prevent oxidative damage. These cellular defense mechanisms against ROS include redox buffering systems and various antioxidant enzymes, such as glutathione (GSH)–generating enzymes, including glutamate cysteine ligase (GCL) and glutathione reductase (GSR), glutathione S-transferase (GST), and superoxide dismutase (SOD).14 Many of these antioxidant enzymes are under the control of a transcription factor nuclear factor E2-related factor 2 (Nrf2).15,16 Despite previous reports on stimulation of ROS generation by HDACIs in cancer cells,17,18 the source of ROS still remains unclear. Furthermore, the role of antioxidants in cellular defense against HDACIs remains to be investigated. Thus, the study of mechanism of HDACI action in the context of ROS generation is important for the design of drug combination strategies to overcome HDACI resistance.
β-Phenylethyl isothiocyanate (PEITC) is a natural compound found in cruciferous vegetables.19 Recent studies have shown that PEITC effectively disables the glutathione antioxidant system and selectively kills cancer cells with increased ROS generation.19,20 Given that glutathione is the most abundant antioxidant system against ROS stress and that a series of glutathione-related enzymes were up-regulated in leukemia patients who were resistant to vorinostat,13 we hypothesized that PEITC might enhance the antileukemia activity of vorinostat by modulating cellular redox status. The objectives of the study presented here were to determine how HDACIs increase ROS generation in leukemia cells, to characterize the role of Nrf2 and its downstream antioxidant enzymes in protecting cells against HDACI-induced ROS stress, and finally to determine whether the combination of an HDACI with PEITC could lead to synergistic cytotoxic effects against leukemia cells. This study provides important information for the understanding of mechanism of action of HDACIs and resistance to this class of compounds.
Methods
Antibodies and reagents
The following antibodies were used for immunoblotting analysis using standard Western blotting procedures: SOD2, glutathione S-transferase pi (GST-pi), GPX1, GCLC, and Nrf2 from Santa Cruz Biotechnology; and β-actin from Sigma-Aldrich. PEITC, diphenylene iodonium (DPI), 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyltetrazolim (MTT), and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich. Vorinostat and MGCD0103 were provided by Merck (Whitehouse) and MethylGene, respectively.
Cell culture
Human leukemia cells lines (HL60, HL60/LR, U937, and ML1) were cultured at 37°C in an atmosphere of 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 2mM l-glutamine. HL60/C6F cells were maintained in RPMI 1640 medium containing 0.47% glucose, 10% FBS, 50 μg/mL uridine, and 1mM pyruvate. Primary leukemia cells were isolated from peripheral blood samples from patients with acute myeloid leukemia (AML) after proper informed consent was obtained in accordance with the Declaration of Helsinki. This study was performed with approval from the M. D. Anderson Cancer Center ethics review board. Mononuclear cells were isolated from AML patient samples by Ficoll density-gradient centrifugation. Primary leukemia cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2mM l-glutamine, and antibiotics. Normal mononuclear cells were isolated from peripheral blood samples of healthy donors using similar procedures. HEK293 cells were maintained in Dulbecco modified Eagle medium with 10% FBS.
ROS detection
Cellular O2− and H2O2 were measured by flow cytometry using hydroethidine (Het) and CM-H2DCF-DA (Invitrogen), respectively. After incubation with Het (100 ng/mL) or CM-H2DCF-DA (3μM) at 37°C for 1 hour, samples were washed with phosphate-buffered saline and analyzed with a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using BD Biosciences CellQuest Pro Version 4.0.2 software package.
Real-time PCR
Total RNA was extracted using the RNeasy kit (QIAGEN) followed by DNAase (Ambion) treatment according to the manufacturer's instructions. cDNA was prepared using TaqMan Reverse Transcription Reagents (Applied Biosystem). A total of 50 ng of cDNA was subjected to quantitative real-time polymerase chain reaction (PCR) using ABI 7700 sequence detection system and SYBR reagents (Invitrogen). Glyceraldehyde-3-phosphate dehydrogenase was used as the internal control gene. Human gene-specific primers were synthesized by Invitrogen, and sequences are described in Table 1.
NADPH oxidase activity assay
One million myeloid leukemia cells treated with or without vorinostat were suspended in Hanks buffer (Invitrogen). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity was detected by lucigenin-derived chemiluminescence with 100μM NADPH (Sigma-Aldrich) and 10μM lucigenin (Sigma-Aldrich). Chemiluminescence was measured using a luminometer (Turner Designs) for 1 minute. The signal reading was normalized and expressed as arbitrary light units per 1 million cells.
Cytotoxicity assays
Cell death of leukemia cell lines and primary leukemia cells induced by HDACIs and PEITC were analyzed with flow cytometry using annexin V/propidium iodide (PI) assays according to the manufacturer's instructions (BD Biosciences). Results were analyzed using CellQuest Pro software. To test the drug effect on cell growth, a Coulter Z2 Particle Count and Size Analyzer was used to count cell number after drug exposure for 24, 48, and 72 hours. U937 cells in exponentially growing phase were seeded in a 96-well plate. After 72-hour exposure to various concentrations of vorinostat, PEITC, and their combination, cell viability was determined by MTT as described before.21 In primary leukemia cells, cell viability was calculated by multiplying the percentage of viable cells in the vorinostat-treated sample with the percentage of viable cells treated with PEITC. The combined effect is considered additive if the observed viable cell fraction is equal to the expected value. If the observed value is less than the expected value, the combined drug effect is considered more than additive.
Immunofluorescence and confocal microscopy
U937 cells were cytospun, fixed with 3.7% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Cells were incubated with 5% bovine serum albumin for 1 hour at room temperature before incubation with Nrf2 antibody (Santa Cruz Biotechnology) at 4°C overnight. Alexa-Fluor-594 goat-antirabbit (Invitrogen) was added to the samples and incubated at room temperature for 1 hour. Slides were washed with phosphate-buffered saline, mounted, and counterstained with mounting medium with 4,6-diamidino-2-phenylindole (Santa Cruz Biotechnology). Images were captured with a Nikon Eclipse TE2000 confocal microscope and analyzed with Nikon EZ-C1 Version 3.80 software (Nikon).
Measurement of cellular glutathione
A glutathione assay kit (Cayman Chemical) was used to measure total cellular glutathione as described.19 Briefly, cell extracts were sonicated in phosphate buffer and deproteinated with metaphosphoric acid. The level of total glutathione was detected by measuring the product of glutathionylated 5-5′-dithiobis-2-nitrobenzoic acid with an ultraviolet spectrophotometer at 405 nm. Cellular glutathione contents were calculated using standard curves generated in parallel experiments.
Nrf2 transfection
A predesigned pCMV6-XL5 vector containing Nrf2 cDNA (OriGene Technologies) was transfected into HEK293 cells using lipofectamine 2000 (Invitrogen). Cells transfected with vector only were used as control. After transfection for 40 hours, cells were subjected to Western blot analysis to verify Nrf2 protein expression.
Statistical analysis
The statistical significance of differences between 2 experimental groups was determined by 2-tailed Student t test. The cytotoxic combination effect of an HDACI with PEITC was calculated using Calcusyn (Biosoft).
