Abstract
More than one quarter of a million adults throughout the world are diagnosed annually with acute myeloid leukemia (AML). Despite considerable progress during the past 3 decades in the therapy of AML, two-thirds of young adults and 90% of older adults still die of their disease. The reported median age has increased over the past few decades, mostly because of a greater willingness of physicians to diagnose and treat older patients, and now is 72 years. The greatest challenge is in this age group. However, much improvement in therapy is needed for all adults with AML. Recent advances in allogeneic transplantation, a better understanding of prognostic factors, and development of targeted agents have only modestly improved overall outcome when large populations of patients are considered. Although an explosion in knowledge about the molecular pathogenesis of AML has outpaced treatment advances, such insights hold promise for the development of new therapies directed at specific molecular abnormalities that perturb malignant cell survival pathways. The current approach in 2010 to the management of this disease is presented through a discussion of illustrative cases.
Introduction
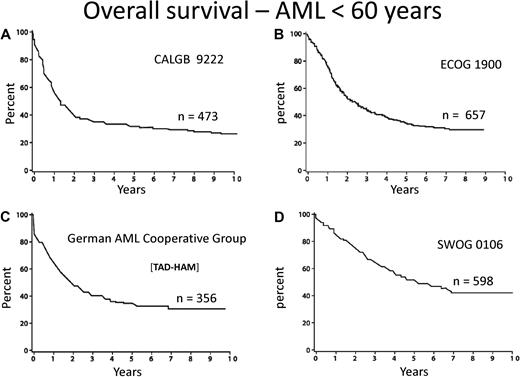
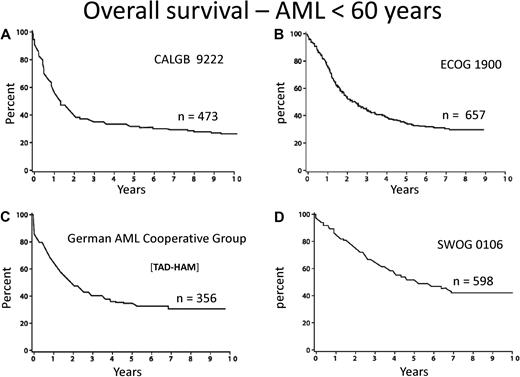
Few diseases other than acute myeloid leukemia (AML) engender so much personal and institutional passion regarding treatment strategies. This is attributable to dramatic progress in deciphering the pathogenesis of the disease, the identification of prognostic factors, and burgeoning treatment options. However, there is a “great divide” between our understanding of the molecular basis and the development of effective treatment. The median age of AML is 72 years, as reported by the Swedish Acute Leukemia Registry, a model for collection of real world data.1 Although some improvement during the last 4 decades is apparent among younger patients, still only approximately 35% of such patients entered on clinical trials are cured of their disease (Figure 1).2-5 Little, if any, progress among older adults has occurred. Indeed, only patients with acute promyelocytic leukemia (APL), a rare subtype, enjoy the excellent outcome and likelihood of cure we all desire. Nevertheless, recent advances in molecular prognostic factors, allogeneic hematopoietic cell transplantation, and drug development provide excitement for the future.
Overall survival from diagnosis for younger adults with AML. Recent publications/presentations from 4 cooperative oncology groups. (A) CALGB 9222. Reprinted from Moore et al2 with permission. (B) ECOG 1900. Reprinted from Fernandez et al4 with permission. (C) German AML Cooperative Group. Reprinted from Buchner et al3 with permission. (D) SWOG 0106.5
Overall survival from diagnosis for younger adults with AML. Recent publications/presentations from 4 cooperative oncology groups. (A) CALGB 9222. Reprinted from Moore et al2 with permission. (B) ECOG 1900. Reprinted from Fernandez et al4 with permission. (C) German AML Cooperative Group. Reprinted from Buchner et al3 with permission. (D) SWOG 0106.5
To some extent, the management of adults with AML appears to be standardized. However, much of the so-called conventional therapy has been established with a lack of data or without rigorous review of the existing evidence; and so, considerable uncertainty remains. Such uncertainty is reflected in the significant diversity in the management of patients with AML, both in induction of older patients and postremission therapy of all patients. The suggested management described herein reflects an approach for the treatment of AML. The recommendations made here, through clinical vignettes describing patients commonly encountered in daily practice, are not a substitute for enrolling patients on carefully designed prospective clinical studies, which remain vital for improving the current and future management of AML. Rather, they represent how we treat adults with AML bolstered by data where they exist and by a dose of healthy skepticism where conventional wisdom prevails, but without definitive supporting evidence.
