Abstract
Malignant cells are capable of influencing the microenvironment in a manner that facilitates tumor cell survival. Bidirectional crosstalk between chronic lymphocytic leukemic (CLL) cells and marrow-derived mesenchymal stromal cells (MSCs) activates both cell types. In this study, we observed that the conditioned medium (CM) obtained from CLL cells was able to induce Akt activation in MSC. Subsequent studies investigated the mechanism of MSC activation mediated by CLL-CM. Platelet-derived growth factor receptors (PDGFRs) were selectively activated in MSCs by CLL-CM and found to be critical receptors for CLL-CM–driven MSC proliferation and MSC Akt activation. The known ligands of PDGFR, platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), were detected in CLL-CM, but PDGF was the predominant ligand involved in the CM-mediated PDGFR activation. Both PDGF and VEGF were found to be elevated in the plasma of CLL patients with a positive association for high-risk factors and more advanced stage. Finally, we demonstrated that PDGF induced MSC VEGF production through a phosphatidylinositol 3-kinase (PI3K)–dependent mechanism. These results show that PDGF-PDGFR signaling influences at least the MSC in the microenvironment of CLL and may play a role in the induction of an angiogenic switch known to be permissive for disease progression.
Introduction
The cancer microenvironment has been implicated as playing a critical role(s) in cancer initiation and progression. There are multiple cellular components present in cancer microenvironments, which in general include immune/inflammatory cells such as lymphocytes, monocytes/macrophages and neutrophils/mast cells, fibroblast-like cells such as cancer-associated fibroblasts, mesenchymal stromal cells (MSCs), and vascular cells such as endothelial cells, pericytes, and smooth muscle cells.1 Chronic lymphocytic leukemia (CLL) has long been recognized as a cumulative disease resulting from a failure of apoptosis; however, recent evidence also points out that CLL is a dynamic disease of leukemic cell proliferation and circulation.2,3 Indeed, proliferation centers in CLL have been identified in lymph nodes, bone marrow, and spleen.4 In vitro experiments have demonstrated the importance of stromal cellular components including bone marrow stromal cells,5,6 nurse-like cells,7 and T cells8,9 in maintaining CLL cell survival and, to a lesser degree, leukemic cell proliferation.9,10 However, the exact mechanism and nature of the interactions between diverse stromal cellular components and CLL leukemic cells remains to be clearly determined.
MSCs comprise one cellular component in the cancer microenvironment, and these cells have recently been studied in terms of the immune regulatory function, cancer promoting function, and wound repair function in a variety of disease models.11 We have established an in vitro culture system to generate bone marrow stromal cells and demonstrated that they are mesenchymal stem cell in nature.10,12 When we examined the MSC-CLL B-cell interaction, we found active bidirectional crosstalk was present between the 2 cell types, and this crosstalk was able to activate both cell types.10 This interaction was associated with both an increase in vascular endothelial growth factor (VEGF) and a decrease in thrombospondin-1 (TSP-1) expression, which taken together is representative of an angiogenic switch,13 a phenomenon known to be associated with disease progression in malignancy.13 Therefore, in our study, we were interested in defining the signals sent from leukemic cells to MSC and how these interactions modify MSC function. Our studies demonstrate that platelet-derived growth factor (PDGF), secreted by CLL cells, activated MSCs via its interaction with the PDGF receptor (PDGFR) present on the MSC membrane. This activation by PDGF was able to enhance MSC proliferation and induce AKT phosphorylation, which was necessary for MSC VEGF production.
Methods
Patient population
Blood and bone marrow biopsies were obtained from CLL patients who had provided written informed consent under a Mayo Clinic Institutional Review Board approved protocol in accordance with the Declaration of Helsinki. All CLL patients had a confirmed diagnosis using the National Cancer Institute Working Group definition.14 Patients in this study cohort were from all Rai stages and had not been treated for at least 6 months prior to blood processing and the use of their blood cells in the experiments described.
Preparation of purified CLL lymphocytes and generation of conditioned medium from CLL cells
CLL cells were isolated from heparinized venous blood by density gradient centrifugation. Purified lymphocytes from CLL patients were cultured in AIM-V medium (Invitrogen) for the laboratory studies described. To generate CLL conditioned medium (CLL-CM), freshly isolated peripheral blood mononuclear cells (PBMCs) from CLL patients were first depleted of platelets by centrifugation at 54g for 20 minutes once or twice. CLL PBMCs isolated in this manner routinely demonstrated < 5% CD61+ cells (a platelet marker). Only CLL PBMCs consisted of > 92% CLL B cells, as revealed by CD19/CD5 2-color staining, were used in this study. These cells were then cultured for 5 days in AIM-V at a concentration of 5 × 106/mL before harvest of cell-free media by centrifugation of the cultured cell suspension at 699g for 5 minutes. For hypoxia experiments, platelet-depleted CLL PBMCs were cultured at 37°C in a humidified atmosphere containing 1% O2 for approximately 16 hours.
Bone biopsy–derived stromal cell cultures
Primary bone biopsy stromal cell cultures were established using a modified version of a previous published technique.12 The stromal cells used in the studies described here were from passages 1 to 6.
Cell migration assay
The migration assay was carried out as previously described15,16 with a slight modification. Briefly, Falcon tissue culture plates with 24 wells along with companion Falcon cell culture inserts were used for the migration assay. MSCs were labeled with calcein AM (5 μg/mL; Invitrogen) for 15 minutes at room temperature, washed, and resuspended in AIM-V medium. CM from CLL cells or AIM-V control medium was plated in the bottom chamber to serve as chemoattractants. Labeled MSC (1 × 104) suspensions were added (400 μL/well) to the upper chamber of the cell culture insert (8-μm pore size filter) and incubated for 16 hours at 37°C with 5% CO2. At the end of the assay, the cell culture inserts were removed, and the 24-well plate containing the migrated MSCs was placed in a multiwell fluorescent plate reader CytoFluor II (PerSeptive Biosystems), and the cells that migrated into the bottom chamber were measured by fluorescence-signal detection. Labeled MSCs (1 × 104) were seeded in a parallel well, and the fluorescence reading of the seeded MSCs was used as the 100% positive control. The percentage of the MSCs that migrated was calculated by dividing the fluorescence intensity of the measured MSC by the positive control.
Cell proliferation and CFU assay
MSCs (0.5-1 × 104) were seeded in a 6-well plate and cultured in 10% α minimum essential medium (αMEM) for 2 days. Subsequently, the medium was changed to either AIM-V medium or CLL-CM mixed with AIM-V at a 1:1 ratio for 10 days. To test the effect of a PDGFR inhibitor on CLL proliferation, 2μM PDGFR tyrosine kinase inhibitor III [Calbiochem; half maximal inhibitory concentration (IC50) = .05μM for α-PDGFR; 0.08μM for β-PDGFR, IC50 ≥ 30μM for epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), Src, PKA, and PKC] was added to the CLL-CM for a subsequent culture period of 10 days. MSCs were cultured in AIM-V medium with escalating doses of PDGF for 10 days; then they were trypsinized and counted with trypan-blue staining to obtain absolute cell numbers.