Results
Increase of major antioxidants and decrease of ROS in pan-HDACI–resistant cell line HL60/LR
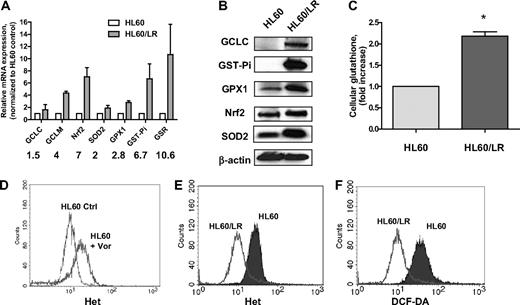
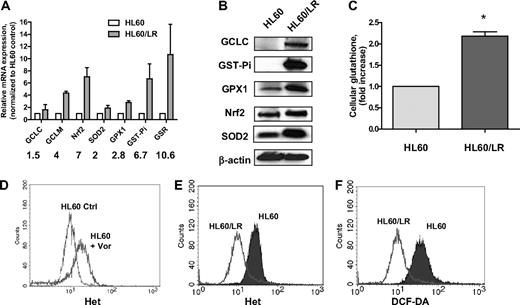
A pan-HDACI–resistant cell clone HL60/LR has been recently generated by continuous exposure of the AML cell line HL60 to the hydroxamate analog LAQ824.22 To study the molecular mechanisms underlying cellular resistance to an HDACI, we first compared the redox parameters, including major antioxidants and ROS levels, in HL60 and HL60/LR cells. Gene expression of various glutathione-related enzymes was significantly up-regulated in HL60/LR cells compared with the parental HL60 cells (Figure 1A). These enzymes include GCL (catalytic subunit GCLC and regulatory subunit GCLM), GST-pi, and GSR. Moreover, SOD2, an enzyme responsible for eliminating O2−, and glutathione peroxidase (GPX), an enzyme responsible for eliminating H2O2, were both increased. Western blotting analysis in Figure 1B confirms the increase of GCLC, GST-Pi, GPX1, and SOD2 in HL60/LR cells at the protein level. Nrf2, a master transcription factor that has been shown to control gene expression of GCLC, GPX, GST, and SOD,16 was also up-regulated at the mRNA and protein levels in HL60/LR cells. Glutathione is the most abundant antioxidant buffer system and the substrate for xenobiotic detoxification enzyme GST-pi.14 As a result of up-regulation of GSH synthesis, enzyme GCLC, and GSH regeneration enzyme GSR, cellular glutathione level was also found to be elevated in HL60/LR cells compared with parental cells (Figure 1C). Previous reports have suggested the role for ROS production in HDACI-induced cytotoxicity.3,18,23 Consistently, incubation with 1μM vorinostat for 18 hours induced acute accumulation of ROS in HL60 cells (Figure 1D). However, when HL60 cells were constantly exposed to LAQ824 and became resistant, ROS levels, including O2− and H2O2, significantly decreased compared with parental cells (Figure 1E-F), which indicated that up-regulation of antioxidants may act as a secondary effect to protect cells from potential ROS injury caused by an HDACI.
Significant increase of a series of antioxidants in HL60/LR cells compared with parental HL60. (A) Comparison of mRNA expression of antioxidant genes in HL60 and HL60/LR cells by real-time PCR analysis. Numbers indicate fold increase in HL60/LR cells. Bars represent mean ± SD from 3 experiments. GCLC indicates glutamate-cysteine ligase, catalytic subunit; and GCLM, glutamate-cysteine ligase, modifier subunit. (B) Cell lysates of HL60 and HL60/LR were analyzed for the indicated antioxidant protein expression using immunoblot assay. β-Actin was used as loading control. (C) Increase of cellular GSH level in HL60/LR cells. Bars represent mean ± SD from 3 experiments. *P < .01 (Student t test). (D) HL60 cells were treated with 1μM vorinostat (Vor) for 18 hours, and cells were labeled with Het for 1 hour followed by flow cytometric analysis to detect O2− levels. Ctrl indicates control cells without treatment. (E-F) Representative histograms of significant decrease of O2− and H2O2 levels in HL60/LR cells versus HL60 as detected by fluorescent probe Het and DCF-DA, respectively.
Significant increase of a series of antioxidants in HL60/LR cells compared with parental HL60. (A) Comparison of mRNA expression of antioxidant genes in HL60 and HL60/LR cells by real-time PCR analysis. Numbers indicate fold increase in HL60/LR cells. Bars represent mean ± SD from 3 experiments. GCLC indicates glutamate-cysteine ligase, catalytic subunit; and GCLM, glutamate-cysteine ligase, modifier subunit. (B) Cell lysates of HL60 and HL60/LR were analyzed for the indicated antioxidant protein expression using immunoblot assay. β-Actin was used as loading control. (C) Increase of cellular GSH level in HL60/LR cells. Bars represent mean ± SD from 3 experiments. *P < .01 (Student t test). (D) HL60 cells were treated with 1μM vorinostat (Vor) for 18 hours, and cells were labeled with Het for 1 hour followed by flow cytometric analysis to detect O2− levels. Ctrl indicates control cells without treatment. (E-F) Representative histograms of significant decrease of O2− and H2O2 levels in HL60/LR cells versus HL60 as detected by fluorescent probe Het and DCF-DA, respectively.
Vorinostat induces ROS generation through NADPH oxidase activation
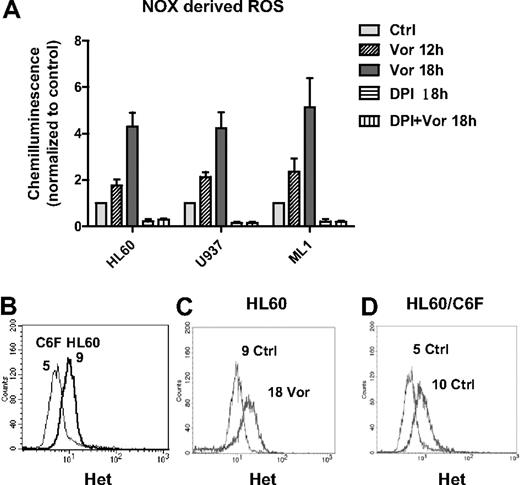
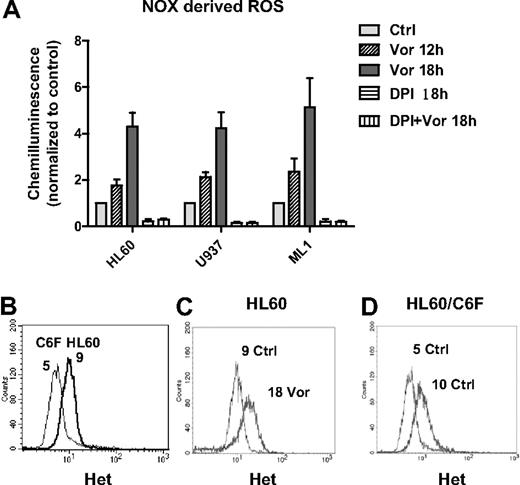
Consistent with previous reports of ROS induction by HDACIs,3,18,24 we observed that vorinostat induced early accumulation of ROS in HL60, U937, and ML1 cells (Figure 1D; and data not shown). However, the initial source of ROS remains unclear. It is known that mitochondria respiratory chain and NADPH oxidase (NOX) are 2 major sources of ROS generation.25,26 To study the source of ROS production induced by vorinostat, we first examined the activity of NOX after vorinostat treatment. As shown in Figure 2A, vorinostat significantly up-regulated NOX activity in a time-dependent manner in various myeloid leukemia cell lines, including U937, HL60, and ML1. DPI, a flavoprotein inhibitor of NOX,27,28 completely inhibited the enzyme activity in these cell lines. Of importance, the increase of NOX activity induced by vorinostat was completely blocked by the same concentration of DPI. These results demonstrate that NOX is involved in ROS generation induced by vorinostat. To test the role of mitochondria in ROS generation induced by vorinostat, we used an HL60-derived cell line (HL60/C6F) with mitochondrial DNA mutation and mitochondrial respiratory deficiency.29 We compared ROS levels in parental HL60 and C6F cells before and after vorinostat treatment. Flow cytometric analysis demonstrated that C6F cells produced only 50% basal O2− level of the parental HL60 (Figure 2B), probably because of mitochondrial respiratory deficiency.29 However, incubation with 1μM vorinostat for 18 hours was able to induce a 2-fold increase of O2− level in both HL60 and C6F (Figure 2C-D), suggesting involvement of a nonmitochondrial source of ROS, probably NOX.