Patient 1
A 43-year-old woman is diagnosed with AML. Her complete blood count at presentation reveals a white blood cell count (WBC) of 23 000/μL with 23% blasts; her hemoglobin is 8.7 g/dL, and the platelet count is 32 000/μL. Her bone marrow is diffusely infiltrated with myeloblasts that express CD34, CD13, and CD33. The karyotype is normal, and evaluation for mutations of the genes encoding for FLT3-ITD, NPM1, and CEBPA is negative. The patient has a human leukocyte antigen (HLA)-identical sibling.
Question: What is the optimal induction and postremission therapy? Is it reasonable to offer standard chemotherapy consolidation and “reserve” an allogeneic transplantation to be used if the patient relapses?
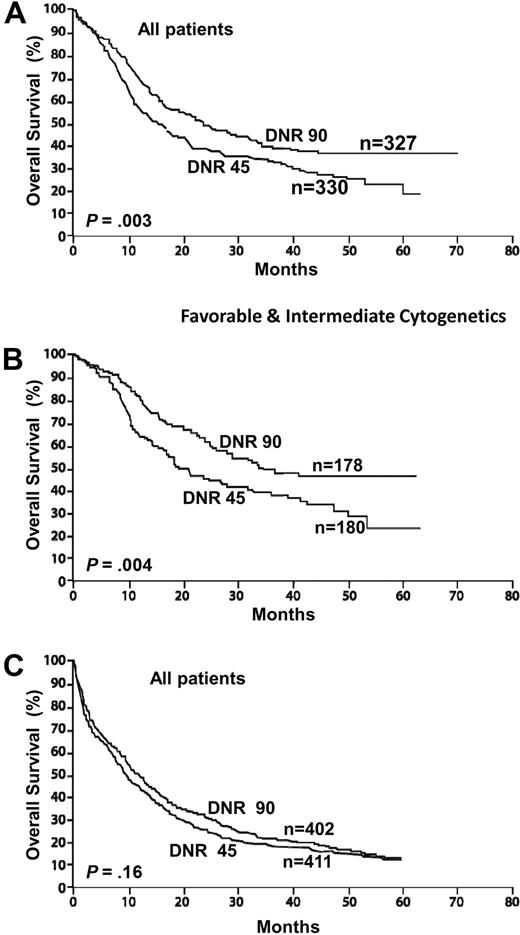
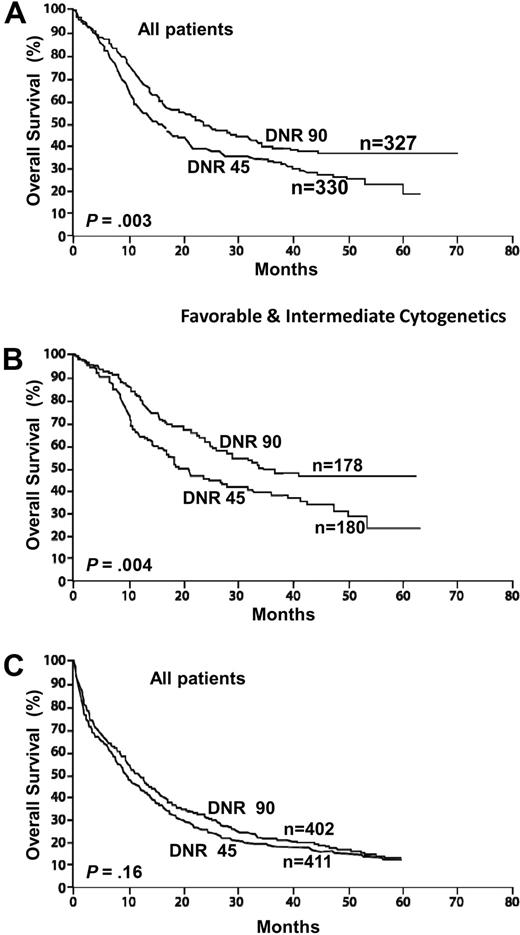
Although in the early 1990s several randomized studies of induction therapy suggested that using idarubicin, mitoxantrone, aclarubicin, or amsacrine demonstrated superior results compared with daunorubicin, there is no evidence that these studies reflected a true biologic advantage rather than a lack of dose equivalence.6 It has now been established that the traditional approved dose of daunorubicin (45 mg/m2 for 3 days) is no longer appropriate as induction therapy for AML. A recent randomized trial for younger patients under age 60 years reported a significantly higher complete remission (CR) rate for patients receiving 90 mg/m2 of daunorubicin compared with 45 mg/m2. The overall survival was also improved with the higher dose of daunorubicin (Figure 2A-B).