For the CFU assay, 500 MSCs were seeded in a 6-well plate and cultured in αMEM containing 10% fetal bovine serum (Invitrogen) for 3 days. Subsequently, the medium was changed to either AIM-V medium or CLL-CM mixed with AIM-V at a 1:1 ratio for 1 month. The cells were then stained with 0.2% Coomassie blue and washed with distilled water 3 times. The colonies of stained cells were counted with a densitometer on automatic mode, and images were taken simultaneously.
Human RTK array.
To determine the profile of RTK activated in MSCs, we used Proteome Profiler human phospho-RTK antibody arrays (R&D Systems) according to the manufacturer's instructions. To block the binding of PDGF or VEGF to PDGFR, neutralization antibody specific for PDGF or VEGF (R&D Systems) or both were introduced into the CLL-CM for 30 minutes at a concentration of 5 μg/mL before the CLL-CM was added to MSC cultures. After exposure of MSCs to CLL-CM or PDGF-AB (5 ng/mL; R&D Systems) or VEGF ligand (5 ng/mL; R&D Systems) for 30 minutes, MSCs were lysed in 1% Nonidet P-40 (NP-40) lysis buffer and approximately 150 μg protein were used in RTK array analysis. Pooled CLL plasma from 4 CLL patients (2 patients with Rai stage III; 2 patients with Rai stage I) or pooled normal plasma from 4 healthy subjects were diluted with AIM-V medium at a ratio of 1:4 and were subsequently used in coculture with MSCs for RTK array analysis. Densitometric analysis was performed using AlphaImager 3400 software (Alpha Innotech).
Immunoprecipitation assay.
To confirm the PDGFRα or -β activation status in MSCs, 0.5 × 106 MSCs (cultured in 10-cm dishes) were incubated with 4-5 mL CLL-CM for different time durations and were subsequently lysed in 1% NP-40 buffer. Cell lysates (approximately 200 μg of protein) were incubated overnight with 10 μL anti-PDGFRα or anti-PDGFRβ (rabbit polyclonal antibodies; Santa Cruz Biotechnology) and 30 μL of protein A–sepharose beads (GE Healthcare Biosciences). The beads were washed twice in 1% NP-40 buffer and boiled in sodium dodecyl sulfate (SDS) sample buffer, and the supernatants were collected to analyze the phosphorylated PDGFR by using an immunoblot assay with antiphosphotyrosine monoclonal antibody (4G10; Upstate). The expression of total PDGFRα and PDGFRβ, phosphorylated Akt, Erk, and β-actin was tested by immunoblot assay as described above.
To test the effect of PDGFR inhibition on Akt phosphorylation of MSC, 2μM PDGFR tyrosine kinase inhibitor III (Calbiochem) was used in culture with the CLL MSCs for 30 minutes before the CM of CLL cells were incubated with MSCs for different durations. Subsequently, the MSCs were lysed in 1% NP-40 buffer, and lysates were used for immunoprecipitation and immunoblot analysis as described above.
Western blot to detect activation of proteins in MSCs.
MSCs were cultured until reaching at least 80% confluence, and the initial medium was replaced with AIM-V for 24 hours. Subsequently, MSCs were exposed to the CLL-CM for different duration in individual experiments and washed once with cold phosphate-buffered saline (PBS) and then lysed in 1% NP-40 buffer. Approximately 5-30 μg of protein from MSC lysate were used for Western blot analysis as described before.12 The membranes were then probed with antibodies specific for various signaling proteins. The antibodies to pErk, pAkt, Erk, and Akt were purchased from Cell Signaling Technology. Anti-PDGF AB was obtained from R&D Systems. To confirm equal loading of the blots, the membrane was reprobed with monoclonal β-actin antibody (Novus).
ELISA
The concentration of the following molecules were evaluated in the platelet-poor plasma or conditioned medium of both CLL patients and healthy subjects using a commercial kit (R&D Systems) according to the manufacturer's protocols: PDGF-AB, VEGF, TSP-1, and basic fibroblast growth factor (bFGF). To test whether PDGF could affect VEGF production by MSCs, 5 ng/mL PDGF-AB (R&D Systems) were added to the culture medium (AIM-V) of MSCs for 72 hours in the presence or absence of phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 used at a concentration of 20μM (Calbiochem) or p38 mitogen-activated protein kinase (MAPK) inhibitor SB202190 used at a concentration of 100nM (BIOMOL). The culture medium was then collected for subsequent enzyme-linked immunosorbent assay (ELISA) analysis of VEGF levels.
Clinical characteristic analysis of CLL patients
The following parameters were analyzed for each patient: age, gender, disease stage at diagnosis according to modified Rai criteria, CD38 expression, ζ-chain–associated protein kinase 70 (ZAP-70) expression,17 immunoglobulin variable heavy chain region (IgVH) gene mutational status,18 CLL fluorescence in situ hybridization (FISH) analysis,19 and history of prior therapy regimens. The 38 patients in this study were from all Rai stages and had not been treated for at least 6 months prior to blood processing (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article.).
Statistical analysis
Statistical significance differences were analyzed by the Wilcoxon signed rank test to compare the means of cell migration, proliferation, and CFU analysis. Mann-Whitney test using 2-tail design was used to compare the means of growth factor levels between different groups. The nonparametric Spearman rank correlation test was used to evaluate the potential correlative relationship between plasma PDGF and VEGF levels.
Results
CLL-CM promotes migration and proliferation of MSCs
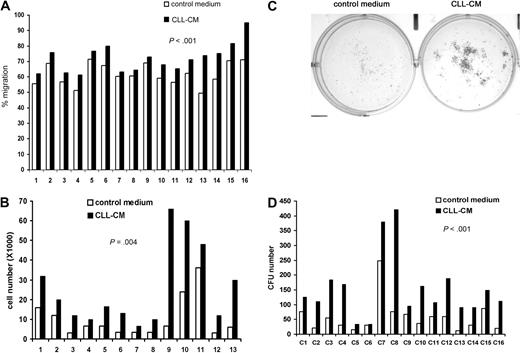
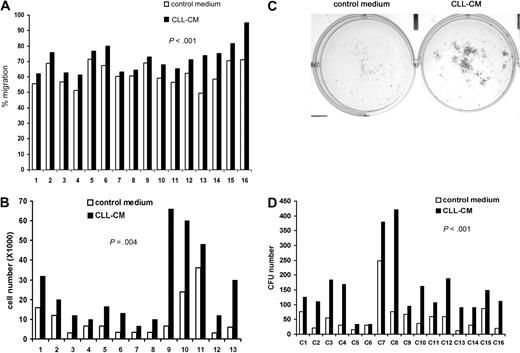
One of our previous findings was that soluble factors secreted from CLL cells were able to activate both Akt and Erk in human MSCs.10 To test whether the soluble factors in CLL-CM are capable of modifying the known intrinsic functions of MSCs, we tested the effect of CM on MSC proliferation and migration. When MSCs were exposed to the CLL-CM, we consistently observed increased migration in MSCs compared with AIM-V control medium (n = 16, mean increase: 9%, P < .001; Figure 1A, the raw data are presented in supplemental Table 2). MSCs from both CLL patients and healthy subjects were used in this study. MSCs cultured with CLL-CM demonstrated significantly more growth compared with control medium AIM-V (n = 13, mean increase: 3.3-fold, P < .001; Figure 1B). To assess the colony formation capacity of MSCs, 500 MSCs were cultured either in AIM-V or CLL-CM for 1 month, and the number of colony units formed (CFU) were counted. Representative pictures of MSC CFU cultured with AIM-V or CLL-CM are demonstrated in Figure 1C. We observed significantly increased CFU in CLL-CM cultured MSCs compared with MSCs cultured with AIM-V control medium (Figure 1D; n = 16, P < .001). The MSCs used in proliferation experiments were different from the ones in CFU assays.