Vorinostat induced NOX-derived ROS generation. (A) HL60, U937, and ML1 cells were treated with vorinostat alone (1μM for HL60, 2μM for U937 and ML1), 2μM DPI alone, or vorinostat plus DPI for the indicated time. Cells were then subjected to NOX activity assay as described in “NADPH oxidase activity assay.” DPI completely blocked the up-regulation of NOX activity induced by vorinostat. Bars represent mean ± SD from 3 experiments. (B) Histogram overlay of baseline O2− levels in HL60 and HL60/C6F. (C-D) Representative histogram overlay of O2− levels in HL60 and HL60/C6F before and after treatment of 1μM vorinostat for 18 hours. Numbers in panels B through D indicate mean values of O2− levels detected by Het. Vor indicates vorinostat.
Vorinostat induced NOX-derived ROS generation. (A) HL60, U937, and ML1 cells were treated with vorinostat alone (1μM for HL60, 2μM for U937 and ML1), 2μM DPI alone, or vorinostat plus DPI for the indicated time. Cells were then subjected to NOX activity assay as described in “NADPH oxidase activity assay.” DPI completely blocked the up-regulation of NOX activity induced by vorinostat. Bars represent mean ± SD from 3 experiments. (B) Histogram overlay of baseline O2− levels in HL60 and HL60/C6F. (C-D) Representative histogram overlay of O2− levels in HL60 and HL60/C6F before and after treatment of 1μM vorinostat for 18 hours. Numbers in panels B through D indicate mean values of O2− levels detected by Het. Vor indicates vorinostat.
Activation of Nrf2 and increase of antioxidant defense genes induced by vorinostat
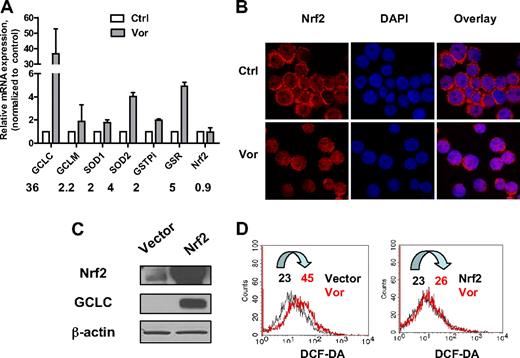
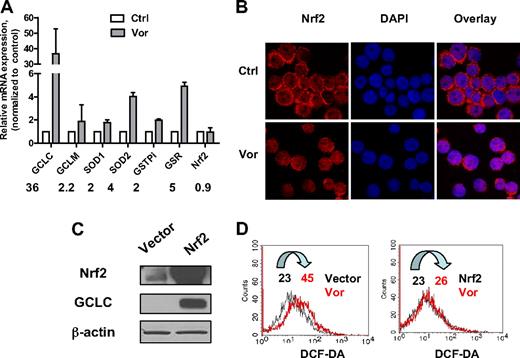
To investigate the effect of vorinostat on major antioxidants, U937 cells were exposed to 2μM vorinostat for 20 hours and gene expression levels were analyzed by real-time PCR. As shown in Figure 3A, exposure to vorinostat significantly increased gene expression of GCLC, the catalytic enzyme responsible for GSH synthesis. The regulatory subunit GCLM was also increased but to a lesser extent. In addition, GSR, the enzyme responsible for GSH regeneration, GST-pi, the enzyme that uses GSH to detoxify a foreign compound, and SOD1 and SOD2, the enzymes that eliminate O2−, were all up-regulated. These results strongly suggested that treatment of leukemia cells with vorinostat also induced up-regulation of genes responsible for cellular defense against oxidative stress as a secondary response.
Effect of vorinostat on Nrf2-regulated antioxidant genes. (A) Up-regulation of mRNA expression of various antioxidants after vorinostat treatment. U937 cells were treated with 2μM vorinostat for 20 hours and mRNA expression of the indicated genes before and after treatment were compared by real-time PCR analysis. Numbers indicate fold change in treated cells compared with untreated cells. Bars represent mean ± SD from 3 experiments. (B) Vorinostat induced translocation of Nrf2 from cytosol to nucleus. U937 cells were treated with 2μM vorinostat for 18 hours and then cytospun. Cells were fixed and labeled for Nrf2 protein (red) and nucleus (blue, 4,6-diamidino-2-phenylindole) as described in “Immunofluorescence and confocal microscopy.” Images were taken by Nikon Eclipse TE2000 confocal microscope with 40×/1.30 numeric aperture oil objective lens. Ctrl indicates control cells without treatment; and Vor, vorinostat. (C) HEK293 cells were transfected with empty vector only or vector with Nrf2 cDNA. Overexpression of Nrf2 and increase of GCLC were verified by Western blot analysis 40 hours after transfection. (D) Nrf2 prevented ROS induced by vorinostat. Cellular H2O2 levels before and after 5μM vorinostat incubation for another 24 hours in HEK293 cells transfected with empty vector or Nrf2 were detected by flow cytometric analysis. Vor indicates vorinostat. Numbers indicate mean values of H2O2 levels.
