Abstract
5-Aminoimidazole-4-carboxamide riboside or acadesine (AICAR) induces apoptosis in chronic lymphocytic leukemia (CLL) cells. A clinical study of AICAR is currently being performed in patients with this disease. Here, we have analyzed the mechanisms involved in AICAR-induced apoptosis in CLL cells in which it activates its only well-known molecular target, adenosine monophosphate-activated protein kinase (AMPK). However, AMPK activation with phenformin or A-769662 failed to induce apoptosis in CLL cells and AICAR also potently induced apoptosis in B lymphocytes from Ampkα1−/− mice, demonstrating an AMPK-independent mechanism of cell death. Importantly, AICAR induced apoptosis irrespective of the tumor suppressor TP53 or ataxia telangiectasia mutated (ATM) status via induction of the mitochondrial pathway. Apoptosis was preceded by an increase in mRNA and protein levels of proapoptotic BCL-2 family proteins of the BH3-only subgroup, including BIM, NOXA, and PUMA in CLL cells. Strikingly, B lymphocytes from Noxa−/− or Bim−/− mice were partially protected from the cytotoxic effects of AICAR. Consistently, B cells from Noxa−/−/Bim−/− mice resisted induction of apoptosis by AICAR as potently as B lymphocytes overexpressing transgenic BCL-2. These findings support the notion that AICAR is an interesting alternative therapeutic option for CLL patients with impaired p53 function and resistance to conventional chemotherapy.
Introduction
Available treatments for chronic lymphocytic leukemia (CLL) generally induce remission, although nearly all patients relapse and CLL remains an incurable disease.1,2 5-Aminoimidazole-4-carboxamide riboside or acadesine (AICAR) induces apoptosis in different cell types, including CLL, mantle cell lymphoma (MCL), and splenic marginal zone B-cell lymphoma (SMZL) cells and tumor cell lines, without affecting primary T lymphocytes.3-6 Thus, AICAR is a promising drug for the treatment of B-cell neoplasms. A clinical phase I/II study of AICAR is currently being performed in CLL patients.7
Incorporation of AICAR into the cells and its subsequent phosphorylation to AICA ribotide (ZMP) are necessary to induce apoptosis in CLL lymphocytes.3 The only well-known molecular target of ZMP described to date is adenosine monophosphate-activated protein kinase (AMPK).6,8 Incubation of CLL, MCL, and SMZL cells with AICAR induces phosphorylation of AMPK at Thr172 and activation of AMPK, which correlates with induction of apoptosis.3,4 However, the role of AMPK in AICAR-induced apoptosis has not been defined in CLL cells. Intriguingly, AICAR enters follicular lymphoma cells and induces phosphorylation of AMPK, but most follicular lymphoma samples tested are resistant to AICAR-induced apoptosis.4 Indeed, AMPK activation has been shown to play a role in apoptosis or in survival depending on the cell type investigated.6,8
Two major pathways of apoptosis have been described that result in the activation of a family of proteases (caspases), which are responsible for the biochemical and morphologic changes associated with apoptosis. The intrinsic pathway involves initial mitochondrial alteration resulting from cellular stress or cytotoxic insults, whereas the extrinsic pathway is triggered by ligand-dependent activation of death receptors, members of the tumor necrosis factor receptor family.9 BCL-2 family proteins are regulators of the mitochondrial pathway through controlling the release of cytochrome c and other proteins from mitochondria.9,10 Activation of the multidomain proapoptotic proteins BAX and BAK mediates the release of cytochrome c from mitochondria and the activation of caspases through the mitochondrial pathway. The intrinsic and extrinsic pathways are connected in certain cell types because activation of caspase-8 through death receptors can induce the proteolysis and activation of the proapoptotic BH3-only protein BID.9
Most drugs currently used in the therapy of CLL act, at least partially, through activation of the p53 pathway.1,2 TP53 is mutated in 5% to 10% of CLL cases at diagnosis, but the incidence of mutations increases along with the therapy.2,11 Furthermore, ataxia telangiectasia mutated (ATM) is inactivated in 10% to 20% of CLL cases, thus providing an alternative way for disabling p53 function.12 Genetic alterations in TP53 and ATM are among the worst prognostic factors in CLL patients, and TP53 alterations confer resistance to conventional chemotherapy.1,2 Thus, new approaches to induce apoptosis in cells with altered TP53 or ATM are needed.2,13
It has been previously shown that AICAR induces the release of cytochrome c from mitochondria into the cytosol of CLL cells together with caspase activation without inducing p53 accumulation.3 However, the relevant upstream events remain poorly defined, and it is currently unknown whether AICAR induces apoptosis in CLL samples with altered TP53 or ATM. Indeed, the mechanism of AICAR-induced apoptosis remains to be elucidated in full detail.
BCL-2 family proteins are critical regulators of apoptosis in CLL cells. High levels of BCL-2 have been well documented in CLL cells.10,13 Furthermore, CLL cells express other antiapoptotic BCL-2 family members, such as MCL-1, BCL-XL, and A1/BFL-1, which are involved in the survival of these cells.10,13 Most apoptotic stimuli do not alter BAX or BAK protein levels in CLL cells, and activation of BAX or BAK is mediated by regulation of the BH3-only members of the BCL-2 family proteins through transcriptional or posttranscriptional mechanisms. For example, the BH3-only protein PUMA is a p53-transcriptional target in CLL cells induced by fludarabine or MDM2 inhibitors.14,15 CLL cells also express the BH3-only proteins BID, BMF, NOXA, and BIM,10,13 some of which have been implicated in the control of tumor cell apoptosis.16 The effect of AICAR treatment on the BCL-2 rheostat in CLL has not been investigated.
Here, we analyze the role of AMPK and BCL-2 family proteins in the mechanism of action of AICAR-induced apoptosis in CLL cells and normal mouse B cells, and the effects of AICAR in CLL cells with altered TP53 or ATM.
Methods
CLL samples and cell isolation
Peripheral blood samples from 43 patients with CLL were studied (Table 1). CLL was diagnosed according to standard clinical and laboratory criteria. Blood samples were obtained from Hospital de Bellvitge, Barcelona, Spain. Written informed consent was obtained from all patients in accordance with Hospital de Bellvitge Ethical Committee. Mononuclear cells from peripheral blood samples were isolated by centrifugation on a Ficoll-Hypaque (Seromed) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (Sigma-Aldrich).
Samples from patients 24 and 25 have mutated TP53, whereas cells from patients 26 and 27 have 17p13 deletion harboring the p53 locus. Patient 24 has the M246V mutation, which interferes with wild-type (WT) p53, and patient 25 has a frame-shift mutation in one allele (nucleotide deletion in codon 272) and a 17p13 deletion in the other allele in 86% of cells from peripheral blood lymphocytes.15 Patient 26 has a 17p13 deletion in one allele in 43% of peripheral blood lymphocytes, and patient 27 has a 17p13 deletion in one allele in 94% of peripheral blood lymphocytes.17
Samples from patients 7 and 28 have 11q22 deletion and do not express ATM protein.17,18 Patient 7 has the 11q23 deletion in one allele in 86% of peripheral blood lymphocytes, and patient 29 has the 11q23 deletion determined by genomic multiplex ligation-dependent probe amplification (MLPA; relative copy number value = 0.57).17
Animals and cell isolation
Spleen lymphocytes were obtained from WT or mutant mice 8 to 16 weeks of age. The generation of Ampkα1−/−,19 Bim−/−,20 Puma−/−,21 Noxa−/−,21 Hrk/Dp5−/−,22 Bad−/−,23 Bmf−/−,24 p53−/−,25 and vav-BCL2 transgenic26 mice has been described previously. Double-deficient mice were generated by intercrossing of double-heterozygote animals. Mice were maintained on a 12:12-hour light-dark cycle and received standard rodent chow and water ad libitum. The animal protocol was reviewed and approved by the Animal Research Committee of Universitat de Barcelona and the Austrian Ministry for Education, Research and Culture. Spleen lymphocytes were isolated by centrifugation on a Ficoll-Hypaque (Seromed) or a Lympholyte-M (Cederlane Labs Limited) gradient.