CLL-CM promotes the migration and proliferation of the MSCs. (A) Bar graphs showing MSC migration in response to CLL-CM or control serum-free AIM-V medium. MSCs from 16 different patients were labeled with calcein AM, and the fluorescence intensity of migrated cells measured with a fluorescent plate reader. The percentage of MSC migration was calculated by the fluorescence intensity of the migrated MSC divided by the fluorescence intensity of the same number of MSC seeded in the 24 wells directly as positive control (n = 16, P < .001). (B) The bar graph represents MSC proliferation in response to CLL-CM or control serum-free AIM-V medium (n = 13, P < .001). MSC from 13 different patients were used in this study. (C) Representative photographs of CLL MSC cultured with either AIM-V or CLL-CM for 1 month followed by staining with Coomassie blue for CFU assay. (D) The number of MSC CFU cultured with either CLL-CM or control AIM-V (n = 16, P < .001) is shown in the bar graph. MSCs from 16 different patients were used in the experiments shown in panels A and D. The MSCs used in panels C and D were from patient sources different from the MSCs used in panel B.
CLL-CM promotes the migration and proliferation of the MSCs. (A) Bar graphs showing MSC migration in response to CLL-CM or control serum-free AIM-V medium. MSCs from 16 different patients were labeled with calcein AM, and the fluorescence intensity of migrated cells measured with a fluorescent plate reader. The percentage of MSC migration was calculated by the fluorescence intensity of the migrated MSC divided by the fluorescence intensity of the same number of MSC seeded in the 24 wells directly as positive control (n = 16, P < .001). (B) The bar graph represents MSC proliferation in response to CLL-CM or control serum-free AIM-V medium (n = 13, P < .001). MSC from 13 different patients were used in this study. (C) Representative photographs of CLL MSC cultured with either AIM-V or CLL-CM for 1 month followed by staining with Coomassie blue for CFU assay. (D) The number of MSC CFU cultured with either CLL-CM or control AIM-V (n = 16, P < .001) is shown in the bar graph. MSCs from 16 different patients were used in the experiments shown in panels A and D. The MSCs used in panels C and D were from patient sources different from the MSCs used in panel B.
MSC PDGFR is selectively activated by CLL-CM
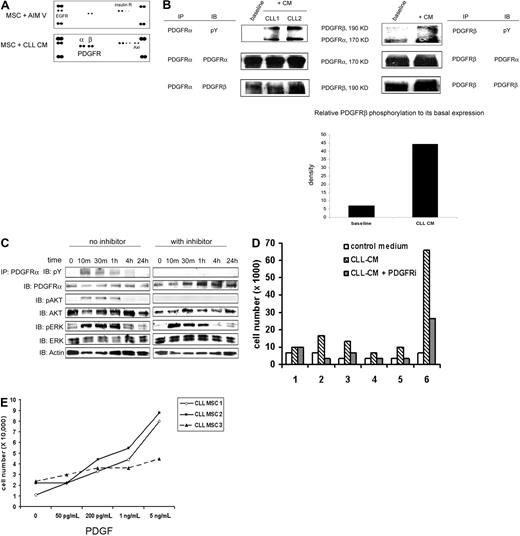
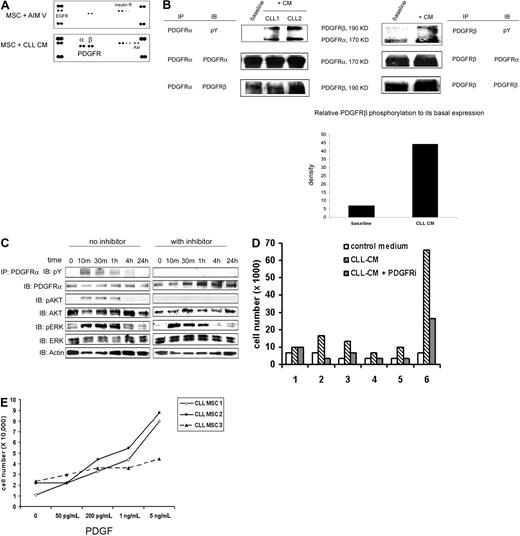
Having demonstrated that soluble factors contained in CLL-CM both modify MSC proliferation and migration, we next investigated the surface receptors on MSCs that can be activated by CLL-CM to then identify the relevant soluble factors. Using an RTK array assay, we found that PDGFRα was selectively activated when MSCs were exposed to CM of cultured CLL B cells for 30 minutes (Figure 2A). PDGFRβ in MSCs was observed to be phosphorylated to a small but certain degree by addition of fresh AIM-V medium but was more potently activated when MSCs were exposed to CLL-CM. PDGFRα appeared to be more selectively phosphorylated compared with PDGFRβ. This finding was validated by demonstrating that PDGFRα and PDGFRβ were indeed phosphorylated as revealed by immunoprecipitating MSC lysate with anti-PDGFRα or anti-PDGFRβ followed by Western blot with antiphosphotyrosine antibody (Figure 2B lanes labeled “+CM”). In addition, we found that a tyrosine-phosphorylated protein of approximately 190 kDa coprecipitated with phosphorylated PDGFRα in the immunoprecipitation experiments using anti-PDGFRα (Figure 2B). After stripping the membrane and immunoblotting with anti-PDGFRβ, we confirmed that the 190-kDa band coprecipitated with PDGFRα was indeed PDGFRβ. PDGFRα was also pulled down when PDGFRβ was immunoprecipitated (Figure 2B). We have also performed the RTK array analysis on MSCs exposed to CLL-CM with or without a platelet-depletion procedure. Selective PDGFRα activation and PDGFRβ activation were still observed in MSC exposed to CLL-CM before and after depletion (supplemental Figure 1). The CD61 percentage varied in different CLL-CM and did not appear to be directly correlated with the intensity of PDGFR activation induced by CLL-CM in RTK analysis. These results suggest CD61+ platelets are not the predominant sources of PDGF production in CLL-CM. We have also observed more activation of Axl receptor in some CM exposed MSCs. The details of Axl activation have recently been published,20 and the mechanism of Axl activation is being further investigated in our group.