Effect of vorinostat on Nrf2-regulated antioxidant genes. (A) Up-regulation of mRNA expression of various antioxidants after vorinostat treatment. U937 cells were treated with 2μM vorinostat for 20 hours and mRNA expression of the indicated genes before and after treatment were compared by real-time PCR analysis. Numbers indicate fold change in treated cells compared with untreated cells. Bars represent mean ± SD from 3 experiments. (B) Vorinostat induced translocation of Nrf2 from cytosol to nucleus. U937 cells were treated with 2μM vorinostat for 18 hours and then cytospun. Cells were fixed and labeled for Nrf2 protein (red) and nucleus (blue, 4,6-diamidino-2-phenylindole) as described in “Immunofluorescence and confocal microscopy.” Images were taken by Nikon Eclipse TE2000 confocal microscope with 40×/1.30 numeric aperture oil objective lens. Ctrl indicates control cells without treatment; and Vor, vorinostat. (C) HEK293 cells were transfected with empty vector only or vector with Nrf2 cDNA. Overexpression of Nrf2 and increase of GCLC were verified by Western blot analysis 40 hours after transfection. (D) Nrf2 prevented ROS induced by vorinostat. Cellular H2O2 levels before and after 5μM vorinostat incubation for another 24 hours in HEK293 cells transfected with empty vector or Nrf2 were detected by flow cytometric analysis. Vor indicates vorinostat. Numbers indicate mean values of H2O2 levels.
It has been suggested that the transcription factor Nrf2 plays a critical role in modulating expression of genes that function as antioxidants, phase 2 detoxification enzymes, and heat shock proteins.15,16 We also studied the mRNA levels of Nrf2. Nrf2 gene expression was not affected after treatment with vorinostat (Figure 3A). It has been shown that, in the normal physiologic state, Nrf2 localizes in the cytoplasm and interacts with Kelch-like ECH-associating protein 1 (Keap1).30 Modification of Keap1 by ROS dissociates Nrf2 from Keap1 and induces the translocation of Nrf2 to nucleus, leading to transactivation of its downstream target genes.31,32 We examined the localization of Nrf2 after vorinostat treatment using immunofluorescence and confocal microscopy. Nrf2 protein and nucleus were detected as red and blue fluorescent signals, respectively. As shown in Figure 3B, before vorinostat treatment, U937 cells displayed condense ring-like red fluorescence in cytoplasm around the nucleus. After incubation with 2μM vorinostat for 18 hours, the ring-like red fluorescence decreased in a majority of cells and the red fluorescence increased in the nucleus, which indicates translocation of Nrf2 protein to the nucleus. To confirm the role of Nrf2 in protecting cells against ROS stress induced by vorinostat, HEK293 cells were transfected with pCMV6-XL5 vector containing Nrf2 cDNA. Vector without the Nrf2 sequence was used as control. Introduction of Nrf2 was verified by Western blot analysis 40 hours after transfection (Figure 3C). Expression of GCLC, a gene that has been shown to be regulated by Nrf2,15 was also increased. Incubation with 5μM vorinostat for another 24 hours induced a 2-fold increase of ROS generation in HEK293 cells transfected with empty vector (Figure 3D). However, ROS level was not affected by vorinostat treatment in cells transfected with Nrf2 (Figure 3D), indicating that the increase of ROS stress was rescued by overexpression of Nrf2. Taken together, these observations indicate that Nrf2 and its downstream antioxidants mediate cellular protection against ROS stress induced by vorinostat.
PEITC enhances vorinostat cytotoxicity in myeloid cell lines as well as primary human leukemia cells by modulating cellular redox status
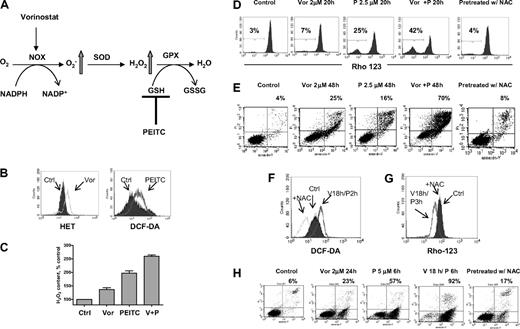
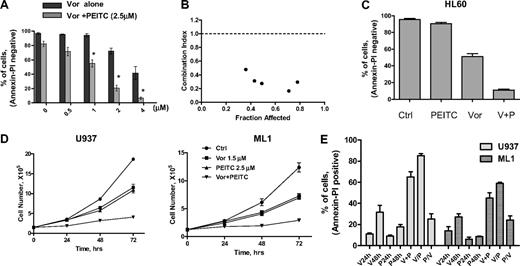
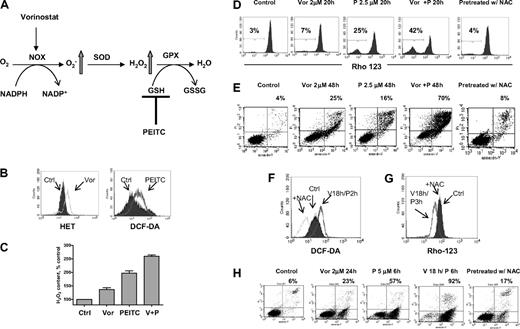
Based on clinical data13 and results shown here, we postulated that PEITC, a novel compound that has been shown to deplete cellular glutathione,19,20 may circumvent resistance to HDACIs and maximize their antileukemia activity. The rationale for our hypothesis is illustrated in Figure 4A. It is known that O2− and H2O2 are 2 major forms of ROS. Vorinostat activates NOX and increases O2−, which is further converted to H2O2 by SOD. H2O2 is eliminated by GPX using GSH as substrate. Addition of PEITC would decrease GSH levels and cause further increase of H2O2. To test such a possibility, a combination of vorinostat and PEITC was first investigated in U937 cells. Previous reports indicated that 5μM PEITC induced selective killing of primary chronic lymphocytic leukemia cells with minimal toxicity to normal lymphocytes.20 We used a lower concentration of PEITC (2.5μM) in combination with vorinostat to treat U937 cells. In Figure 4B, flow cytometric analysis demonstrated that 2μM vorinostat increased O2− generation and 2.5μM PEITC increased H2O2 generation in U937 cells after 18 hours of incubation. The combination of these 2 compounds resulted in additive accumulation of H2O2 (Figure 4C). This led to subsequent loss of mitochondrial transmembrane potential, indicating early induction of apoptotic cell death. As shown in Figure 4D, 2μM vorinostat caused a minimal effect on mitochondrial transmembrane potential after 20 hours of incubation; 2.5μM PEITC induced 25% loss of transmembrane potential after 20 hours. Their combination resulted in more than additive effect as the cell loss was 42%. Most importantly, the combination effect was reversed by pretreatment with 5mM NAC, a GSH precursor. This indicates that accumulation of H2O2 contributes to the cytotoxic effect induced by vorinostat plus PEITC. Annexin V/PI assay (Figure 4E) further confirmed that vorinostat and PEITC had more than an additive cytotoxic effect in U937 cells after a 48-hour incubation and that supplementation with the antioxidant NAC prevented cell death. We then tested a higher concentration of PEITC (5μM) with vorinostat. U937 cells were treated with 2μM vorinostat for 18 hours followed by 5μM PEITC for another 2 hours. Such a combination resulted in a significant accumulation of ROS, which was also prevented by pretreatment with 5mM NAC (Figure 4F). Correspondingly, mitochondrial transmembrane potential was decreased after treatment with such a combination. The loss of mitochondrial transmembrane potential was also completely reversed by pretreatment with the same concentration of NAC (Figure 4G). Treatment with 2μM vorinostat for 18 hours followed by 5μM PEITC treatment for 6 hours resulted in significant apoptotic cell death (92%), as vorinostat and PEITC alone caused 23% and 57% cell death alone, respectively. Consistently, pretreatment with 5mM NAC also protected the cell death induced by the combination (Figure 4H). U937 cells were then treated with various concentrations of vorinostat with a fixed concentration of PEITC (2.5μM), which by itself was a marginally toxic concentration as shown in Figure 4E. In this experiment, PEITC significantly enhanced the cytotoxicity induced by vorinostat at various concentrations (Figure 5A). An MTT assay was used to further test the combination effect of vorinostat and PEITC. The median-effect analysis program (Calcusyn) was used to calculate the drug combination index. The combination index value was less than 1.0 (Figure 5B), indicating a synergistic interaction between vorinostat and PEITC. HL60 cells were more sensitive to the combination of vorinostat and PEITC than a single agent (Figure 5C).