AML: intensifying induction therapy (overall survival from diagnosis). Randomized study conducted by ECOG in adults younger than 60 years comparing daunorubicin (DnR) 45 versus 90 mg/m2 for 3 days, both with cytarabine 100 mg/m2 for 7 days. (A) All study patients. (B) Patients with favorable and intermediate cytogenetics. Reprinted from Fernandez et al4 with permission. (C) Similar randomized study conducted by HOVON/SAKK in older adults (older than 60 years). Reprinted from Lowenberg et al55 with permission.
AML: intensifying induction therapy (overall survival from diagnosis). Randomized study conducted by ECOG in adults younger than 60 years comparing daunorubicin (DnR) 45 versus 90 mg/m2 for 3 days, both with cytarabine 100 mg/m2 for 7 days. (A) All study patients. (B) Patients with favorable and intermediate cytogenetics. Reprinted from Fernandez et al4 with permission. (C) Similar randomized study conducted by HOVON/SAKK in older adults (older than 60 years). Reprinted from Lowenberg et al55 with permission.
Approximately 70% of young adults undergoing induction therapy are expected to achieve a CR. The published data on responses to induction vary considerably between the cooperative trial groups, reflecting different criteria for assessment of remission as well as different inclusion criteria for clinical studies. For example, studies in which patients with antecedent hematologic disorders or therapy-related AML are included would have inferior results compared with those that exclude such patients. A dose of 90 mg/m2 of daunorubicin is clearly safe and should become the standard of care, although doses between 60 mg/m2 and 90 mg/m2 may be as effective.
This patient is in the intermediate-risk category given that her karyotype is normal.7 Advances in the molecular classification of AML, particularly among patients with a normal karyotype, have recently refined this risk group from the traditional 50% to 70% among AML patients7-9 to no more than 25% to 30%.10 In this patient, the absence of unfavorable mutations, such as FLT3-ITD, or the more favorable mutations, such as NPM1 and CEBPA, suggests that this patient remains best classified in the intermediate-risk category,11 recognizing that even in this group further discrimination is likely in the coming years with the use of genetic profiling and further molecular characterization.
Allogeneic hematopoietic cell transplantation (allo-HCT) provides the most potent antileukemic effect of any postremission strategy in AML, as demonstrated by the lowest rates of relapse in all clinical studies. For patients, such as this who have an HLA-identical sibling donor, an allo-HCT should be offered, preferably if the patient remains negative for minimal residual disease (MRD) before transplantation.12 Despite substantial transplantation-related mortality of 15% to 20%, the reduction in the relapse rate significantly outweighs the transplantation-associated risk and is considered standard of care for such a patient (Table 1).10 An exception to this approach may be made for patients whose leukemia cells express more favorable mutations at diagnosis. Several recent reports have indicated a more favorable outcome among patients with a normal karyotype for those who present with mutations of NPM1 or CEBPA. One recent analysis suggested that patients whose cells are NPM1+/FLT3-ITD− belong more appropriately in the favorable-risk group and may not benefit from an allo-HCT.11 Although fairly widely accepted and having moved into routine practice in many centers,13 the data supporting such a practice are based on only 38 patients with a donor.11 The CEBPA mutation also confers a more favorable prognosis for patients with a normal karyotype11,14 ; therefore, the same consideration as applicable to NPM1+ should be given, although there have been no specific reports that have demonstrated this. Of note, recent data suggest that the more favorable prognosis in this group is limited to patients with the biallelic CEBP mutations.15
Although there are multiple reports of the use of reduced-intensity conditioning (RIC) regimens in AML, there have been no prospective comparisons with standard regimens, particularly in younger adults. Therefore, at present, RIC should be reserved for older adults with AML or those with significant comorbidities, which preclude conventional myeloablative conditioning for transplantation. The standard of care for younger adults remains a fully myeloablative conditioning regimen, for which abundant data exist.