PDGFR activated by CLL-CM is upstream of Akt activation and is important for CLL-CM–driven MSC proliferation. (A) RTK array analysis of CLL MSC stimulated by CLL-CM for 30 minutes. The results are representative of more than 3 independent experiments. (B) PDGFRα and PDGFRβ phosphorylation were confirmed by immunoprecipitation of the CM-stimulated MSC lysates (incubation time, 30 minutes) with anti-PDGFRα or anti-PDGFRβ, followed by immunoblot analysis with antiphosphotyrosine antibody. After stripping the membrane, the upper 190-kDa protein coprecipitated with PDGFRα was confirmed to be PDGFRβ. The 170-kDa protein coprecipitated with PDGFRβ was found to be PDGFRα. The density of PDGFRβ phosphorylation and total protein expression was measured by densitometry analysis, and relative density was calculated by calculating the ratio of PDGFRβ phosphorylation to its total protein expression. The bar graph demonstrated the increased PDGFRβ phosphorylation when MSCs were exposed to CLL-CM. The results are representative of independent experiments (n = 3). CM, conditioned medium; pY, anti-phosphotyrosine antibody. (C) Both PDGFRα and Akt in CLL MSCs were phosphorylated by CLL-CM within 10 minutes of exposure. PDGFR tyrosine kinase inhibitor III (2μM) was able to block both PDGFRα and Akt phosphorylation. pAKT, phosphorylated AKT; pERK, phosphorylated ERK. Two gels performed from one immunoprecipitation experiment were run and shown. Individual gels are shown in separate rectangle. (D) PDGFR inhibitor was able to partially block the proliferative effect of CLL-CM on MSCs. Six different CLL MSC sources were used in this experiment. (E) MSC proliferation increased in a dose-dependent manner to escalating doses of PDGF. Three different CLL MSCs were used in this experiment.
PDGFR activated by CLL-CM is upstream of Akt activation and is important for CLL-CM–driven MSC proliferation. (A) RTK array analysis of CLL MSC stimulated by CLL-CM for 30 minutes. The results are representative of more than 3 independent experiments. (B) PDGFRα and PDGFRβ phosphorylation were confirmed by immunoprecipitation of the CM-stimulated MSC lysates (incubation time, 30 minutes) with anti-PDGFRα or anti-PDGFRβ, followed by immunoblot analysis with antiphosphotyrosine antibody. After stripping the membrane, the upper 190-kDa protein coprecipitated with PDGFRα was confirmed to be PDGFRβ. The 170-kDa protein coprecipitated with PDGFRβ was found to be PDGFRα. The density of PDGFRβ phosphorylation and total protein expression was measured by densitometry analysis, and relative density was calculated by calculating the ratio of PDGFRβ phosphorylation to its total protein expression. The bar graph demonstrated the increased PDGFRβ phosphorylation when MSCs were exposed to CLL-CM. The results are representative of independent experiments (n = 3). CM, conditioned medium; pY, anti-phosphotyrosine antibody. (C) Both PDGFRα and Akt in CLL MSCs were phosphorylated by CLL-CM within 10 minutes of exposure. PDGFR tyrosine kinase inhibitor III (2μM) was able to block both PDGFRα and Akt phosphorylation. pAKT, phosphorylated AKT; pERK, phosphorylated ERK. Two gels performed from one immunoprecipitation experiment were run and shown. Individual gels are shown in separate rectangle. (D) PDGFR inhibitor was able to partially block the proliferative effect of CLL-CM on MSCs. Six different CLL MSC sources were used in this experiment. (E) MSC proliferation increased in a dose-dependent manner to escalating doses of PDGF. Three different CLL MSCs were used in this experiment.
PDGFR activation driven by CLL-CM is required for Akt phosphorylation and MSC proliferation
To delineate the activation pattern of PDGFR and possible downstream signal proteins, we tested the time-dependent phosphorylation of PDGFR and Akt in MSC after CLL-CM incubation. Both PDGFRα and Akt were activated in MSCs within 10 minutes of exposure to the CM of CLL B cells (Figure 2C). When CLL MSCs were pretreated with the PDGFR tyrosine kinase inhibitor III, an inhibitor known to specifically block the ATP binding site of PDGFR, neither PDGFRα nor Akt activation were detectable when MSCs were exposed to CM from CLL B cells (Figure 2C). Of note, the PDGFRβ phosphorylation assessed by Western blot was also significantly reduced (data not shown). These results suggest that Akt is likely activated downstream of the PDGFR signaling pathway in CLL MSCs. Erk was also found to be activated in MSCs within 10 minutes of exposure to the CLL-CM (Figure 2C); however, the phosphorylation of Erk in MSCs was not affected by exposure to the PDGFR inhibitor, suggesting the Erk activation is secondary to a different signal event/pathway than by PDGFR activation.
When MSCs were exposed to the CLL-CM with the addition of PDGFR inhibitor III, the proliferation mediated by CLL-CM was mostly abrogated by PDGFR inhibition (n = 6, P = .05; Figure 2D). In addition, when MSCs were cultured with escalating doses of PDGF, we observed increased proliferation in a dose-dependent manner (Figure 2E). We used a PDGF dose in the range of 50-200 pg/mL (similar to the ones we observed in CLL-CM) and in the range of 1-5 ng/mL (similar to the ones we observed in CLL plasma). MSCs were observed to have moderately increased proliferation (approximately 2- to 3-fold increase) when cultured with 50-200 pg/mL PDGF and more proliferation (approximately 3- to 8-fold) when cultured with 5 ng/mL PDGF. These findings add evidence that PDGF-PDGFR signaling is important for MSC proliferation mediated by CLL-CM.
However, we have also attempted to use different individual inhibitors (including PDGFR and VEGFR inhibitors) to block the migration and could not completely block the observed migration increase (data not shown). These results imply to us that multiple factors are responsible for the observed increase in migration capacity induced by CLL-CM.