Combination of vorinostat and PEITC induced ROS-mediated apoptosis. (A) Illustration of biochemical strategy to enhance cellular ROS accumulation. Vorinostat induced NOX-derived O2−, which is further converted to H2O2. PEITC was used to inhibit H2O2 elimination by GSH system and thus caused further ROS stress. (B) Treatment with 2μM vorinostat and 2.5μM PEITC for 18 hours caused significant increase of O2− and H2O2 in U937 cells, respectively, as detected by fluorescent probes Het and DCF-DA. (C) Quantitative analysis of H2O2 accumulation induced by 2μM vorinostat alone (Vor), 2.5μM PEITC alone, and vorinostat plus PEITC (V + P). Bars represent mean ± SD from 3 experiments. (D) Change of mitochondrial transmembrane potential in U937 cells treated with the indicated compounds was detected by flow cytometry using rhodamine-123. Numbers indicate percentage of cells with loss of transmembrane potential. Pretreatment with 5mM NAC 1 hour before coadministration of vorinostat and PEITC completely blocked the loss of transmembrane potential induced by such combination. (E) Apoptosis induced by the indicated compounds and protection of cell death by pretreatment with 5mM NAC 1 hour before was detected by double staining of annexin V/PI. Numbers indicate percentage of cell death population. (F-G) Incubation with 2μM vorinostat for 18 hours followed by 5μM PEITC for another 2 hours (V18h/P2h) also induced significant increase of H2O2 in U937 cells and subsequent decrease of mitochondrial transmembrane potential (V18h/P3h, vorinostat for 18 hours followed by PEITC for 3 hours). Pretreatment with 5mM NAC 1 hour before inhibited both increase of H2O2 and loss of mitochondrial transmembrane potential (+NAC). (H) Incubation with 2μM vorinostat for 18 hours followed by 5μM PEITC for 6 hours resulted in tremendous apoptosis as demonstrated by annexin V/PI assay. Pretreatment with 5mM NAC significantly prevented the cell death induced by the combination treatment. Numbers indicate percentage of apoptotic cell death population.
Combination of vorinostat and PEITC induced ROS-mediated apoptosis. (A) Illustration of biochemical strategy to enhance cellular ROS accumulation. Vorinostat induced NOX-derived O2−, which is further converted to H2O2. PEITC was used to inhibit H2O2 elimination by GSH system and thus caused further ROS stress. (B) Treatment with 2μM vorinostat and 2.5μM PEITC for 18 hours caused significant increase of O2− and H2O2 in U937 cells, respectively, as detected by fluorescent probes Het and DCF-DA. (C) Quantitative analysis of H2O2 accumulation induced by 2μM vorinostat alone (Vor), 2.5μM PEITC alone, and vorinostat plus PEITC (V + P). Bars represent mean ± SD from 3 experiments. (D) Change of mitochondrial transmembrane potential in U937 cells treated with the indicated compounds was detected by flow cytometry using rhodamine-123. Numbers indicate percentage of cells with loss of transmembrane potential. Pretreatment with 5mM NAC 1 hour before coadministration of vorinostat and PEITC completely blocked the loss of transmembrane potential induced by such combination. (E) Apoptosis induced by the indicated compounds and protection of cell death by pretreatment with 5mM NAC 1 hour before was detected by double staining of annexin V/PI. Numbers indicate percentage of cell death population. (F-G) Incubation with 2μM vorinostat for 18 hours followed by 5μM PEITC for another 2 hours (V18h/P2h) also induced significant increase of H2O2 in U937 cells and subsequent decrease of mitochondrial transmembrane potential (V18h/P3h, vorinostat for 18 hours followed by PEITC for 3 hours). Pretreatment with 5mM NAC 1 hour before inhibited both increase of H2O2 and loss of mitochondrial transmembrane potential (+NAC). (H) Incubation with 2μM vorinostat for 18 hours followed by 5μM PEITC for 6 hours resulted in tremendous apoptosis as demonstrated by annexin V/PI assay. Pretreatment with 5mM NAC significantly prevented the cell death induced by the combination treatment. Numbers indicate percentage of apoptotic cell death population.
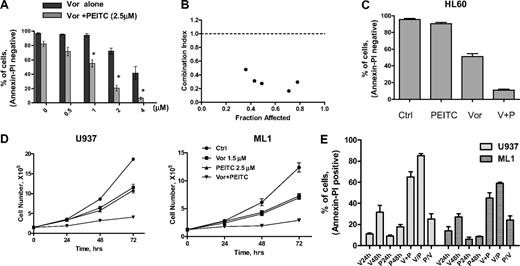
Synergistic activity between vorinostat and PEITC in various myeloid leukemia cell lines. (A) Dose-dependent induction of apoptosis in U937 by vorinostat in the absence and presence of PEITC (2.5μM, 48 hours) was analyzed with annexin V/PI assay. Data represent mean ± SD from 3 experiments. Addition of 2.5μM PEITC significantly enhanced the cytotoxicity of vorinostat alone. *P < .01 (Student t test). (B) Combination index (CI) of vorinostat and PEITC in U937 cells were analyzed by median dose-effect method using Calcusyn Version 2.0 software (Biosoft). U937 cells were incubated with various concentrations of vorinostat (0.3-4μM) and PEITC (0.5-3μM) for 72 hours. Drug effect on cell viability was determined by MTT assay as described in “Cytotoxicity assays.” CI = 1 indicates additive effect; CI < 1 indicates synergistic effect; and CI > 1 indicates antagonist effect. (C) HL60 cells were treated with 1μM vorinostat alone, 1μM PEITC alone, or vorinostat plus PEITC (V + P) for 48 hours, and killing effect was analyzed with annexin V/PI assay. Bars represent mean ± SD from 3 experiments. (D) Growth inhibition by vorinostat and PEITC in U937 and ML1 cells. Cells in exponential growth were treated with vorinostat or/and PEITC at the indicated concentration. Cell culture was continued for up to 72 hours, and total cell numbers were counted every 24 hours using a Coulter Z2 Particle Counter & Size Analyzer. (E) Sequence-dependent effect of vorinostat combined with PEITC in U937 and ML1 cells. U937 and ML1 cells were both treated with 2μM vorinostat and 2.5μM PEITC concomitantly for 48 hours (V + P) or treated with vorinostat for 24 hours followed by PEITC for another 24 hours (V/P), or treated with PEITC for 24 hours followed by vorinostat for another 24 hours (P/V). Cytotoxic effect of each compound alone or their combination was analyzed with annexin V/PI assay.