Although there are no prospective trials that have addressed the need for any postremission consolidation chemotherapy before an allo-HCT, 2 retrospective analyses from large international registries suggest that there is no benefit to adding any consolidation therapy before an allo-HCT.16,17
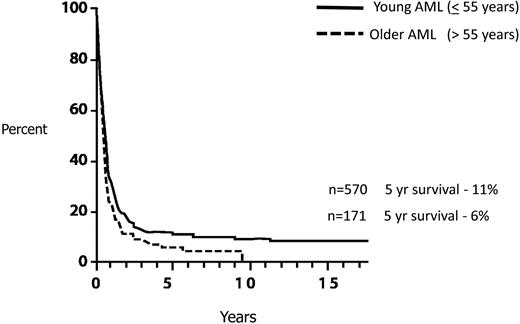
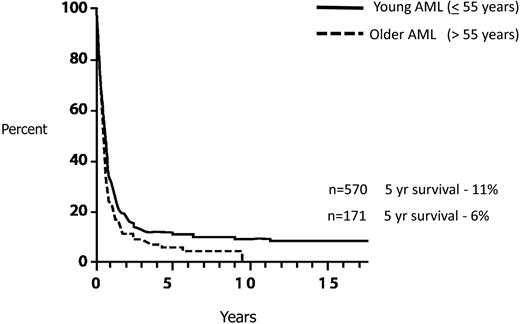
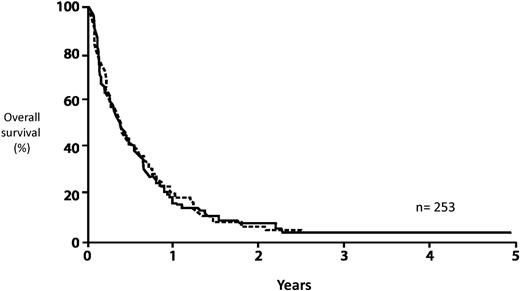
Finally, although the concern for the high transplantation-related morbidity and mortality is appropriate, this should not lead to delaying an allo-HCT in first complete remission (CR1) and reserving such treatment for patients in the event of a relapse. Reports indicating a successful outcome after relapse with a curative potential of approximately 30%18,19 are highly selective and relate only to patients who have survived their relapse and are fit enough to receive a transplantation in second remission. The predictive overall survival of relapsed AML patients is exceedingly poor, no more than approximately 10%20-22 (Figure 3). In our view, delaying transplantation until after relapse is a misleading strategy, although we recognize that no trial has ever randomized patients with donors between immediate and delayed transplantation.
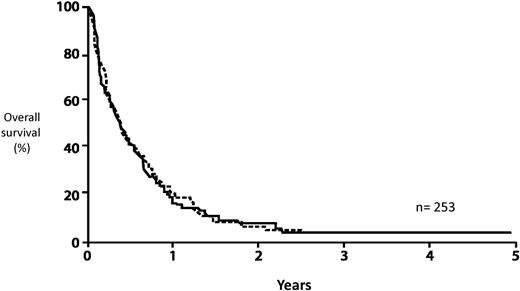
AML: survival from relapse by age. Data based on 2441 patients entered on 8 consecutive ECOG studies.22 Reprinted from Rowe et al22 with permission.
Proposed treatment: This patient should receive induction therapy with daunorubicin 90 mg/m2 for 3 days together with cytarabine 100 mg/m2 for 7 days. A dose of daunorubicin between 60 mg/m2 and 90 mg/m2 is also reasonable. As postremission therapy, the patient should be referred for an allogeneic transplantation from her HLA-identical sibling and a conventional myeloablative conditioning should be used. “Reserving” an allogeneic transplantation for relapse is definitely not recommended. If possible, any consolidation chemotherapy before the allogeneic transplantation should be avoided.
Patient 2
A 54-year-old man presents with gingival hypertrophy and bleeding. At presentation his WBC is 39 000/μL with 60% monoblasts; the hemoglobin is 7.9 g/dL, and the platelet count is 6000/μL. His bone marrow is diffusely infiltrated with monoblasts. Cytogenetic analysis shows a normal karyotype, and the leukemic cells express the mutated FLT3-ITD. He does not have any siblings. He received standard induction therapy. His day 14 bone marrow demonstrated some cytoreduction but unequivocal residual leukemia, after which he received a second cycle of identical induction therapy and achieved CR.
Question: Should this patient be referred for an alternative donor transplantation? What would be the postremission strategy in the absence of the mutated FLT3-ITD?
This patient's course raises several important issues. First, historically, patients with monocytic leukemia were considered to have a poor prognosis, and those who did not clear their blasts by day 14 were also considered to be in a poorer risk category, irrespective of subsequent response to therapy. However, although monocytic leukemia presents with unique clinical features, such as extramedullary tissue infiltration and central nervous system involvement, once a CR is achieved, there is no evidence that with contemporary therapy the ultimate prognosis is determined by this unique morphology alone.23
Although a day 14 bone marrow generally predicts for a lesser likelihood of achieving a CR with induction, recent data from the Eastern Cooperative Oncology Group (ECOG) suggest that patients who receive a second cycle of induction therapy on day 14, based on the presence of unequivocal residual leukemia, and subsequently achieve a CR, have a prognosis that is similar to those achieving CR with one cycle of induction.24 Thus, the presence of residual leukemia on day 14 in and of itself should not alter the postremission strategy if the patient responds successfully to the induction therapy. The choice of postremission therapy should be based solely on the cytogenetic and molecular determinants at diagnosis and possibly on MRD after induction therapy, as determined by refined molecular or immunophenotypic analyses.25,26 Although the presence of MRD is of concern to any treating physician, at present we do not alter the postremission strategy based on such findings.