Reagents
AICAR was synthesized by Kyowa-Hakko. A-769662 was obtained from Abbott Laboratories. Dimethyl sulfoxide and phenformin were purchased from Sigma-Aldrich. Anti-Fas, clone CH11 (Fas ligand) was from Upstate Biotechnology. IETD.fmk was from R&D Systems. Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) were from Bender MedSystems. Benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD-fmk) was from Bachem AG. 5-Iodotubercidin was from BIOMOL Research Laboratories. Nutlin-3a was provided by Hoffmann-La Roche. Annexin V–phycoerythrin, anti–CD45R/B220-phycoerythrin, anti–CD45R/B220-allophycocyanin, and anti–CD3e-FITC were from BD Biosciences PharMingen. Anti–TCR β-FITC was obtained from eBioscience. Annexin V–AlexaFluor-647 was from BioLegend. 7-Amino-actinomycin D (7-AAD) was purchased from Alexis-Axxora.
Cell culture
Human lymphocytes were cultured immediately after thawing or isolation at a concentration of 0.5 to 4 × 106 cells/mL in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum, 2mM l-glutamine, 100 U/mL penicillin, and 100 ng/mL streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Mouse lymphocytes were cultured after isolation from the spleen at a concentration of 1.5 × 106 cells/mL in Iscove modified Dulbecco medium supplemented with 10% heat-inactivated fetal calf serum, 2mM l-glutamine, 55μM β-mercaptoethanol, 100 U/mL penicillin, and 100 ng/mL streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Analysis of cell viability by flow cytometry
Cell viability was measured by exposure of phosphatidylserine and membrane integrity. Data were analyzed using FACSCalibur and CellQuestPro software (BD Biosciences).
CLL cell viability was measured as the percentage of annexin V and PI double-negative cells, and it is expressed as the percentage of nonapoptotic cells. A total of 2.5 × 105 CLL cells were incubated for 24 or 48 hours with the indicated factors. CLL cells were washed and incubated with annexin-binding buffer and annexin V for 15 minutes in the dark. Cells were then incubated with PI and analyzed by flow cytometry.
Mouse B-cell viability was measured as the percentage of annexin V and 7-AAD double-negative cells and CD45R/B220+ cells, being expressed as the percentage of nonapoptotic cells. A total of 5 × 105 lymphocytes were incubated for 12 or 24 hours with the indicated compounds. Cells were washed and incubated with phosphate-buffered saline with CD45R/B220 and CD3ϵ or TCR-β for 15 minutes in the dark. Cells were then washed and incubated with annexin-binding buffer, annexin V, and 7-AAD for 15 minutes in the dark.
RT-MLPA
RNA content was analyzed by reverse transcriptase (RT)–MLPA using SALSA MLPA KIT R011 Apoptosis mRNA from MRC-Holland for the simultaneous detection of 38 messenger RNA molecules.27 In brief, RNA samples (200 ng total RNA) were first reverse transcribed using a gene-specific probe mix. The resulting cDNA was annealed overnight at 60°C to the MLPA probe mix. Annealed oligonucleotides were ligated by adding Ligase-65 (MRC-Holland) and incubated at 54°C for 15 minutes. Ligation products were amplified by PCR (35 cycles, 30 seconds at 95°C; 30 seconds at 60°C, and 1 minute at 72°C) with one unlabeled and one FAM-labeled primer. The final PCR fragments amplified were separated by capillary electrophoresis on a 48-capillary ABI-Prism 3730 Genetic Analyzer (Applied Biosystems/Hitachi). Peak area and height were measured using GeneScan v3.0 analysis software (Applied Biosystems). The sum of all peak data was set at 100% to normalize for fluctuations in total signal between samples, and individual peaks were calculated relative to the 100% value. The mRNA levels of all the genes were standardized to those of β-glucoronidase.
Western blot analysis
Cells were lysed with Laemmli sample buffer or RIPA extraction buffer, and Western blot analysis was performed as described previously3 using the following antibodies: BCL-2 (Dako Denmark), BAX and BIM (BD Biosciences PharMingen), BID (R&D Systems), BNIP3 (Calbiochem-Novabiochem), BNIP3L and NOXA (Abcam), ERK and phospho-ACC (Ser79, Upstate Biotechnology), MCL-1 (Santa Cruz Biotechnology), MOAP1 (Abnova), and phospho-AMPK (Thr172) and PUMA (Cell Signaling Technology). Antibody binding was detected using a secondary antibody conjugated to horseradish peroxidase and the enhanced chemiluminescence detection system (GE Healthcare).
Cytochrome c release measurements
Cells (25 × 106) were harvested, washed once, and gently lysed for 30 seconds in 80 μL ice-cold lysis buffer (250mM sucrose, 1mM ethylenediaminetetraacetic acid, 0.05% digitonin, 25mM Tris, pH 6.8, 1mM dithiothreitol, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 1mM benzamidine, and 0.1mM phenylmethylsulfonyl fluoride). Lysates were centrifuged at 12 000g at 4°C for 3 minutes to obtain the supernatants (cytosolic extracts free of mitochondria) and the pellets (fraction containing mitochondria). Supernatants (50 μg of protein) were electrophoresed on a 15% polyacrylamide gel and analyzed by Western blot using anticytochrome c antibody (7H8.2C12, BD Biosciences PharMingen) and enhanced chemiluminescence detection system.
Statistical analysis
Statistical analysis was performed using the Student t test, and P values less than .05 were considered statistically significant. Data were analyzed using the SPSS Version 14.0 software package.
Results
Activation of AMPK does not induce apoptosis in CLL cells
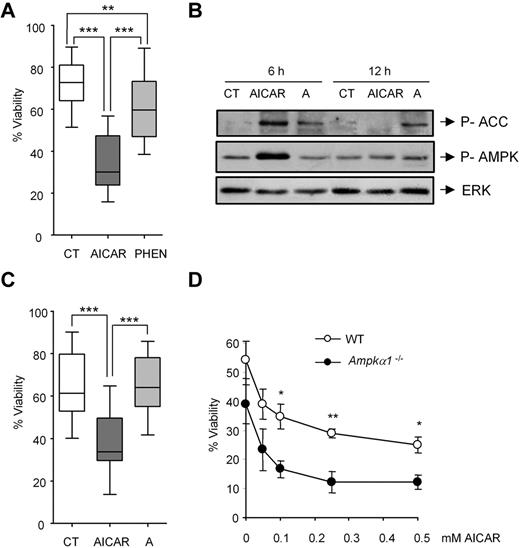
To study the role of AMPK in the induction of apoptosis, we assessed whether AMPK activators can induce apoptosis in CLL cells. Treatment of tumor cells with phenformin28 or AICAR for 9 hours induced AMPK phosphorylation (not shown). However, when we studied the effect of 0.5mM phenformin or 0.5mM AICAR for 24 hours in 11 CLL samples, we noted that only AICAR induced efficient apoptosis in all the tumor cell samples (P < .005; Figure 1A), whereas phenformin did not induce apoptosis in most CLL samples tested (8 of 11; P < .01; Figure 1A).