PDGF is present in CLL-CM and is responsible for PDGFR activation in MSCs
Having demonstrated that PDGFR was activated by the CLL-CM, we tested whether the known ligands of PDGFR, PDGF, and VEGF21 are present in the CLL-CM. As expected, variable levels of PDGF and VEGF were detected in the tested CLL-CM (Figure 3A; n = 12, mean ± SEM: 113.7 ± 23.6 pg/mL for PDGF; n = 12, mean ± SEM: 186.94 ± 54.57 pg/mL for VEGF). Because both PDGF and VEGF have been demonstrated to activate the PDGFR, we designed blocking experiments using anti-PDGF or anti-VEGF or both to neutralize these ligands in the CLL-CM during MSC exposure. Neutralization antibodies to PDGF or to both PDGF and VEGF were able to clearly reduce the PDGFRα and -β activation in MSCs mediated by CLL-CM (Figure 3B). However, the neutralizing VEGF antibody did not affect the level of PDGFRα activation (ie, phosphorylation levels) induced by CLL-CM, but mildly reduced the activation of PDGFRβ (Figure 3B). To support the role of PDGF in PDGFR activation, we also found that both PDGFRα and -β were potently activated (ie, phosphorylated) by PDGF ligand (PDGF-AB); however, VEGF addition only minimally induced activation of PDGFRβ. Thus, PDGF-AB induced a similar pattern of PDGFR activation to the one mediated by CLL-CM, indicating that PDGF is the likely ligand in CLL-CM inducing the PDGFR activation in MSCs (Figure 3B). Densitometry analysis of these blots confirmed the visually observed pattern of PDGFR activation tested in different conditions described for these experiments (Figure 3B).
PDGF but not VEGF in the CLL-CM is able to activate MSC PDGFR. (A) Variable levels of PDGF and VEGF were detected in CLL-CM. The line in each dot plot represents the mean level of detected growth factor. (B) RTK array analysis of CLL MSC stimulated by CLL-CM for 30 minutes in the presence or absence of neutralizing antibody to PDGF, VEGF, or both. RTK array analysis of CLL MSC was performed in the presence of either PDGF-AB or VEGF-A ligand. (C) Immunoblot analysis demonstrating the presence of PDGF-AB in CLL B cells. PDGF-AB was up-regulated when CLL B cells were cultured under hypoxic conditions after overnight culture in 3 of 4 patients tested. CLL 1-4, 4 CLL patient lysates used in the immunoblot. Four different CLL samples were run on separate gels and are shown. Each gel is shown in separate rectangle.
PDGF but not VEGF in the CLL-CM is able to activate MSC PDGFR. (A) Variable levels of PDGF and VEGF were detected in CLL-CM. The line in each dot plot represents the mean level of detected growth factor. (B) RTK array analysis of CLL MSC stimulated by CLL-CM for 30 minutes in the presence or absence of neutralizing antibody to PDGF, VEGF, or both. RTK array analysis of CLL MSC was performed in the presence of either PDGF-AB or VEGF-A ligand. (C) Immunoblot analysis demonstrating the presence of PDGF-AB in CLL B cells. PDGF-AB was up-regulated when CLL B cells were cultured under hypoxic conditions after overnight culture in 3 of 4 patients tested. CLL 1-4, 4 CLL patient lysates used in the immunoblot. Four different CLL samples were run on separate gels and are shown. Each gel is shown in separate rectangle.
Because we had detected PDGF in CLL-CM, we next tested whether PDGF was present in CLL cells using immunoblot. As demonstrated in Figure 3C, we were able to detect a variable level of PDGF in CLL cell lysates by Western blot. When CLL cells were exposed to hypoxic conditions,22 previously shown to increase PDGF levels, PDGF expression did appear to be increased in CLL cells. To test whether normal B cells also express PDGF, we negatively sorted B lymphocytes from PBMCs of healthy donors and subsequently evaluated the lysates of normal B cells by Western blot analysis for PDGF expression. In normal blood B cells, PDGF expression was also detected (data not shown).
CLL plasma contains significantly elevated PDGF and VEGF compared with healthy subjects
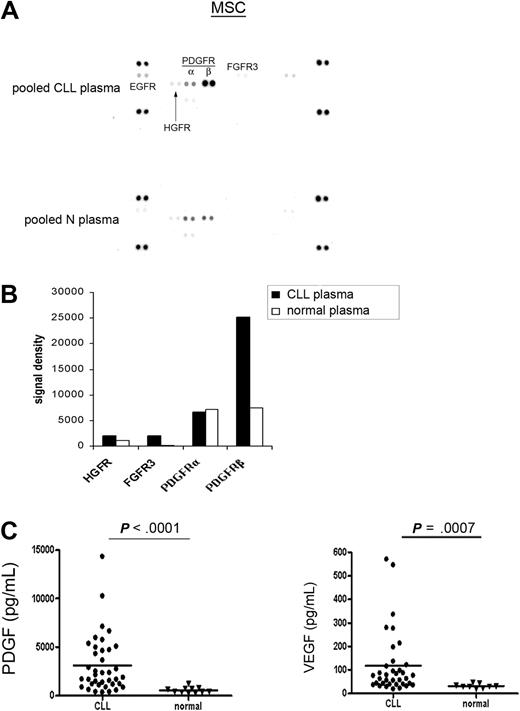
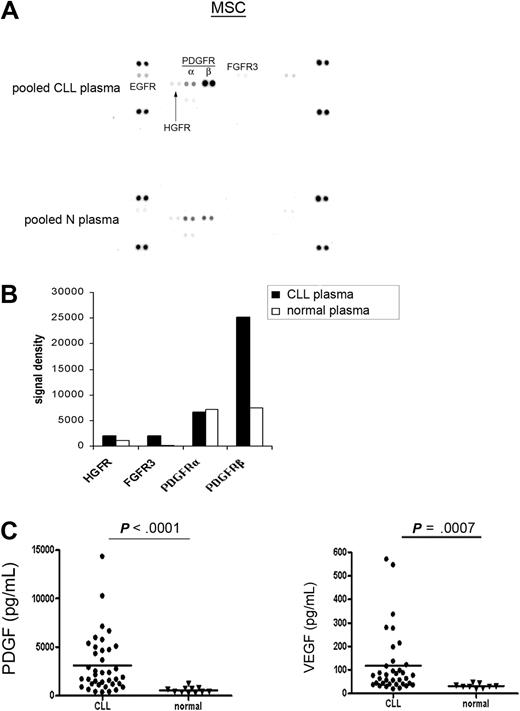
The above experiments demonstrated the selective activation of PDGFR in MSCs mediated by CLL-CM. We next wanted to assess whether CLL plasma is capable of activating the surface receptors in MSCs in a similar manner. To minimize the individual variation likely present in CLL plasma, we used pooled CLL and normal plasma from 4 individual subjects of each cohort. Four CLL plasmas isolated from 2 Rai stage III and 2 Rai stage I patients were diluted with AIM-V medium at a ratio of 1:4 and were subsequently used for incubation with MSCs for RTK array analysis. As demonstrated in Figure 4A-B, we observed more potent PDGFR activation when MSCs were exposed to CLL plasma compared with normal plasma. When MSCs were preincubated with PDGFR inhibitor III, the PDGFR activation by plasma was blocked (data not shown). These results add evidence that PDGF in the plasma can activate PDGFR in MSCs. FGFR3 and hepatocyte growth factor receptor (HGFR) activation also appeared to be evident in this assay, which is consistent with the previous finding that bFGF was elevated in CLL plasma compared with normal plasma.23 Based on these experiments, we assumed that the PDGF level in CLL plasma could be elevated compared with normal plasma. Therefore, we tested PDGF levels in 38 CLL patients from all Rai stages and found the PDGF level is significantly elevated in CLL plasma compared with normal plasma (Figure 4C; CLL plasma PDGF: n = 38, mean ± SEM: 3121.0 ± 478.9 pg/mL; normal plasma PDGF: n = 11, 554.4 ± 88.6 pg/mL, P < .0001). Similarly, we found elevated VEGF levels in CLL plasma compared with normal plasma (Figure 4C; CLL plasma: n = 36, 117.1 ± 22.2 pg/mL; normal plasma: n = 9, 31.0 ± 3.1 pg/mL, P = .0007). The clinical and prognostic characteristics of the patients used in this study are presented in supplemental Table 1.