The presence of the mutated FLT3-ITD confers a poor prognosis for this patient.27,28 The practical issue is whether to offer a transplantation from an alternative donor, either a matched unrelated donor (MUD), a genetically haploidentical donor, or an umbilical cord donor.
Although the indications for an alternative donor transplantation have not been properly defined, its performance is nevertheless becoming more widespread as the clinical experience is increasing. The only prospective data demonstrating the beneficial effect of a MUD transplantation have been in patients with unfavorable-risk AML.29 Historically, the hesitation to offer an alternative donor transplantation was based on the higher morbidity and mortality compared with sibling transplantations, possibly altering unfavorably the risk-benefit balance for AML patients in CR1. Recent publications of an almost identical outcome after an 8 of 8 MUD transplantation,13,30,31 that is confirmed also by molecular high-resolution typing, are encouraging. However, such data need to be cautiously interpreted because they probably reflect a selection bias in that the eligibility criteria for a MUD transplantation are significantly more stringent than for a sibling donor transplantation. Furthermore, although there is a perception, based on a sound rationale, that immunologic graft-versus-leukemia effect may be particularly potent using MUD because of a higher likelihood of allelic disparity at minor histocompatibility antigens,32 a recent study from the Center for the International Blood and Marrow Transplant Research described somewhat surprising results regarding the outcome of myeloablative MUD transplantations as well as a well-matched cohort of HLA-identical sibling transplantations.33 There was an increased relapse rate in MUD transplantations for AML patients in CR1 and the leukemia-free survival was also significantly improved for patients receiving a sibling transplantation. Unexpectedly, although the presence of graft-versus-host disease is associated with reduced relapse of AML, it does not appear that such an effect is dependent on the degree of genetic disparity and the best donor remains the most closely matched donor. It is of interest that similar observations were recently reported in chronic myeloid leukemia34 and in a Center for the International Blood and Marrow Transplant Research study of reduced intensity HCT in older patients with AML.35
In the absence of a sibling donor, this high-risk patient would be offered the option of a transplantation from a fully MUD, although there are no prospective data that establish this as standard of care. In the absence of the FLT3-ITD mutation the patient who does not have a sibling donor would receive postremission therapy without allogeneic transplantation.
There is much controversy regarding the optimal postremission therapy, including the number of cycles of intensive chemotherapy, the best agent and even regarding the preferred doses.36 In our opinion, a patient not on a clinical study would receive 2 cycles of consolidation therapy with high-dose cytarabine followed by an autologous transplantation. The rationale for using an autologous transplantation is based on the fundamental concept that the optimal approach to postremission therapy is based on the regimens with the most potent antileukemic activity, provided this effect is not abrogated by unacceptably high mortality. In the majority of major prospective studies published over the past decade, a lower relapse rate was reported for patients undergoing an autologous transplantation compared with chemotherapy. In a meta-analysis of 6 trials, including 4410 patients, auto-HCT was associated with modest improvement of 10% to 18% in disease-free survival.37 The hesitation to use an autologous transplantation was the relatively high treatment-related mortality reported in older studies that in most instances used bone marrow as the source for hematopoietic cells.38,39 Currently, the mortality rate associated with an autologous transplantation, in experienced centers using hematopoietic cells collected from the peripheral blood, is less than 2%,40,41 which offers a compelling argument for adding autologous transplantation to chemotherapy-based consolidation.
Although for the majority of patients MUD transplantation is the preference when a sibling donor is not available, there are other alternatives for which data are available. In experienced centers, a transplantation from a genetically haploidentical donor can be performed with overall results that are similar to those reported for MUD transplantation.42 An important advantage with this form of transplantation is the almost universal availability of a donor, with minimal delay to transplantation. Similarly, double unrelated umbilical cord transplantation is increasingly used, and rapidly accumulating data suggest that this is also an option when a sibling donor or MUD is not available.43
Proposed treatment: The decision for induction or postremission therapy should be based on cytogenetic and molecular determinants and is not altered by the presentation with the monocytic variant morphology or by the fact that remission was only achieved after 2 cycles of induction. As postremission therapy, this patient should be referred for MUD transplantation. In the absence of FLT3-ITD mutation, or other high-risk feature, this patient with a normal karyotype would receive 2 cycles of consolidation therapy with high-dose cytarabine followed by an autologous transplantation.