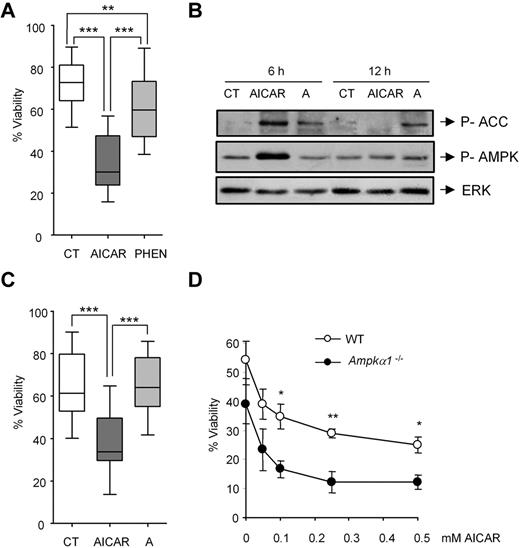
AICAR induces apoptosis independently of AMPK. (A) CLL cells were untreated (CT) or treated with 0.5mM AICAR or with 0.5mM phenformin (PHEN) for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (n = 11). ***P < .005, AICAR-treated versus untreated cells or versus PHEN-treated cells. **P < .01, PHEN-treated versus untreated cells. (B) CLL cells were incubated without (CT) or with 0.5mM AICAR or 100μM A-769662 (A) for 6 and 12 hours. Phospho-ACC and phospho-AMPK were analyzed by Western blot. ERK was used as a control of protein loading. A representative patient is shown (n = 5). (C) CLL cells were untreated (CT) or treated with 0.5mM AICAR or with 100μM A-769662 (A) for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (n = 18). ***P < .005, AICAR-treated versus untreated or versus A-treated cells. In the boxplots (A,C), the top line represents the 75% quartile; bottom line, the 25% quartile; and middle line, median. The ends of the whiskers represent the minimum and maximum of all the data. (D) WT and Ampkα1−/− B lymphocytes were incubated for 12 hours with AICAR in a range of concentrations (0.05, 0.1, 0.25, and 0.5mM). Viability was measured by flow cytometry, and it is expressed as the percentage of the viability. Data are mean ± SEM from 3 independent experiments for each genotype. **P < .01, *P < .05, Ampkα1−/− versus WT B lymphocytes.
AICAR induces apoptosis independently of AMPK. (A) CLL cells were untreated (CT) or treated with 0.5mM AICAR or with 0.5mM phenformin (PHEN) for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (n = 11). ***P < .005, AICAR-treated versus untreated cells or versus PHEN-treated cells. **P < .01, PHEN-treated versus untreated cells. (B) CLL cells were incubated without (CT) or with 0.5mM AICAR or 100μM A-769662 (A) for 6 and 12 hours. Phospho-ACC and phospho-AMPK were analyzed by Western blot. ERK was used as a control of protein loading. A representative patient is shown (n = 5). (C) CLL cells were untreated (CT) or treated with 0.5mM AICAR or with 100μM A-769662 (A) for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (n = 18). ***P < .005, AICAR-treated versus untreated or versus A-treated cells. In the boxplots (A,C), the top line represents the 75% quartile; bottom line, the 25% quartile; and middle line, median. The ends of the whiskers represent the minimum and maximum of all the data. (D) WT and Ampkα1−/− B lymphocytes were incubated for 12 hours with AICAR in a range of concentrations (0.05, 0.1, 0.25, and 0.5mM). Viability was measured by flow cytometry, and it is expressed as the percentage of the viability. Data are mean ± SEM from 3 independent experiments for each genotype. **P < .01, *P < .05, Ampkα1−/− versus WT B lymphocytes.
Because phenformin activates AMPK indirectly,28 we tested the effect of the direct AMPK activator A-76966229 on CLL cell survival. Incubation with 100μM A-769662 for 6 and 12 hours induced the phosphorylation of the AMPK substrate acetyl-CoA carboxylase (ACC), which indicates activation of AMPK that, by itself appeared only mildly activated at 12 hours, as shown previously for other cell types at this concentration.30 Incubation with 0.5mM AICAR induced the phosphorylation of AMPK and ACC at 6 hours, which returned to control levels at 12 hours (Figure 1B). Treatment of 18 individual CLL samples with 100μM A-769662 or 0.5mM AICAR for 24 hours revealed that A-769662 failed to induce apoptosis in most CLL samples analyzed (16 of 18; P = .995; Figure 1C), suggesting that AICAR-mediated cell killing may not require AMPK.
Mouse lymphocytes31 and CLL cells (not shown) only express the catalytic isoform AMPKα1 but not related AMPKα2. To clarify the role of AMPK in AICAR-induced apoptosis, we assessed the effect of AICAR in B cells derived from the spleen of WT or Ampkα1−/− mice. Lymphocytes were incubated for 12 and 24 hours with graded doses of AICAR (0.05, 0.1, 0.25, and 0.5mM), which induced apoptosis in a dose-dependent manner in both WT (P < .05) and Ampkα1−/− (P < .05) B lymphocytes alike (Figure 1D). Interestingly, B lymphocytes from Ampkα1−/− mice displayed increased sensitivity to AICAR treatment, in line with previous results showing that these cells are more susceptible to metabolic mitochondrial stress.32
Altogether, our results show that the activation of AMPK per se is not sufficient to induce apoptosis in CLL cells and that AICAR-mediated cell death can occur efficiently in the absence of AMPK.