Synergistic activity between vorinostat and PEITC in various myeloid leukemia cell lines. (A) Dose-dependent induction of apoptosis in U937 by vorinostat in the absence and presence of PEITC (2.5μM, 48 hours) was analyzed with annexin V/PI assay. Data represent mean ± SD from 3 experiments. Addition of 2.5μM PEITC significantly enhanced the cytotoxicity of vorinostat alone. *P < .01 (Student t test). (B) Combination index (CI) of vorinostat and PEITC in U937 cells were analyzed by median dose-effect method using Calcusyn Version 2.0 software (Biosoft). U937 cells were incubated with various concentrations of vorinostat (0.3-4μM) and PEITC (0.5-3μM) for 72 hours. Drug effect on cell viability was determined by MTT assay as described in “Cytotoxicity assays.” CI = 1 indicates additive effect; CI < 1 indicates synergistic effect; and CI > 1 indicates antagonist effect. (C) HL60 cells were treated with 1μM vorinostat alone, 1μM PEITC alone, or vorinostat plus PEITC (V + P) for 48 hours, and killing effect was analyzed with annexin V/PI assay. Bars represent mean ± SD from 3 experiments. (D) Growth inhibition by vorinostat and PEITC in U937 and ML1 cells. Cells in exponential growth were treated with vorinostat or/and PEITC at the indicated concentration. Cell culture was continued for up to 72 hours, and total cell numbers were counted every 24 hours using a Coulter Z2 Particle Counter & Size Analyzer. (E) Sequence-dependent effect of vorinostat combined with PEITC in U937 and ML1 cells. U937 and ML1 cells were both treated with 2μM vorinostat and 2.5μM PEITC concomitantly for 48 hours (V + P) or treated with vorinostat for 24 hours followed by PEITC for another 24 hours (V/P), or treated with PEITC for 24 hours followed by vorinostat for another 24 hours (P/V). Cytotoxic effect of each compound alone or their combination was analyzed with annexin V/PI assay.
A cell proliferation assay was also used to test the effect of the combination on leukemia cell growth. A subtoxic concentration of vorinostat (1.5μM) was used in combination with 2.5μM PEITC to treat both U937 and ML1 cells. The number of cells was counted for 3 consecutive days by Coulter Counter. Vorinostat or PEITC alone caused a similar delay of cell growth in both U937 and ML1 cells, but the combination significantly enhanced the cell growth inhibition compared with a single agent alone (Figure 5D).
We then tested the effect of different sequences of vorinostat/PEITC administration on cell viability. Both U937 and ML1 cells were treated with 2μM vorinostat and 2.5μM PEITC concomitantly, or with vorinostat before and after PEITC. Figure 5E shows an increase of cell death induced by coadministration of vorinostat and PEITC after 48 hours compared with single agents alone. Pretreatment of vorinostat followed by PEITC induced 10% to 20% additional apoptosis. However, pretreatment of PEITC followed by vorinostat exhibited 30% to 40% less killing compared with simultaneous treatment. Therefore, the combination effect of vorinostat and PEITC is schedule-dependent. The combination with PEITC was able to increase ROS levels and reverse vorinostat resistance in HL60/LR cells (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Treatments of of 2μM and 4μM vorinostat combined with 2.5μM PEITC caused 70% and 140% increase of ROS production, respectively. Incubation with 4μM vorinostat for 48 hours induced only 25% cell death. A combination with 2.5μM PEITC, which killed 19% cells, enhanced cell death to 70% (supplemental Figure 1B).
We also tested the combination effect using MGCD0103, a nonhydroxamic acid class I–specific HDACI,33 with PEITC. A similar synergistic cytotoxic effect was also observed in several leukemia cell lines (data not shown).
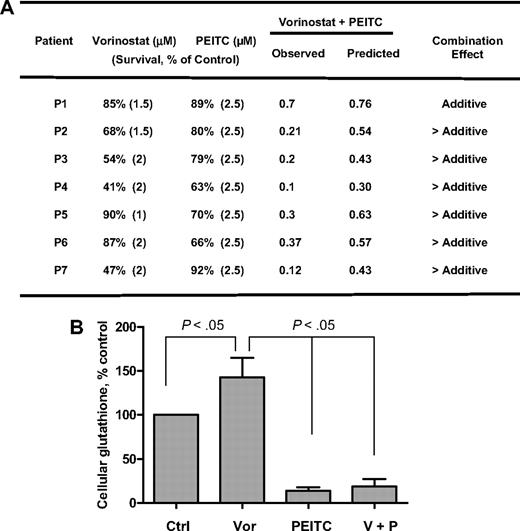
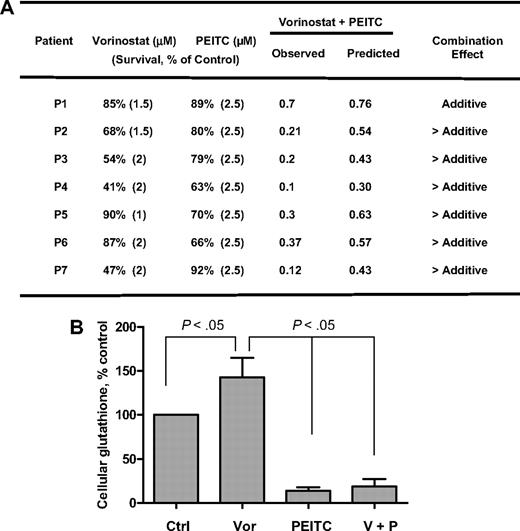
Finally, the effects of vorinostat, PEITC, and their combination were tested in primary leukemia cells isolated from 7 AML patients with high blast counts in peripheral blood. As shown in Figure 6A, fresh leukemia cells were treated with 1μM, 1.5μM, and 2μM vorinostat alone, 2.5μM PEITC alone, and their combination for 48 hours. Vorinostat caused 10% to 53% cell death in different patient samples. PEITC at a fixed concentration of 2.5μM caused 8% to 37% cell death in these samples. The results shown in Figure 6A indicate that the combination effect is more than additive in 6 of 7 patient samples. Analysis of cellular glutathione in 4 patient samples revealed that 2μM vorinostat caused a 40% increase of GSH on average after 16 hours, probably because of secondary defense response to ROS stress induced by vorinostat. Yet addition of 2.5μM PEITC for 18 hours dramatically decreased cellular glutathione (Figure 6B). These data indicate that cells treated with vorinostat may rely on glutathione buffering system against ROS stress for cell survival, suppression of glutathione may cause further oxidative stress to the point that triggers subsequent cell death as shown in Figure 6A. We also tested the combination in 4 normal peripheral blood samples and found that vorinostat (1-2μM) induced similar cell death in normal cells as in AML cells. However, in contrast to 8% to 37% cell death in AML cells, 2.5μM PEITC did not have any effect in normal cells, which is consistent with the selective killing of PEITC in neoplastic cells previously reported.20
Cytotoxicity of vorinostat, PEITC, and their combination in fresh myeloid leukemia cells. (A) Fresh primary leukemia cells isolated from 7 patients with AML were each treated with various concentrations of vorinostat (1μM, 1.5μM, and 2μM) and 2.5μM PEITC alone or their combination for 48 hours. Cell viability was determined by annexin V/PI assay. The expected viable cell fraction (percentage) was calculated by multiplying percentage viable cells in the vorinostat-treated sample with the percentage of viable cells in the PEITC-treated sample. The combination effect is considered additive when the observed viable cell fraction is equal to the expected value. When observed value is less than the expected value, the combination effect is considered more than additive. (B) Total glutathione levels in primary AML cells treated with 2μM vorinostat or/and 2.5μM for 16 hours. Values are normalized to the levels in untreated cells. Bars represent mean ± SD (n = 4 AML patient samples).