Patient 3
A 43-year-old man presents with a one-week history of weakness and progressive dyspnea. His WBC at presentation is 260 000/μL; the hemoglobin is 8 g/dL, and the platelet count is 32 000/μL. Cytogenetic analysis reveals t(8;21)(q22;q22), and molecular analysis reveals only the presence of the c-KIT mutation.
Question: What is the best emergent management? Is standard induction appropriate? Is central nervous system prophylaxis recommended? What is the appropriate postremission therapy?
This patient presents with a very high WBC count, where apart from any long-term prognostic considerations, there are emergent issues. Hyperleukocytosis in AML is associated with leukostasis with potentially lethal central nervous system and pulmonary complications. The optimal emergent management is uncertain, and one approach is to initiate immediate induction therapy. An alternative strategy consists of daily leukapheresis with the concurrent administration of hydroxyurea at doses of 2 to 6 g/day. Although not substantiated by any data, it is customary in our institutions to continue this approach and wait for the initiation of induction therapy until the WBC has fallen below 40 000 to 50 000/μL. It is presumed, but not proven, that this increases the likelihood of achieving CR with a single cycle of chemotherapy. Once induction therapy is initiated, standard doses should be given with no modification.
The issue of prophylaxis for the central nervous system is controversial in any patient with AML and is often considered in a patient who presents initially with a high WBC.44 Although there are theoretical considerations for administering prophylaxis, in our institutions this is not customarily performed for any patient with AML, in the absence of any symptoms related to the central nervous system.
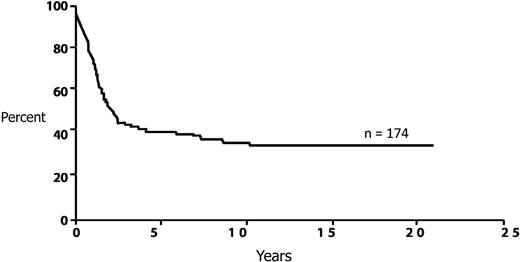
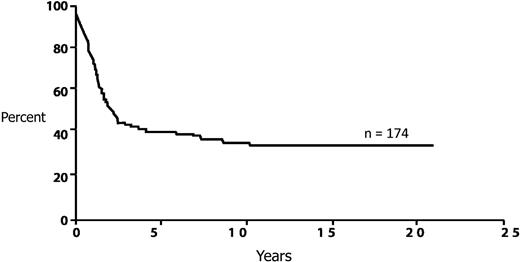
This patient presents with t(8;21)(q22;q22) karyotype. Although frequently described as associated with a favorable prognosis, this is a misnomer, considering that the long-term survival rate of patients is less than 50% in series reporting large numbers of patients45 (Figure 4). Despite this prognosis, multiple prospective studies as well as meta-analyses have not established any benefit to an allogeneic transplantation in patients with t(8;21)(q22;q22).13,46,47 The reduction in relapse is abrogated by the transplantation-related mortality. This recommendation probably is not altered by the presence of other cytogenetic abnormalities.10,48 However, there are increasing number of reports that have suggested that patients with core-binding factor translocations with mutations in c-KIT have a very high relapse rate, almost comparable with patients with unfavorable risk cytogenetics.49-51 For this reason, it is important to routinely obtain all the common molecular markers, which would also include FLT3-ITD, NPM1, and CEBPA.
Long-term survival from diagnosis for AML patients with t(8;21). Adapted from Appelbaum et al45 with permission.
Long-term survival from diagnosis for AML patients with t(8;21). Adapted from Appelbaum et al45 with permission.
The high WBC count has also been reported to be associated with c-KIT mutations, especially in patients with t(8;21).52 These reports in adult patients need to be cautiously interpreted because of the small numbers, and it should be noted that a recent publication in pediatric patients could not confirm the poorer prognosis for patients with c-KIT mutations.53 Nevertheless, given the preponderance of data in adults, this patient would be referred for a matched sibling transplantation or an alternative donor transplantation in the absence of an available HLA-matched sibling.
Proposed treatment: The patient should receive urgent leukapheresis together with hydroxyurea until the WBC is less than 50 000/μL. At that point, standard induction therapy should be given. Central nervous system prophylaxis is not routinely administered. For postremission therapy, the patient should be referred for an allogeneic transplantation from an HLA-identical sibling or from an alternative donor.