AICAR induces apoptosis through the mitochondrial pathway in CLL cells
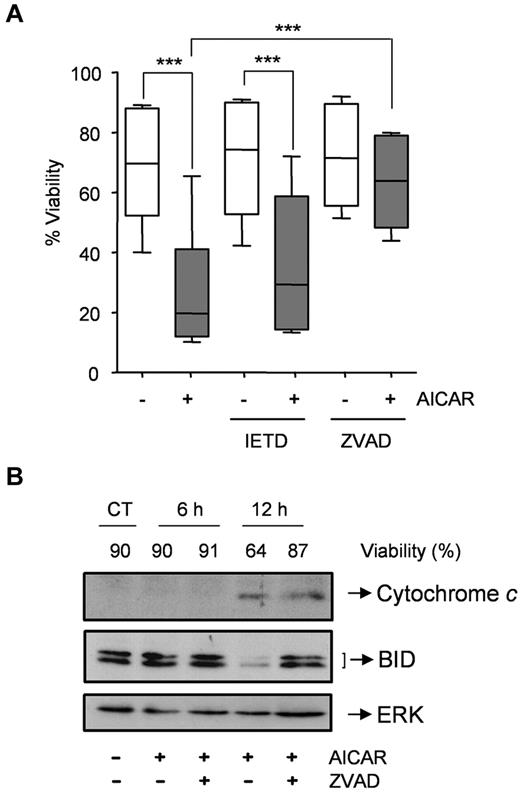
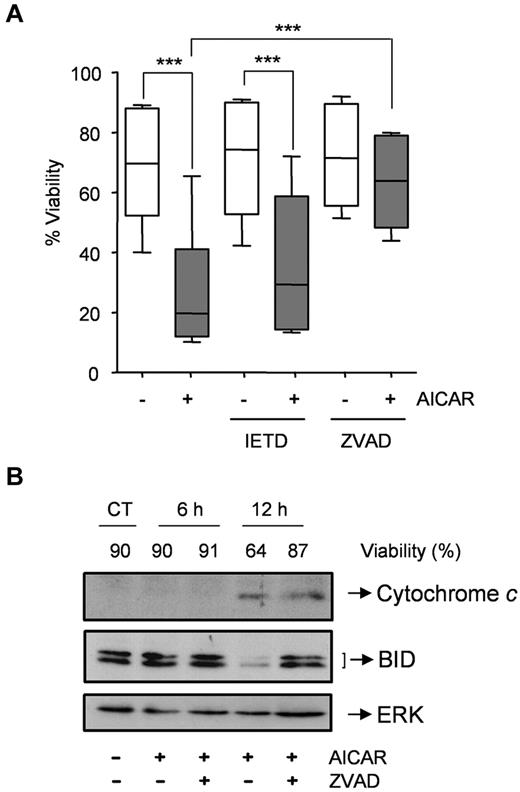
Next, we examined the relative contribution of the 2 major apoptosis-signaling pathways (ie, death receptor vs mitochondrial) to cell death induced by AICAR. Thus, CLL cells were preincubated with or without the caspase-8 inhibitor IETD.fmk or the pan-caspase inhibitor Z-VAD.fmk and then 0.5mM AICAR was added. Remarkably, IETD.fmk did not protect CLL cells from AICAR-induced apoptosis (P = .195; Figure 2A) at a concentration (50μM) that potently inhibited the induction of apoptosis by Fas ligand in Jurkat cells (not shown). On the contrary, Z-VAD.fmk blocked AICAR-induced apoptosis (P < .001; Figure 2A). Moreover, Z-VAD.fmk delayed processing of the BH3-only protein BID and tumor cell killing downstream of cytochrome c release (Figure 2B). Appearance of tBID was only observed in 1 of 5 samples analyzed and was reverted by Z-VAD.fmk (not shown). Together, these data indicate that the extrinsic pathway is not critical for AICAR-induced apoptosis.
AICAR induces apoptosis through the mitochondrial pathway in CLL cells. (A) Cells were preincubated without (−) or with 50μM IETD.fmk or 200μM Z-VAD.fmk for 30 minutes, and 0.5mM AICAR (+) was added for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability. The top line represents the 75% quartile, and the bottom line represents the 25% quartile, with the middle line showing the median. The ends of the whiskers represent the minimum and maximum of all the data (n = 6). ***P < .005, AICAR-treated versus untreated or versus Z-VAD.fmk + AICAR, or IETD + AICAR versus IETD-treated cells. (B) Cells were preincubated without (CT) or with 200μM Z-VAD.fmk for 30 minutes, and 0.5mM AICAR was added for 6 and 12 hours. Control extracts were obtained at 12 hours. Cytochrome c and BID were analyzed in cytosolic extracts by Western blot, and viability was measured by flow cytometry. ERK was used as a control of protein loading. A representative sample is shown (n = 2).
AICAR induces apoptosis through the mitochondrial pathway in CLL cells. (A) Cells were preincubated without (−) or with 50μM IETD.fmk or 200μM Z-VAD.fmk for 30 minutes, and 0.5mM AICAR (+) was added for 24 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability. The top line represents the 75% quartile, and the bottom line represents the 25% quartile, with the middle line showing the median. The ends of the whiskers represent the minimum and maximum of all the data (n = 6). ***P < .005, AICAR-treated versus untreated or versus Z-VAD.fmk + AICAR, or IETD + AICAR versus IETD-treated cells. (B) Cells were preincubated without (CT) or with 200μM Z-VAD.fmk for 30 minutes, and 0.5mM AICAR was added for 6 and 12 hours. Control extracts were obtained at 12 hours. Cytochrome c and BID were analyzed in cytosolic extracts by Western blot, and viability was measured by flow cytometry. ERK was used as a control of protein loading. A representative sample is shown (n = 2).
AICAR induces apoptosis irrespective of TP53 or ATM mutational status in CLL cells
Activation of the intrinsic BCL-2 regulated apoptosis signaling pathway often relies on the presence of functional p53. Hence, we studied the effect of AICAR in CLL samples with mutated/deleted TP53 to assess whether AICAR-induced apoptosis is dependent on functional p53 in CLL cells. Patient samples with mutated TP53 (patients 24 and 25) or with 17p13 deletion harboring the p53 locus (patients 26 and 27) were previously described15,17 and used for this analysis.
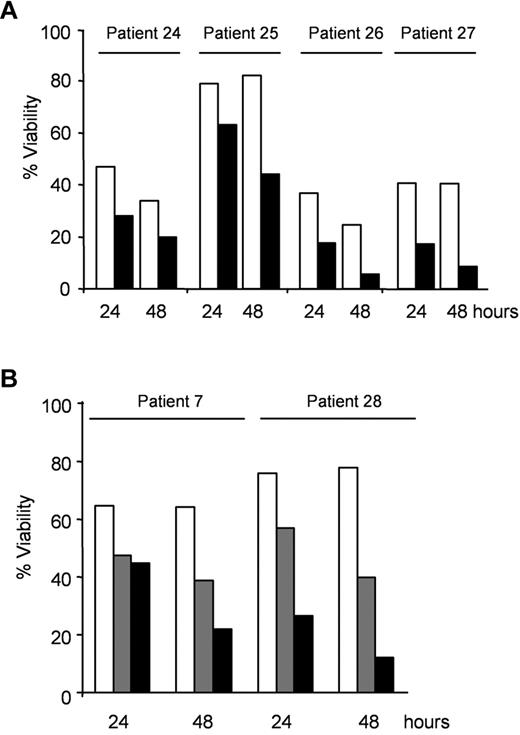
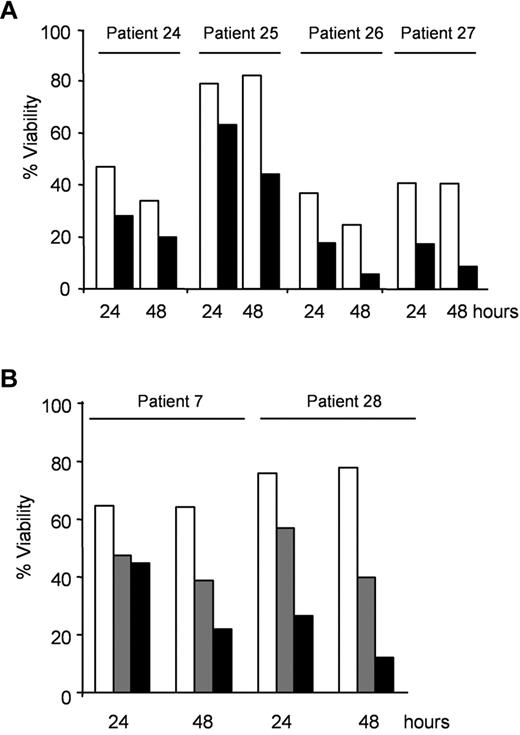
First, CLL cells with mutated or deleted TP53 were treated with 0.5mM AICAR for 24 and 48 hours. Importantly, AICAR induced apoptosis in CLL cells with mutated or deleted TP53 (P < .005; Figure 3A) as effectively as in tumors cells harboring WT TP53 (Table 1, patients without 11q23 or 17p13 deletion). To assess TP53 status, samples from patients 24, 25, 26, and 27 were incubated with 5μM nutlin-3a, the MDM2 inhibitor, which has been shown to induce apoptosis and accumulation of p53 in WT TP53 cells but not in mutated TP53 cells.15 Nutlin-3a did not induce apoptosis in these samples with mutated or deleted TP53 (not shown). In agreement with these results, AICAR induced apoptosis in B lymphocytes from the spleen of p53−/− or WT mice alike (not shown).