PDGF and VEGF are elevated in CLL plasma versus normal plasma. (A) RTK array analysis of the CLL MSC stimulated by either pooled CLL or normal plasma. (B) Densitometry analysis revealed that PDGFR activation was more evident in CLL MSCs when they were exposed to CLL plasma versus normal plasma. (C) Plasma PDGF levels in CLL patients were significantly elevated compared with normal subjects (CLL plasma, n = 38; mean ± SEM, 3121.0 ± 478.9 pg/mL; normal plasma, n = 11, 554.4 ± 88.6 pg/mL; P < .0001). Similarly, significantly elevated VEGF levels were found in CLL plasma compared with normal plasma (CLL plasma, n = 36, 117.1 ± 22.2 pg/mL; normal plasma, n = 9, 31.0 ± 3.1 pg/mL; P = .0007). The characteristics of the patients used in this study were presented in supplemental Table 1. The horizontal line in each dot plot represents the mean level of the tested growth factor.
PDGF and VEGF are elevated in CLL plasma versus normal plasma. (A) RTK array analysis of the CLL MSC stimulated by either pooled CLL or normal plasma. (B) Densitometry analysis revealed that PDGFR activation was more evident in CLL MSCs when they were exposed to CLL plasma versus normal plasma. (C) Plasma PDGF levels in CLL patients were significantly elevated compared with normal subjects (CLL plasma, n = 38; mean ± SEM, 3121.0 ± 478.9 pg/mL; normal plasma, n = 11, 554.4 ± 88.6 pg/mL; P < .0001). Similarly, significantly elevated VEGF levels were found in CLL plasma compared with normal plasma (CLL plasma, n = 36, 117.1 ± 22.2 pg/mL; normal plasma, n = 9, 31.0 ± 3.1 pg/mL; P = .0007). The characteristics of the patients used in this study were presented in supplemental Table 1. The horizontal line in each dot plot represents the mean level of the tested growth factor.
PDGF promotes VEGF production in CLL MSC in a PI3K-dependent manner
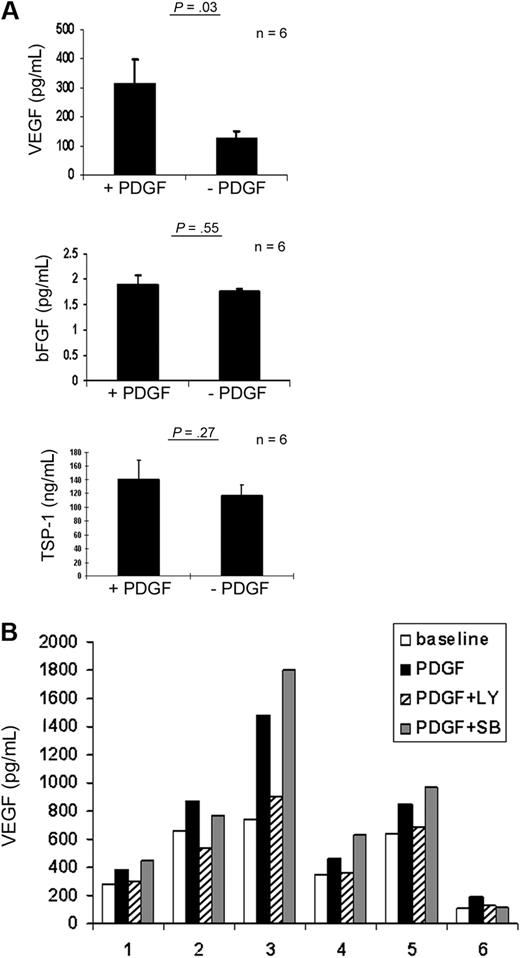
Because we have previously found that a switch in pro- versus antiangiogenic cytokine levels can occur with an increase in proangiogenic cytokines when CLL B cells were added to MSCs,12 we tested whether PDGF can similarly modify MSC angiogenic cytokine levels. When PDGF was added to the CLL MSC culture medium, we found that VEGF production increased significantly (Figure 5A; n = 6, mean increase: 3.3-fold, P = .03) in the culture medium of the MSCs harvested after a 72-hour culture period. This effect appeared to be specific because neither TSP-1 nor bFGF production were affected by PDGF in the CLL-CM (Figure 5A). Subsequently, we used 2 chemical inhibitors that block either PI3K or p38 MAPK signals to test the pathways necessary for increased VEGF production mediated by PDGF. Only the PI3K inhibitor LY294002 was able to abrogate the increased VEGF production mediated by PDGF (n = 6, P = .04, PDGF versus PDGF+LY). p38 MAPK inhibitor SB202190 did not affect the level of VEGF production mediated by PDGF (P = .25, PDGF versus PDGF+SB). This latter finding indicates the significance of the PI3K-AKT pathway in PDGF-driven VEGF production in MSCs (Figure 5B).
PDGF promotes VEGF production in CLL MSCs in a PI3K-dependent manner. (A) Addition of PDGF to MSC culture promotes VEGF production (n = 6; mean increase, 3.3-fold; P = .03). TSP-1 and bFGF production in MSCs were not altered by addition of PDGF. (B) PI3K blockade with LY294002 was able to reduce the increased production of VEGF in MSCs mediated by PDGF (P = .04, PDGF versus PDGF+LY; P = .25, PDGF versus PDGF+SB). SB, SB202190; LY, LY294002. Six MSCs derived from the marrow biopsies of 6 different patients were tested in 6 individual experiments shown in the bar graph.
PDGF promotes VEGF production in CLL MSCs in a PI3K-dependent manner. (A) Addition of PDGF to MSC culture promotes VEGF production (n = 6; mean increase, 3.3-fold; P = .03). TSP-1 and bFGF production in MSCs were not altered by addition of PDGF. (B) PI3K blockade with LY294002 was able to reduce the increased production of VEGF in MSCs mediated by PDGF (P = .04, PDGF versus PDGF+LY; P = .25, PDGF versus PDGF+SB). SB, SB202190; LY, LY294002. Six MSCs derived from the marrow biopsies of 6 different patients were tested in 6 individual experiments shown in the bar graph.