Patient 4
A 70-year-old woman presents with AML. At diagnosis, her WBC is 2400/μL; her hemoglobin is 10.2 g/dL, and the platelet count is 17 000/μL. Cytogenetic analysis was not available.
Question: What is the optimal induction and postremission therapy for this patient? How would cytogenetics affect the management of such a patient?
This patient presents with what is probably the most important challenge in managing patients with AML. Given that the median age is 72 years, this is a group with a much higher incidence of AML and among whom the overall survival remains approximately 10%, at best. There has been much discussion, controversy, and a lack of accurate data, given the widely disparate treatment approaches for such patients. Less than 10% of younger AML patients are referred for cooperative group trials, and among older patients the number is far below 5%. In addition, patients referred to a tertiary cancer center and then entered on clinical trials are a highly select subgroup.54 In a recent elegant population-based study from the Swedish Acute Leukemia Registry, a compelling case is made for the administration of standard intensive therapy for all fit older patients rather than adopting a purely palliative approach.1 The approach in our center is unequivocal in offering all AML patients induction therapy, unless presenting with prohibitive comorbidities. There are several important principles in the management of such an older patient. Once the decision is made to treat, then standard doses of induction should be given. Attenuation of induction is contraindicated. A low dose will not reduce the toxicity and is more likely to lead to ineffective therapy with a similar degree of myelosuppression. Fit older adults tolerate chemotherapy at least as well as younger patients, but they do not tolerate prolonged aplasia. A recent randomized trial from the HOVON/SAKK Collaborative Group confirmed the safety of higher doses of daunorubicin, up to 90 mg/m2 for 3 days in older adults.55 In one report, 2 sequential studies of older patients were compared. No significant survival benefit was reported in the study that included postremission therapy compared with the study that offered no such therapy.56 The reticence by many to treat older adults with standard doses of induction chemotherapy has often been based on a mistaken perception that such doses could not be tolerated. In the HOVON/SAKK study, the higher dose of daunorubicin led to a more rapid initial response as well as a higher response rate than a more conventional dose of 45 mg/m2, although there was no significant improvement in the overall survival (Figure 2C). For this reason, this patient would receive a dose of 60 mg/m2 for 3 days as induction recognizing that higher doses may be preferable and may in time become the standard of care. The achievement of CR remains of paramount clinical significance, and this is an important endpoint also in older patients,57 particularly when considering quality of life.1
Several suggestions have been made on how to treat patients who are older than 75 years with a suboptimal performance status. Our own approach would be to avoid using an arbitrary age cut-off and offer standard induction therapy to any patient who we think will tolerate intensive induction chemotherapy. In line with Juliusson et al,1 we would not withhold induction therapy for any patient based on age alone. The presence of comorbidities encompasses a broad spectrum, ranging from those that should be treated with supportive care only, which composes the administration of blood products and antibiotics, to therapy with hydroxyurea and escalating to low-dose cytarabine or some of the new hypomethylating agents, the farnesyltransferase inhibitors or, preferably always, a clinical trial exploring an investigational agent.
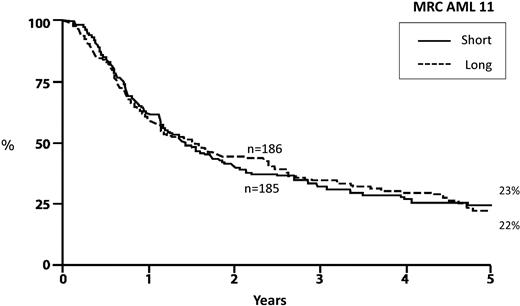
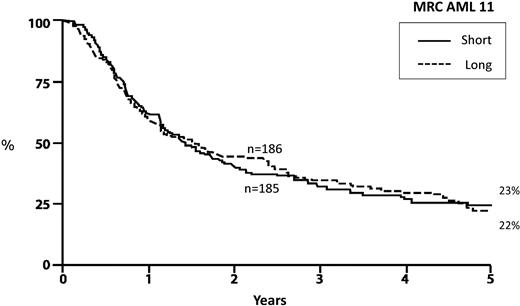
There is enormous uncertainty and controversy regarding the optimal postremission therapy in older patients. In contrast to younger adults, the value of postremission therapy has never been unequivocally established for older patients.58 Despite this, it is common practice and virtually every published clinical trial for older patients with AML includes one or more courses of consolidation therapy. The Medical Research Council (MRC) in Great Britain conducted a large study of 1314 older patients that attempted to determine whether the addition of multiple cycles of consolidation is superior to a single cycle. In this study, patients received standard induction therapy and, if in remission, received the identical course of induction as their first course of consolidation therapy. Patients were then randomized to receive 3 further cycles of consolidation or only observation. The outcomes in both groups were identical, demonstrating that there is no particular value in intensifying postremission therapy beyond a single course of consolidation59 (Figure 5). However, the MRC study did not address whether or not any consolidation is required in older adults. This important issue remains open.