AICAR induces apoptosis independently of TP53 or ATM in CLL cells. (A) Cells with mutated TP53 (patients 24 and 25) or with 17p13 deletion (patients 26 and 27) were untreated (□) or treated with 0.5mM AICAR (■) for 24 and 48 hours. (B) Cells with 11q23 deletion and without ATM protein (patient 7 and 28) were untreated (□) or treated with 0.5mM (▩) and 1mM (■) AICAR for 24 and 48 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (A-B).
AICAR induces apoptosis independently of TP53 or ATM in CLL cells. (A) Cells with mutated TP53 (patients 24 and 25) or with 17p13 deletion (patients 26 and 27) were untreated (□) or treated with 0.5mM AICAR (■) for 24 and 48 hours. (B) Cells with 11q23 deletion and without ATM protein (patient 7 and 28) were untreated (□) or treated with 0.5mM (▩) and 1mM (■) AICAR for 24 and 48 hours. Viability was measured by flow cytometry, and it is expressed as the percentage of the viability (A-B).
Next, the effect of AICAR was examined in CLL cells with ATM alterations. CLL cells from patients 7 and 28 have 11q23 deletion, which involves the ATM gene, and do not express ATM protein.17,18 CLL cells with altered ATM were treated with 0.5 or 1mM AICAR for 24 and 48 hours. Remarkably, AICAR also induced apoptosis in CLL cells with 11q23 deletion and without ATM protein expression (Figure 3B). Taken together, our results demonstrate that AICAR potently induces apoptosis in CLL cells regardless of its p53 or ATM status. In agreement with these data, there is no correlation between AICAR-induced apoptosis and the most common genetic abnormalities in CLL cells (presence of 11q23 deletion, trisomy 12, 13q14 deletion, or 17p13 deletion, supplemental Figure 1 [available on the Blood Web site; see the Supplemental Materials link at the top of the online article]).
AICAR induces the expression of proapoptotic BCL-2 family members in CLL cells
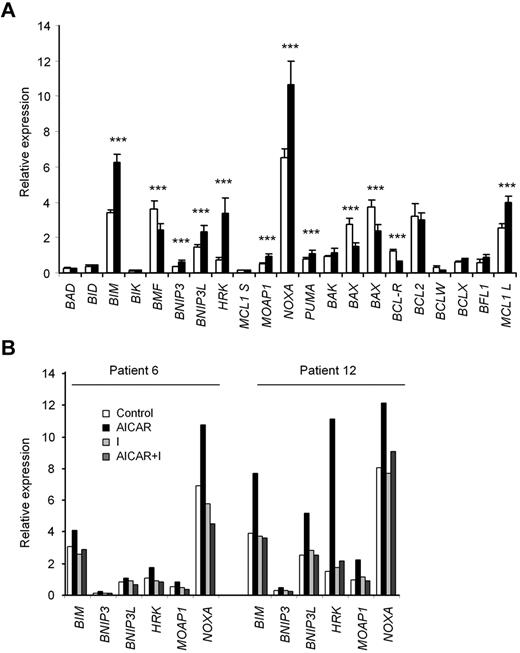
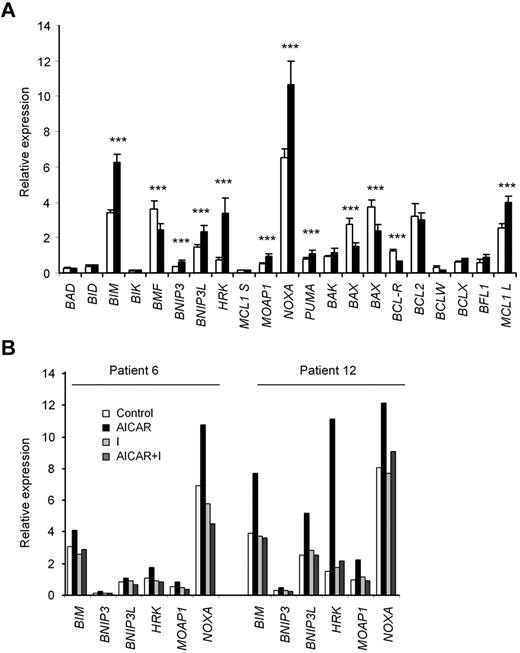
RT-MLPA was used to analyze changes in the expression of all known BCL-2 family members.27 Incubation of CLL cells with 0.5mM AICAR for 24 hours significantly modified the mRNA levels of several apoptosis-related genes (Figure 4A). Importantly, increases in the proapoptotic genes BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA could play a role in AICAR-induced apoptosis. Of note, the mRNA levels of these proapoptotic genes were not modified concomitantly in all CLL samples analyzed; consequently, not all of them were induced in the same tumor sample (Figure 4B). Interestingly, the mRNA levels of the BH3-only BCL-2 family members BIM, HRK, and NOXA were most strongly and most frequently coregulated. In addition, the mRNA levels of BIM, NOXA, and PUMA reached statistically significant increases in relative mRNA levels already at 6 hours after AICAR treatment (supplemental Figure 2).
Analysis of apoptosis-related gene expression induced by AICAR in CLL cells. (A) Cells were untreated (□) or treated (■) with 0.5mM AICAR for 24 hours. Cells were lysed, and the mRNA levels of the BCL-2 family members were analyzed by RT-MLPA. Data are mean ± SEM (n = 19). ***P < .005, AICAR-treated versus untreated cells. (B) Cells were preincubated without (Control) or with 0.2μM 5-iodotubercidin (I) for 30 minutes, and 0.5mM AICAR was added for 24 hours. Cells were lysed, and the mRNA levels of BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA were analyzed by RT-MLPA. Two representative samples are shown (n = 3).
Analysis of apoptosis-related gene expression induced by AICAR in CLL cells. (A) Cells were untreated (□) or treated (■) with 0.5mM AICAR for 24 hours. Cells were lysed, and the mRNA levels of the BCL-2 family members were analyzed by RT-MLPA. Data are mean ± SEM (n = 19). ***P < .005, AICAR-treated versus untreated cells. (B) Cells were preincubated without (Control) or with 0.2μM 5-iodotubercidin (I) for 30 minutes, and 0.5mM AICAR was added for 24 hours. Cells were lysed, and the mRNA levels of BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA were analyzed by RT-MLPA. Two representative samples are shown (n = 3).