PDGF levels are strongly correlated with VEGF levels in late stage, ZAP-70, CD38 positive, and progressive CLL patients requiring therapy
Having demonstrated that PDGF was capable of inducing VEGF production in MSCs, we wanted to explore whether there is a relationship between PDGF and VEGF levels in the plasma of CLL patients. Using the nonparametric Spearman correlation analysis, we found there was a positive correlation between PDGF and VEGF levels among the tested 38 patients (n = 38, r = 0.5, P = .004). Then we performed patient subgroup analysis and found that PDGF appeared to correlate strongly with VEGF in CLL patients who had progressive disease-required therapies, Rai stage II-IV, CD38 positive, or ZAP-70 positive patients (Figure 6A-D). However, PDGF did not correlate with VEGF in ZAP-70 negative (r = 0.37, P = .10, n = 22), Rai stage 0-I (r = −0.04, P = .9, n = 15), CD38 negative (r = 0.39, P = .05, n = 30) patients, and patients with indolent disease not requiring therapies (r = 0.3, P = .1, n = 26). In addition, PDGF levels were not significantly different in ZAP-70 positive or CD38 positive CLL patients versus CLL patients with negative ZAP-70 or CD38 (data not shown) and were not different between patients with progressive diseases requiring therapy versus patients with indolent disease (data not shown).
Plasma PDGF is positively correlated with VEGF levels in patients with progressive disease requiring therapy, late Rai stage, ZAP-70, and CD38 positive CLL patients. (A-D) In CLL patients with progressive disease requiring therapy (r = 0.82, P = .003), Rai stage II-IV (r = 0.88, P < .001), CD38 positive patients (r = 0.81, P = .02), and ZAP-70 positive patients (r = 0.52, P = .06), plasma PDGF strongly associated with VEGF levels. Linear regression analysis of PDGF versus VEGF levels was represented by the line in each dot plot.
Plasma PDGF is positively correlated with VEGF levels in patients with progressive disease requiring therapy, late Rai stage, ZAP-70, and CD38 positive CLL patients. (A-D) In CLL patients with progressive disease requiring therapy (r = 0.82, P = .003), Rai stage II-IV (r = 0.88, P < .001), CD38 positive patients (r = 0.81, P = .02), and ZAP-70 positive patients (r = 0.52, P = .06), plasma PDGF strongly associated with VEGF levels. Linear regression analysis of PDGF versus VEGF levels was represented by the line in each dot plot.
We have performed correlative analysis between PDGF levels and platelet count given that platelets are an important source of PDGF production in vivo. PDGF appeared to correlate with platelet count in 38 patients (r = 0.6, P < .01). However, in the subgroup analysis, PDGF correlated with platelet count most strongly in CD38 negative (r = 0.74, P < .001, n = 30) and ZAP-70 negative (r = 0.75, P < .001, n = 22) patients. In contrast, platelet and PDGF levels did not correlate in the CLL patients with positive CD38 expression (r = 0.25, P = .5) or ZAP-70 positive expres-ion (r = 0.37, P = .19). The correlation between platelet and PDGF was not affected by either Rai stage (Rai 0-I, r = 0.5, P = .04; Rai II-IV, r = 0.57, P = .004) or therapy status (patients without therapies: r = 0.61, P < .001; patients required therapies: r = 0.7, P = .015). Taken together, PDGF correlates positively with VEGF in patients with progressive features (CD38+, ZAP-70+, and late Rai stage). In contrast, platelet levels correlate positively with PDGF more in patients with CD38 negative or ZAP-70 negative patients.
Discussion
We have previously demonstrated that bidirectional crosstalk promotes cellular activation in both MSCs and CLL B cells. The current study investigated the mechanism of MSC activation that could be generated by the leukemic CLL B cell. To that end, we have identified the important role of the PDGF-PDGFR (α and β receptors) signal pathway in MSC function and VEGF production in CLL disease. In addition, PDGFR-activation was shown to be necessary for CLL-CM–mediated MSC proliferation and Akt phosphorylation. Subsequently, using approaches designed to neutralize PDGF or VEGF or both, PDGF was shown to be the predominant ligand required for PDGFR-activation of MSCs when driven by CLL-CM. The plasma level of both PDGF and VEGF was found to be significantly elevated compared with the levels seen in healthy controls. Addition of PDGF in MSC culture increased VEGF production in MSCs more than 3-fold, and this effect was dependent on PI3K activity in MSCs. We believe this is the first report to demonstrate that CLL cells are capable of secreting PDGF and subsequently modifying MSC function. In total, we believe the modification of MSC function by CLL cell-generated PDGF may have a profound influence on CLL disease progression. In relation to this, our studies found that the plasma PDGF and VEGF levels in CLL patients are positively correlated in patients with the following high-risk features: late Rai stage, ZAP-70, and CD38 positive, as well as in patients with more aggressive disease requiring therapies.
Because of our previous finding that soluble factors secreted from CLL cells are capable of activating Akt and Erk in MSCs, we assessed whether the soluble factors in CLL-CM can regulate 2 basic MSC functions: proliferation and migration. We found the migration and proliferation capacities of MSCs were modulated by CLL-CM. To assess the MSC ability to generate colonies, we observed significantly more CFU numbers in MSCs cultured with CLL-CM compared with AIM-V. These findings are consistent with previous findings that culture medium from breast cancer cells is capable of promoting MSC migration and proliferation.15
The role of MSCs on tumor growth is controversial. Injected primary human MSCs exerted a potent tumor-suppressive effect on sarcoma growth in vivo through direct cell contact.24 Similarly, human skin-derived stem cells, similar to MSCs, were able to inhibit tumor growth in a mouse model of brain tumor.25,26 Other studies showed that coinjection of MSCs with tumor cells promotes tumor growth in vivo and metastasis.27 Recently, Mishra et al15 found that MSCs can be differentiated into a phenotype consistent with the so-called cancer-associated fibroblast. It is important to understand whether tumor cells or leukemic cells can alter MSC function through specific receptor activation to regulate their function. Our work in CLL found that PDGFRα and PDGFRβ were selectively activated by CLL-CM, and Akt is activated downstream of the PDGFR phosphorylation mediated by CLL-CM. Blocking PDGFR activation using a specific inhibitor was able to abrogate the CLL-CM–driven MSC proliferation, indicating the key role of PDGFR activation in CLL-CM–mediated MSC proliferation and Akt activation. In support of the important role of the PDGF-PDGFR signal in hematologic malignancy, PDGF has also been reported to be important in the prosurvival pathway in large granular lymphocytic (LGL) leukemia.28,29
PDGF and VEGF have been reported to function as the ligands for PDGFR activation in MSCs. In this regard, we detected both PDGF and VEGF in CLL-CM. The blocking experiments designed to neutralize PDGF or VEGF or both revealed that PDGF is the predominant ligand for PDGFR activation in CLL-CM–exposed MSCs. Subsequently, we tried to assess which RTKs are activated in MSCs when exposed to CLL plasma or normal plasma. We found predominant PDGFR activation, as well as HGFR and bFGF receptor phosphorylation in CLL plasma-exposed MSCs compared with the normal plasma-exposed MSCs. These results suggested that there may be different levels of these 3 growth factors in CLL compared with normal plasma. In this study, we have focused on studying the PDGF and VEGF regulation. When we measured the levels of plasma PDGF and VEGF, we found that mean levels of PDGF and VEGF were significantly elevated in CLL plasma compared with the controls. However, there is a portion of CLL patients who do not have elevated PDGF and VEGF levels compared with normal controls. The reasons for the varied expression of PDGF and VEGF within the CLL cohorts are unclear currently.