Cytotoxicity of vorinostat, PEITC, and their combination in fresh myeloid leukemia cells. (A) Fresh primary leukemia cells isolated from 7 patients with AML were each treated with various concentrations of vorinostat (1μM, 1.5μM, and 2μM) and 2.5μM PEITC alone or their combination for 48 hours. Cell viability was determined by annexin V/PI assay. The expected viable cell fraction (percentage) was calculated by multiplying percentage viable cells in the vorinostat-treated sample with the percentage of viable cells in the PEITC-treated sample. The combination effect is considered additive when the observed viable cell fraction is equal to the expected value. When observed value is less than the expected value, the combination effect is considered more than additive. (B) Total glutathione levels in primary AML cells treated with 2μM vorinostat or/and 2.5μM for 16 hours. Values are normalized to the levels in untreated cells. Bars represent mean ± SD (n = 4 AML patient samples).
Discussion
Vorinostat is a hydroxamic acid analog and a pan-HDACI. Clinically, vorinostat has been approved for the treatment of cutaneous T-cell lymphoma and has modest single-agent activity in advanced AML and myelodysplastic syndrome.13 HDACIs induce acetylation of histones and other proteins and restore epigenetically suppressed genes inducing reexpression of functional molecular pathways. HDACIs also induce apoptosis, autophagy, cell-cycle arrest, and senescence.1 Despite their well-characterized effects in vitro and in vivo, the activity of this class of compounds has been modest in leukemia. We had previously reported a phase 1 study of vorinostat in a patient with advanced leukemias and myelodysplastic syndrome.13 Overall, 17% patients achieved response. We detected that increased baseline expression of 17 antioxidant genes correlated with vorinostat resistance. The objectives of this study were to investigate the molecular mechanism of action of HDACIs in the context of ROS generation and the role of antioxidants in resistance to HDACIs. The understanding of mechanisms of resistance to HDACIs will allow development of effective combination strategies to maximize the cytotoxic effect of this class of compounds.
A stable pan-HDACI–resistant cell line, HL60/LR, has been generated by culturing HL60 cells under constant selection pressure of the hydroxamic acid analog LAQ824.22 We first compared the redox status of parental HL60 cells and HL60/LR cells. A series of GSH-related enzymes were significantly up-regulated both at the mRNA and protein levels in HL60/LR cells. These included: GCLC, the rate-limiting enzyme for GSH synthesis; GSR, the enzyme responsible for GSH regeneration; GPX, an antioxidant that uses GSH as substrate to eliminate H2O2; and GST-pi, a key enzyme that uses GSH as substrate to detoxify xenobiotics. Probably because of an increase of enzymes responsible for GSH synthesis and regeneration, total cellular glutathione level was also increased in the resistance cells. Moreover, SOD, the only enzyme responsible for elimination of O2− by converting it to H2O2, was also markedly elevated in HL60/LR cells. It is known that GSH is the major component of cellular thiol buffer and protects cells against oxidants, such as H2O2, and xenobiotics, such as chemotherapeutic agents.14 These findings suggest that during cellular selection with an HDACI, the antioxidant system may have emerged to protect HL60/LR cells from ROS stress. Indeed, here we showed that vorinostat induced an early increase of O2− after 18 hours (Figure 1D). However, both O2− and H2O2 levels decreased after a long-term incubation with LAQ824 (Figure 1E-F). Thus, up-regulation of SOD and GSH is probably secondary to accumulation of oxidative stress induced by HDACI and may protect cells from ROS-mediated cytotoxicity. Consistent with these findings in the pan-HDACI–resistant cell model, SOD and a series of GSH-related enzymes were also found to be elevated in patients who did not have a response to vorinostat in a phase 1 clinical trial.13 Taken together, these findings suggest that high levels of antioxidants, specifically SOD and GSH, correlate with both de novo and acquired resistance to HDACIs.
Expression of SOD, GPX, GST-Pi, and GCLC is regulated by the transcription factor Nrf2.15 Nrf2 localizes in the cytoplasm under normal conditions where it interacts with Keap1. Oxidative modification of Keap 1 causes it to dissociate from Nrf2, allowing Nrf2 translocation to the nucleus where it binds to antioxidant responsive elements.15 We postulated that vorinostat-induced ROS may cause Nrf2-mediated up-regulation of antioxidants. Confocal microscopic analysis demonstrated translocation of Nrf2 from cytoplasm to nucleus in U937 cells after treatment of vorinostat (Figure 2B). Overexpression of Nrf2 in HEK293 cells reversed the increase of ROS induced by vorinostat (Figure 2C), which further confirmed the role of Nrf2 in cellular defense against ROS stress induced by this compound.
The source of HDACI-induced ROS remains unclear. Here, we show that treatment of vorinostat caused activation of the ROS-generating enzyme NOX in a time-dependent manner in different myeloid leukemia cell lines. Vorinostat induced ROS in a mitochondrial deficient cell line HL60/C6F to the same extent as in the parental HL60. This indicates that NOX is central for vorinostat-mediated ROS generation. NOX is essential for Nrf2-mediated induction of GCLC in response to ROS stress.34 Our data indicate that overexpression of Nrf2 resulted in up-regulation of GCLC and subsequent inhibition of ROS generation induced by vorinostat.
It should be noted that earlier studies have reported that vorinostat can induce expression of Trx binding protein 2 in prostate and lung cancer models.17,18 Trx binding protein 2 binds to and inactivates thioredoxin (Trx), another important antioxidant that scavenges ROS. Therefore, inhibition of Trx may also contribute to ROS stress induced by HDACIs. Interestingly, we also found that Trx protein expression was significantly decreased in HL60/LR cells compared with parental HL60 cells,22 yet ROS production remained suppressed in HL60/LR cells. These findings suggest that, in HDACI-resistant cells, the redox mechanism involving Trx may not be functioning, and during selection with an HDACI other antioxidant mechanisms emerged to cope with ROS stress. Up-regulation of a series of glutathione-associated enzymes in HL60/LR cells demonstrated the critical role of the glutathione system in protecting cells from ROS-mediated cell injury induced by HDACIs.