Next, we analyzed whether the changes in the mRNA expression profile induced by AICAR were dependent on its intermediate metabolite ZMP. CLL cells were preincubated with or without the adenosine kinase inhibitor 5-iodotubercidin to inhibit ZMP accumulation3 and then treated with 0.5mM AICAR. 5-Iodotubercidin completely blocked all mRNA changes induced by AICAR (Figure 4B). Moreover, the pan-caspase inhibitor Z-VAD.fmk did not inhibit these mRNA changes (not shown), demonstrating that these inductions precede the activation of caspases.
As AICAR-induced apoptosis appears independent of AMPK, the changes in BCL-2 family members induced by AICAR should be independent of AMPK. Therefore, we analyzed by RT-MLPA the changes induced in CLL cells using the AMPK activator A-769662 on the mRNA profile of apoptosis-related genes. CLL cells were incubated with 100μM A-769662 or 0.5mM AICAR for 24 hours. A-769662 did not induce any significant change in the mRNA levels of the genes that were modulated by treatment with AICAR (supplemental Figure 3). Thus, selective activation of AMPK is not sufficient to induce proapoptotic genes as induced by AICAR in CLL cells.
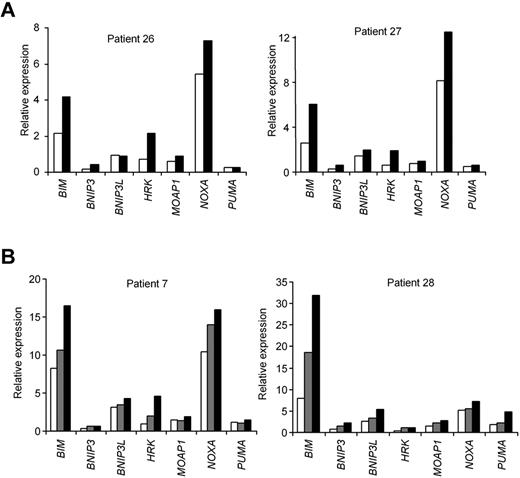
Consistent with our observations on cell death, incubation with 0.5mM AICAR for 24 hours induced the same mRNA profile in CLL cells with mutated or deleted TP53 (Figure 5A) as in TP53 WT CLL cells (supplemental Figure 4). Of note, nutlin-3a did not change the p53-dependent genes in these samples with mutated or deleted TP53 (not shown). Furthermore, incubation with AICAR for 24 hours induced the same mRNA profile in CLL cells without ATM protein (Figure 5B) than in CLL cells without 11q23 deletion (supplemental Figure 4).
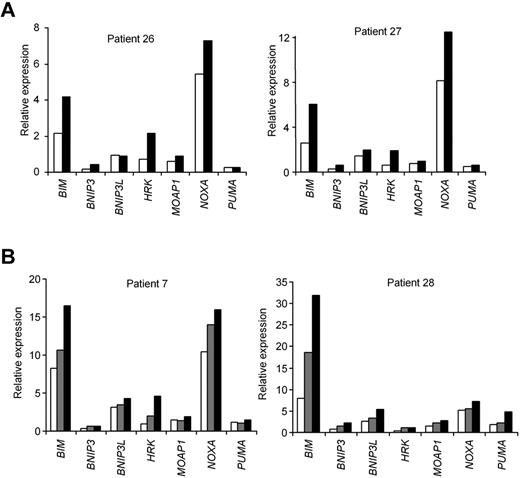
AICAR-induced mRNA profile is irrespective of TP53 or ATM in CLL cells. (A) Cells from patients 26 and 27 (with 17p13 deletion) and (B) patients 7 and 28 (with 11q23 deletion and without ATM protein) were untreated (□) or treated with 0.5mM (▩) or 1mM AICAR (■) for 24 hours. Cells were lysed, and the mRNA levels of BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA were analyzed by RT-MLPA.
AICAR-induced mRNA profile is irrespective of TP53 or ATM in CLL cells. (A) Cells from patients 26 and 27 (with 17p13 deletion) and (B) patients 7 and 28 (with 11q23 deletion and without ATM protein) were untreated (□) or treated with 0.5mM (▩) or 1mM AICAR (■) for 24 hours. Cells were lysed, and the mRNA levels of BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA were analyzed by RT-MLPA.
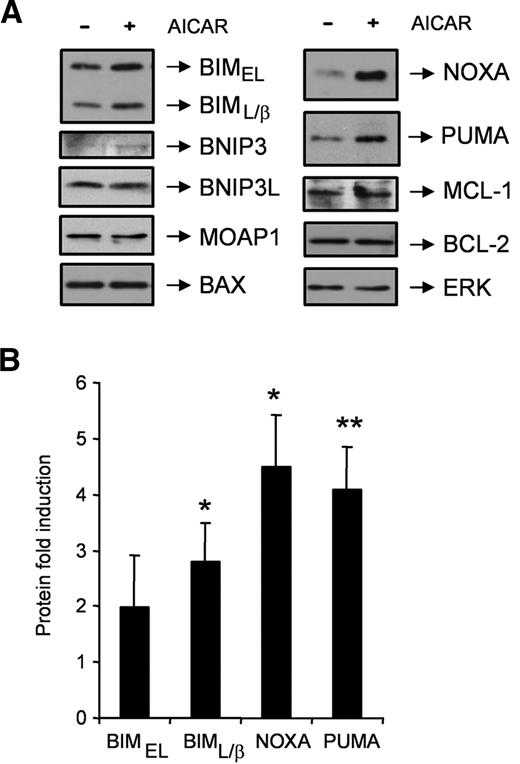
Importantly, we analyzed changes in the protein expression of several BCL-2 family members after 9 hours of incubation with AICAR in CLL cells (Figure 6). Western blot analysis confirmed an induction of the BIM, NOXA, and PUMA protein levels by AICAR in all the samples analyzed (Figure 6B). In contrast, the protein levels of the BCL-2 family members BNIP3, BNIP3L, MOAP1, BAX, BCL-2, or MCL-1 did not change after incubation with AICAR (Figure 6A). The levels of HRK could not be analyzed because, to our knowledge, no antibodies exist that reliably recognize human HRK protein.
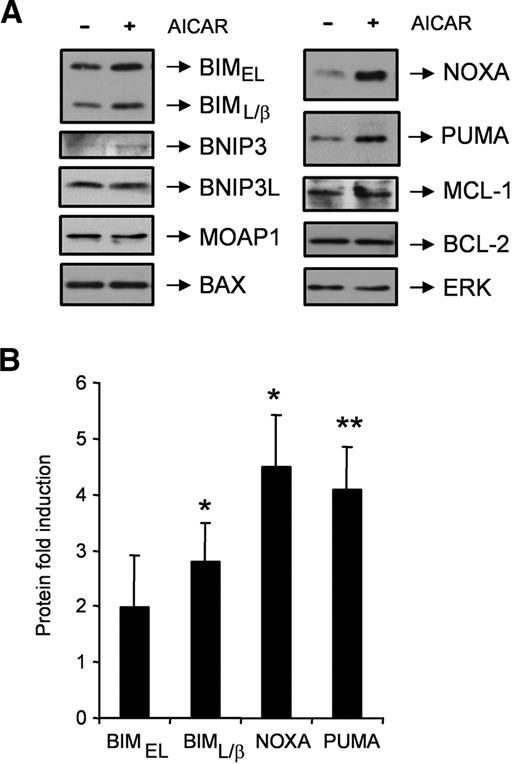
AICAR induces BIM, NOXA, and PUMA proteins in CLL cells. (A) Cells were untreated (−) or treated (+) with 0.5mM AICAR for 9 hours. Cells were lysed and analyzed by Western blot. Total levels of the BCL-2 family members BIM, BNIP3, BNIP3L, MOAP1, BAX, NOXA, PUMA, MCL-1, and BCL-2 were analyzed. ERK was used as a control of protein loading. One representative sample is shown (n = 6). (B) BIMEL, BIML/β, NOXA, and PUMA protein levels from AICAR-treated cells for 9 hours were quantified by densitometry and normalized by the ERK protein levels. Data are mean ± SEM (n = 6), expressed as the fold induction compared with untreated cells. **P < .01, *P < .05, AICAR-treated versus untreated cells.