Increased VEGF expression has been well-established in association with disease progression in a variety of cancers,30 including CLL.30 Because we found that there is an angiogenic switch, an increase of VEGF and decrease of TSP-1, with the coculture of CLL MSCs,12 we wanted to explore whether PDGF had any regulatory role in the VEGF, TSP-1, and bFGF production of MSCs. Indeed, we found that PDGF addition was able to increase VEGF production by approximately 3-fold, whereas TSP-1 and bFGF production from MSCs were not affected by PDGF. This finding of PDGF stimulation for VEGF is consistent with prior reports.31-33 Using several different inhibitors, we found that the PI3K pathway is necessary for PDGF-driven VEGF production in MSCs. Previous work has shown that PDGF promotes VEGF production in endothelial cell and ovarian cancer cells.34,35 Our observations now indicate that this can occur for human MSCs in CLL disease. Given the known importance of VEGF in regulating angiogenesis and its potential role in CLL disease progression,30 as well as the demonstrated regulatory role of PDGF in VEGF production in MSCs as shown in this report, it was reasonable to analyze the correlation of plasma PDGF with VEGF level. We did indeed observe a positive correlation between PDGF and VEGF levels in CLL plasma, especially evident in CLL patients with progressive disease requiring therapy, patients with late stage, or those who are ZAP-70 or CD38 positive. These results suggest that PDGF secreted by CLL cells is a key regulator of VEGF production, thus indirectly regulating angiogenesis and CLL disease progression. Because PDGF is able to enhance MSC functions, we also speculate that this latter activity can enhance the ability of MSCs to nurture leukemic CLL B-cell resistance to drug-induced cytotoxicity.
Finally, we found that PDGF expression in CLL cells increased when CLL cells underwent hypoxic culture conditions, demonstrating that PDGF production by cancer cells can be modified by microenvironmental conditions. Thus, if heavily infiltrated marrows in CLL are subject to hypoxic conditions, this could increase the tissue site concentration of PDGF. Given the secretion of PDGF by CLL B cells, we also wished to know whether a functional PDGF-PDGFR autocrine-signal pathway is present in CLL cells. PDGFRα was not detectable by either reverse transcription polymerase chain reaction (RT-PCR) or immunoblot in multiply tested CLL patients (data not shown). PDGFRβ expression was detectable by RT-PCR but not by immunoblot (data not shown). In addition, when CLL cells were exposed to recombinant PDGF ligands, no PDGFR activation was detected using a sensitive RTK array analysis (data not shown), strongly suggesting that a PDGF-PDGFR pathway may not be functional in CLL cells. We do not believe that MSCs produce significant amounts of PDGF. MSCs cultured alone produced minimal amounts of PDGF (supplemental Figure 2). In addition, when CLL-CM is added, we have observed decreasing levels of PDGF in the MSC culture medium over time (supplemental Figure 2). Therefore, we believe CLL leukemic cells secrete PDGF, which mainly functions in a paracrine fashion toward MSCs, through a PDGF-PDGFR-PI3K-Akt pathway to modify their function and in particular their proangiogenic factor production.
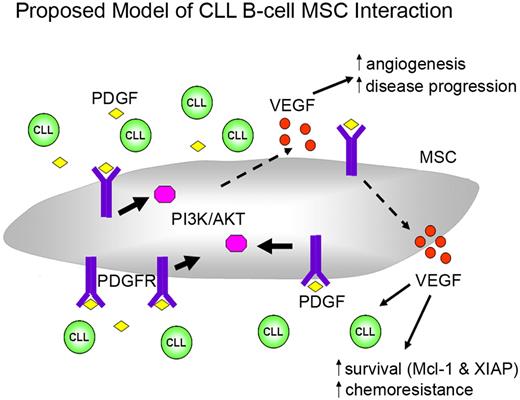
Based on our findings, we propose a model of CLL-stroma interaction (Figure 7), which facilitates leukemic B-cell survival. Under normoxic, but even more so with hypoxic conditions, CLL cells secrete PDGF into the microenvironment. Then PDGF can bind to the PDGFR on MSCs to trigger downstream PI3K-Akt activation. PDGFR-PI3K-Akt activation further triggers downstream programs that result in increased VEGF production, which subsequently leads to increased survival/drug resistance in CLL cells, as well as promoting neovascularization, which we and others have shown is related to disease progression.36,37 Paracrine PDGF signaling also has an important role in recruiting and modifying tumor stroma.38,39 Therefore, we believe that PDGF-PDGFR signaling is part of an increasing complex and important network in CLL-stroma interaction. Future studies will be needed to determine how to effectively target this pathway to maximize treatment strategies for treating CLL patients or perhaps even in preventing disease progression in early stage CLL patients.
Proposed model of leukemic CLL B cell and stromal cell interaction. This model indicates that CLL secrete PDGF into the microenvironment. PDGF can then bind to the PDGFR in MSCs to trigger downstream PI3K-Akt activation. PDGFR-PI3K-Akt activation further triggers downstream signal pathways that result in increased VEGF production, which subsequently leads to increased survival/drug resistance in CLL cells, as well as promoting neovascularization. See “Discussion” for more details regarding this proposed model.
Proposed model of leukemic CLL B cell and stromal cell interaction. This model indicates that CLL secrete PDGF into the microenvironment. PDGF can then bind to the PDGFR in MSCs to trigger downstream PI3K-Akt activation. PDGFR-PI3K-Akt activation further triggers downstream signal pathways that result in increased VEGF production, which subsequently leads to increased survival/drug resistance in CLL cells, as well as promoting neovascularization. See “Discussion” for more details regarding this proposed model.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from the CLL Global Research Foundation and National Institutes of Health/National Cancer Institute grant nos. CA116237 (to N.E.K.) and CA136591 (to D.F.J.). We greatly appreciate the generosity of the Donner Foundation and Ed Spenser in providing additional funding.
National Institutes of Health
Authorship
Contribution: W.D. designed and performed the experiments, analyzed and interpreted the results, and wrote the paper; T.R.K., R.C.T., and J.C.B. performed the experiments and interpreted the results; W.W. and S.M.S. provided statistical and clinical database support; D.F.J. provided scientific input, review, and discussion; and N.E.K. designed the research, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neil E. Kay, Division of Hematology, Mayo Clinic, Rochester, MN 55905; e-mail: kay.neil@mayo.edu.