The rates of endogenous ROS production and elimination are 2 major factors that determine overall cellular ROS levels. A combination of 2 compounds that stimulate endogenous ROS generation and inhibit ROS elimination would be a logical strategy to create severe cellular oxidative stress. We hypothesized that PEITC, a compound that has been reported to deplete GSH, could circumvent antioxidant protection and enhance the antileukemia activity of vorinostat. The selective cytotoxic effect of PEITC against neoplastic cells19,20 also makes it a potential candidate for combination with vorinostat. Here we demonstrated that vorinostat induced significant O2− generation through NOX. PEITC induced significant increase of H2O2 by eliminating glutathione. Their combination effectively enhanced cellular ROS accumulation and significantly increased apoptotic cell death compared with vorinostat or PEITC alone. NAC, an antioxidant, effectively suppressed ROS accumulation and protected cells from drug-induced apoptotic cell death, indicating a critical role of ROS in mediating the cytotoxic effect induced by combination. Importantly, PEITC was also able to increase ROS levels and reverse vorinostat resistance in HL60/LR cells.
The cytotoxic effect of the combination was schedule dependent. The effect of vorinostat followed by PEITC incubation was more effective than coadministration of vorinostat plus PEITC, whereas PEITC followed by vorinostat incubation exhibited less cell killing activity than concomitant treatment. It has been shown that PEITC depletes glutathione by formation of glutathione conjugate in leukemia cells, and such reaction may be rapid and reversible.35,36 It is probable that pretreatment of vorinostat caused ROS stress in leukemia cells, which were highly dependent on the major antioxidant component GSH for survival. PEITC may react rapidly with GSH and further provide an oxidative trigger for apoptotic cell death. If leukemia cells were pretreated with PEITC followed by vorinostat, the peak of glutathione conjugation would have occurred before vorinostat-induced up-regulation of the GSH buffer system. We also tested the activity of the combination in primary AML cells and normal mononuclear cells. We confirmed the synergistic cytotoxicity effect also in these primary cells.
To analyze whether the findings observed here were specific for vorinostat, we also used MGCD0103, a benzamide that inhibits class I HDACI. Similar synergistic interactions, compared with those observed with vorinostat, were detected. This indicates that the synergistic effect is not limited to one class of HDACIs.
Finally, it should be noted that previous reports have indicated that Nrf2 is a target of acetylation.37 Therefore, it is possible that HDACIs may modulate acetylation status of Nrf2 and contribute to Nrf2-dependent antioxidant response. The future study of Nrf2 acetylation status after treatment with HDACIs is of importance to further define the role of Nrf2 in resistance to HDACIs.
There are several limitations to this study: (1) It is possible that ROS-mediated cytotoxic effects shown here may not be specific to HDACIs. This is of importance as combinations of PEITC with other chemotherapy agents could have similar effects and therefore broaden the implications of the results shown here. (2) We did not significantly investigate the role of nonhistone acetylation induced by HDACI in this paper. Acetylation of critical modulators of apoptosis, such as p53, could be important to explain the apoptotic effects observed in this study and should be further investigated in the future. (3) Finally, the role of NOX in HDACI induced-ROS needs to be further investigated as well and confirmed in other model systems.
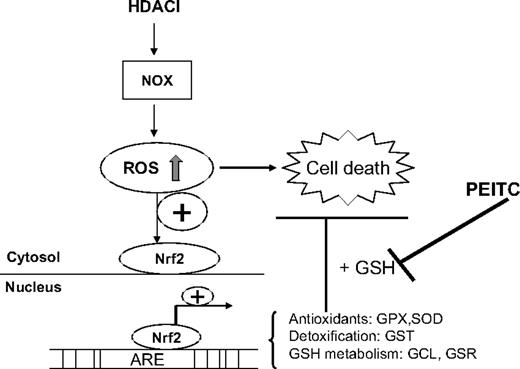
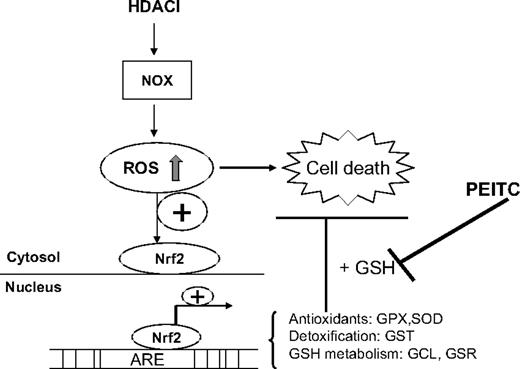
In conclusion, as shown in our proposed model in Figure 7, we have identified a redox-mediated pathway that contributes to the antileukemia activity of HDACIs and resistance of leukemia cells to HDACIs. Disruption of antioxidant-mediated cytoprotective pathway by PEITC significantly enhances the antileukemia activity of HDACIs. The combination of HDACIs and PEITC results in synergistic and selective cytotoxic effect against leukemia cells and thus has significant clinical implications. Based on our observations, we are currently pursuing a phase 1 study of HDACIs in combination with inhibitors of antioxidant pathway currently used in the clinic.
Proposed model of combination effect of an HDACI and PEITC on leukemia cells. An HDACI activates NOX and induces ROS stress, which contributes to cellular damage and cytotoxicity. As a secondary response, increase of ROS also results in translocation of Nrf2, a transcription factor, from cytosol to nucleus, leading to up-regulation of its downstream targets, including antioxidants and phase 2 detoxification genes. Among these genes, the effectiveness of GST to detoxify foreign compounds depends on GSH levels, which is determined by GCL, the key enzyme for glutathione synthesis and GSR, the enzyme responsible for glutathione regeneration. GPX relies on glutathione as a substrate to eliminate H2O2. As such, GSH system is critical for cellular defense against oxidative injury. Addition of PEITC, the compound capable of depleting cellular GSH, inhibits such defense system and potentiates the antileukemia activity of HDACIs.
Proposed model of combination effect of an HDACI and PEITC on leukemia cells. An HDACI activates NOX and induces ROS stress, which contributes to cellular damage and cytotoxicity. As a secondary response, increase of ROS also results in translocation of Nrf2, a transcription factor, from cytosol to nucleus, leading to up-regulation of its downstream targets, including antioxidants and phase 2 detoxification genes. Among these genes, the effectiveness of GST to detoxify foreign compounds depends on GSH levels, which is determined by GCL, the key enzyme for glutathione synthesis and GSR, the enzyme responsible for glutathione regeneration. GPX relies on glutathione as a substrate to eliminate H2O2. As such, GSH system is critical for cellular defense against oxidative injury. Addition of PEITC, the compound capable of depleting cellular GSH, inhibits such defense system and potentiates the antileukemia activity of HDACIs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Leukemia & Lymphoma Society of America, the Ruth Arnold Fund, and the M. D. Anderson Cancer Center (G.G.-M.).
Authorship
Contribution: Y.H. designed and performed research, analyzed data, and wrote the manuscript; W.L., G.C., H.Z., Y.J., Y.W., H.Y., and W.Z. performed research and analyzed data; W.F. and K.B. provided critical reagents and analyzed data; M.K. analyzed data and helped with manuscript preparation; and P.H. and G.G.-M. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermo Garcia-Manero, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ggarciam@mdanderson.org.