AICAR induces BIM, NOXA, and PUMA proteins in CLL cells. (A) Cells were untreated (−) or treated (+) with 0.5mM AICAR for 9 hours. Cells were lysed and analyzed by Western blot. Total levels of the BCL-2 family members BIM, BNIP3, BNIP3L, MOAP1, BAX, NOXA, PUMA, MCL-1, and BCL-2 were analyzed. ERK was used as a control of protein loading. One representative sample is shown (n = 6). (B) BIMEL, BIML/β, NOXA, and PUMA protein levels from AICAR-treated cells for 9 hours were quantified by densitometry and normalized by the ERK protein levels. Data are mean ± SEM (n = 6), expressed as the fold induction compared with untreated cells. **P < .01, *P < .05, AICAR-treated versus untreated cells.
AICAR induces apoptosis by triggering the joint induction of BIM and NOXA
To study a rate-limiting role of BIM, NOXA, and PUMA in AICAR-induced apoptosis, we assessed the effect of drug treatment in B lymphocytes lacking Bim, Puma, Noxa, Bim and Puma, or Bim and Noxa and compared it with the effects observed in mice overexpressing human BCL-2 in all hematopoietic cells. Lymphocytes were incubated for 12 hours with AICAR in a range of concentrations (0.05, 0.1, 0.25, and 0.5mM). AICAR induced apoptosis, as in CLL samples,3 preferentially in B cells, although 4 times higher doses were required to kill mouse T cells (supplemental Figure 5). Moreover, the pan-caspase inhibitor Z-VAD.fmk blocked AICAR-induced apoptosis in mouse B cells from spleen (supplemental Figure 6). In line with previous results in CLL cells, RT-MLPA analysis showed that AICAR induced a significant increase in the mRNA levels of Bim and Noxa in mouse lymphocytes (not shown).
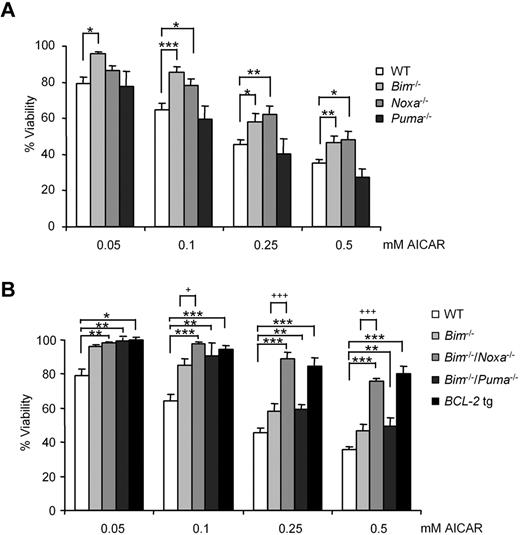
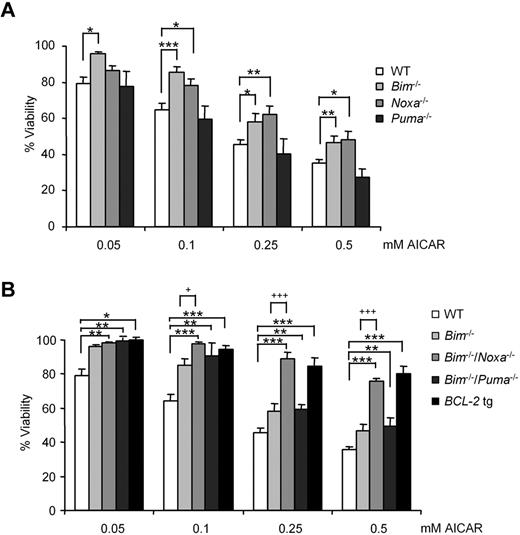
B cells from WT or Puma−/− animals died with the same kinetics, whereas Bim−/− or Noxa−/− B lymphocytes were partially protected from the effects of AICAR (Figure 7A). Consistent with this observation, B lymphocytes derived from Bim−/−/Noxa−/− animals resisted AICAR-induced apoptosis most effectively, whereas cells from Bim−/−/Puma−/− behaved like Bim-deficient cells (Figure 7B). Notably, the protection provided by combined loss of Bim and Noxa was comparable with the one achieved by overexpression of human BCL-2 as a transgene in mouse B lymphocytes (Figure 7B). Loss of other BH3-only proteins, including Bad, Bmf, or Hrk/Dp5, provided no protective effect (not shown).
B lymphocytes from Noxa−/−/Bim−/− mice resisted induction of apoptosis by AICAR. (A) B cells from WT, Bim−/−, Noxa−/−, and Puma−/− and (B) B cells from WT, Bim−/−, Bim−/−/Noxa−/−, Bim−/−/Puma−/−, and vav-BCL2 transgenic (BCL-2 tg) mouse spleen were incubated for 12 hours with AICAR in a range of concentrations (0.05, 0.1, 0.25, and 0.5mM). Viability was measured by flow cytometry, and it is expressed as the percentage of the viability of untreated cells. Data are mean ± SEM from 3 to 6 independent experiments for each genotype. ***P < .005, **P < .01, *P < .05, mutant versus WT B lymphocytes. +++P < .005, +P < .05, mutant versus Bim−/− B lymphocytes.
B lymphocytes from Noxa−/−/Bim−/− mice resisted induction of apoptosis by AICAR. (A) B cells from WT, Bim−/−, Noxa−/−, and Puma−/− and (B) B cells from WT, Bim−/−, Bim−/−/Noxa−/−, Bim−/−/Puma−/−, and vav-BCL2 transgenic (BCL-2 tg) mouse spleen were incubated for 12 hours with AICAR in a range of concentrations (0.05, 0.1, 0.25, and 0.5mM). Viability was measured by flow cytometry, and it is expressed as the percentage of the viability of untreated cells. Data are mean ± SEM from 3 to 6 independent experiments for each genotype. ***P < .005, **P < .01, *P < .05, mutant versus WT B lymphocytes. +++P < .005, +P < .05, mutant versus Bim−/− B lymphocytes.
Taken together, these observations suggest that AICAR-induced induction of BIM and NOXA is most critical for cell killing, whereas other transcriptional changes observed appear dispensable. Furthermore, BIM and NOXA account for all BCL-2 blockable cell death triggered by AICAR.
Discussion
In the present study, we show that AMPK activators, such as phenformin28 or A-769662,29 do not induce apoptosis in most CLL samples analyzed. These results suggest that AMPK activation on its own is insufficient to induce apoptosis in CLL cells and that in these tumor cells AICAR induces apoptosis largely through an AMPK-independent mechanism. Consistently, AICAR induces apoptosis in both WT and Ampkα1−/− B lymphocytes from mouse spleen alike. In addition, AMPK seems to act as a survival factor in mouse B lymphocytes because Ampkα1−/− B cells showed higher sensitivity to AICAR-induced apoptosis than WT cells. Specific inhibitors of AMPK could induce apoptosis or enhance the apoptotic effect of AICAR in CLL cells. Activation of AMPK inhibits proliferation8 and induces cytoprotective autophagy33,34 in different cell types. Thus, although the apoptotic effect of AICAR is independent of AMPK in CLL cells, AMPK could play a role in limiting cell proliferation in the CLL-proliferating centers.
It has been described that AICAR induces p53 through AMPK activation.35,36 However, AICAR does not affect p53 phosphorylation or accumulation in CLL cells,3 suggesting that it could induce apoptosis through a p53-independent mechanism. Furthermore, a combination of AICAR with the MDM2 inhibitor nutlin-3a does not enhance its apoptotic activity.15 Importantly, in the present study, we demonstrate that AICAR induces apoptosis and the same mRNA changes in CLL cells with mutated/deleted TP53 or deleted ATM, as in WT TP53 or ATM CLL cells. These data demonstrate that AICAR induces apoptosis in CLL cells regardless of their TP53 and ATM status and hence constitutes an interesting treating option for patients presenting an inactivated ATM/p53 signaling axis.
We found that AICAR induces apoptosis through activation of the mitochondrial pathway because inhibition of caspase-8 did not inhibit AICAR-induced apoptosis and caspase inhibition failed to inhibit the cytochrome c release induced by AICAR. Furthermore, we found that AICAR induces a significant increase in BIM, BNIP3, BNIP3L, HRK, MOAP1, NOXA, and PUMA mRNA levels in the tumor samples analyzed, but not all genes were induced concomitantly. Importantly, our findings demonstrate that BCL-2 family changes induced by AICAR are dependent on ZMP accumulation and independent of AMPK activation in CLL cells.
AICAR induces the accumulation of BIM, NOXA, and PUMA proteins in the CLL samples analyzed. Remarkably, B lymphocytes from mice lacking both Bim and Noxa mice are strongly resistant to AICAR-induced apoptosis. Previously, increases in NOXA protein levels have been shown to be implicated in the induction of apoptosis by aspirin,37 2-phenylacetylenesulfonamide,38 and bortezomib39 in CLL cells, whereas BIM protein seems to play a role in the cytotoxic effect of glucocorticoids40 and forodesine.41 Moreover, both proteins have been involved in the apoptotic effect of HDAC inhibitors42 in CLL cells. The exact mechanisms of transcriptional induction of BIM and NOXA by AICAR in B lymphocytes are presently unknown and will be the subject of our future investigations. Candidate transcriptional activators of NOXA, besides p53,43 which does not appear critical here, include p73, HIF-1α, E2F-1, and c-MYC.44,45 In addition, several transcriptional factors, including FOXO3a, E2F-1, and c-Jun, have been involved in the regulation of BIM.46 Whether PUMA plays a critical role in AICAR-induced apoptosis in human CLL but not mouse B cells remains to be investigated.
Of interest for future therapeutic application is the observation that AICAR induces NOXA, which primarily neutralizes the BCL-2 prosurvival homolog MCL-1.47 MCL-1 expression levels were defined as the key-resistance factor hampering the use of currently developed BH3-mimetics that act like BH3-only proteins, such as ABT-737 or ABT-263 in CLL.48,49 Although we noted that Bim−/−/Noxa−/− and vav-BCL2 transgenic mice are highly resistant to AICAR treatment, most CLL cell samples tested were highly sensitive to AICAR treatment. This could be surprising because CLL cells frequently express high levels of BCL-2 and seem to depend on it for survival.10,13 In CLL cells, BIM appears to be sequestered by BCL-2, rendering these cells “primed for death.”50 In the context of AICAR treatment, the observed increase in BIM is then sufficient to push these cells “over the edge” because NOXA, induced in the same setting, acts as a sensitizer that helps to prevent MCL-1 from also sequestering BIM. In B cells from mice overexpressing human BCL-2, the levels of BCL-2 most probably exceed those in CLL cells and possibly can buffer all available as well as induced BIM, that, in WT cells may require NOXA for sensitization to cell death by blocking MCL-1, as suggested by our findings using Bim−/−/Noxa−/− mice. So, cell death inhibition in vav-BCL2 transgenic versus Bim−/−/Noxa−/− mice may actually differ at the molecular level but yields similar results in terms of overall survival in vitro.
In conclusion, present results demonstrate that AICAR induces apoptosis by a p53- and AMPK-independent mechanism through up-regulation of BIM and NOXA in CLL cells. AICAR is currently in a clinical trial for CLL patients7 and might be a new therapeutic option for treatment of B-cell neoplasms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alba Pérez Perarnau and Camila Rubio, and Dr Esther Castaño (Scientific-Technical Services of the Unitat de Bellvitge at the Universitat de Barcelona) for helpful discussions and suggestions; Professors Andreas Strasser, Jerry Adams, Manuel Serrano, and the late Stan Korsmeyer for mutant mouse strains; Kathrin Rossi for animal care; Irene Gaggl for mouse genotyping; Unitat de Biologia and the Unitat de Genòmica from Scientific-Technical Services at the Universitat de Barcelona for their technical support; and Abbott Laboratories for kindly providing A-769662.
This study was supported by Ministerio de Ciencia e Innovación and FEDER (SAF2007-60964), Instituto de Salud Carlos III (RTICC RD06/0020), AGAUR-Generalitat de Catalunya (2009SGR-00395; J.G.), the Austrian Science Fund (Y212-B13; START; A.V.), and ADVANCELL-Advanced In Vitro Cell Technologies SA. D.M.G.-G. is the recipient of a research fellowship from the Ministerio de Ciencia e Innovación; D.I.-S. has a postdoctoral contract from Fundació Bosch i Gimpera.
Authorship
Contribution: A.F.S. and D.M.G.-G. performed research, analyzed data, and wrote the paper; D.I.-S., L.C.-M., A.M.C., and M.d.F. performed research; C.C. contributed with analytical tools; E.G.-B. and E.A. contributed with patient samples and data; V.L. assisted with mice material; B.V. supplied Ampkα1−/− mice and revised the data; A.B. supplied Hrk/Dp5−/− mice and revised the data; G.P. analyzed the data; A.V. supervised experiments of D.M.G.-G. using mice that lack Bim, Noxa, Puma, Bim/Noxa, Bim/Puma, Bad, Bmf, or p53 or animals expressing a vav-BCL2 transgene, analyzed the data, and edited the manuscript; J.G. designed and supervised the research, analyzed the data, and wrote the paper; and all authors revised the manuscript critically and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.C. and J.G. patented the use of AICAR for B-cell neoplasms treatment, and this patent was licensed to ADVANCELL-Advanced In Vitro Cell Technologies SA in 2004. C.C. currently works as Executive VP of Advancell Therapeutics at ADVANCELL-Advanced In Vitro Cell Technologies SA. The remaining authors declare no competing financial interests.
Correspondence: Joan Gil, Departament de Ciències Fisiològiques II, IDIBELL-Universitat de Barcelona, Campus de Bellvitge, Pavelló de Govern, 4a planta, E-08907 L'Hospitalet de Llobregat, Barcelona, Spain; e-mail: jgil@ub.edu.
REFERENCES
Author notes
A.F.S. and D.M.G.-G. contributed equally to this study.