Overall survival from postremission randomization: AML in patients more than 55 years of age. Randomization after induction and 1 cycle of intensification to 3 cycles of consolidation (long) versus observation (short). Adapted from Goldstone et al59 with permission.
Overall survival from postremission randomization: AML in patients more than 55 years of age. Randomization after induction and 1 cycle of intensification to 3 cycles of consolidation (long) versus observation (short). Adapted from Goldstone et al59 with permission.
There is information from prospective trials regarding cytogenetics that may also affect the decision regarding the optimal postremission therapy. Between 25% and 30% of patients who present with unfavorable cytogenetics can achieve a CR.60,61 However, despite the administration of intensive consolidation therapy, the 5-year survival is less than 5%60-62 (Figure 6). Therefore, for such patients, it is hard to justify the administration of consolidation, and a strong case can be made for putting such patients on a clinical trial of novel investigational therapies. Despite this, most current investigations, which include older patients, prescribe postremission therapy, irrespective of the cytogenetics at presentation.
AML in older adults (older than 60 years): long-term survival for patients with unfavorable cytogenetics. Patients received induction and consolidation therapy on trial and were randomized to receive either TAD-HAM or HAM-HAM with results that were superimposable. Reprinted from Büchner et al62 with permission.
AML in older adults (older than 60 years): long-term survival for patients with unfavorable cytogenetics. Patients received induction and consolidation therapy on trial and were randomized to receive either TAD-HAM or HAM-HAM with results that were superimposable. Reprinted from Büchner et al62 with permission.
It is clear that older patients cannot tolerate the same doses of consolidation therapy that are administered to younger patients, often resulting from gastrointestinal toxicity and, if high-dose cytarabine is used, central nervous system toxicity. Typically, doses are decreased for patients between 55 and 70 years of age and are further reduced for those older than 70 years, although there is considerable arbitrariness in such a recommendation.
The advent of RIC regimens as preparative regimens before allogeneic HCT may, for the first time in decades, make a significant impact on the long-term survival of such patients with AML.63 Although there is a paucity of prospective data regarding RIC transplantations, recent studies emphasize the feasibility of this procedure, the curative potential and tolerability in older patients.35,63-67 Despite reports that more than one-third of patients can achieve a long-term survival with this regimen,35 prospective data are needed to establish the true long-term survival rates in a nonselect population. Indeed, very few older patients ultimately undergo such a procedure even in sophisticated centers.68 RIC transplantation has become common practice in many centers, but attempts at accruing a significant number of patients into prospective clinical studies of RIC have fallen short. Whenever possible, patients should be entered on a clinical trial that prospectively evaluates the role of RIC transplantation. Nevertheless, given the emerging data in our centers and others, patients not entered on a clinical study would be offered RIC transplantation from either a matched sibling donor or MUD.
Several issues are completely unresolved when considering such an approach. First, should any consolidation be administered before RIC transplantation? Although for patients receiving standard ablative conditioning allogeneic transplantation there are data suggesting that there is no benefit for the administration of any prior consolidation in CR1,14,15 no such data exist for patients undergoing RIC transplantation. Although a strong rationale exists for administering some form of intensification before RIC, in an attempt to minimize the leukemia burden and allow time for generation of the graft-versus-leukemia effect, in practice the design of cooperative group studies is not uniform. In a recently published HOVON study for older patients, RIC was offered after one cycle of consolidation,55 whereas in a newly designed study by the ECOG, E2906, RIC allogeneic transplantation is offered after successful achievement of CR before any consolidation. This is the approach taken by us, which, although unproven, is driven by an attempt to reduce the transplantation-related toxicity.
A second unresolved issue is whether RIC should be offered also to patients with unfavorable cytogenetics. This is the group that, even among younger AML patients, has a poor prognosis.7,8,47 There are almost no data on RIC transplantations performed in patients with unfavorable cytogenetics because younger patients with unfavorable cytogenetics almost always undergo myeloablative conditioning. In practice, in very experienced centers, an older patient with a good performance status who achieved a CR would be referred for an RIC transplantation, but for these patients we administer one course of consolidation therapy, acknowledging that there are no published data to support such an approach.
Proposed treatment: This woman should receive induction therapy with a dose of daunorubicin that is not less 60 mg/m2 for 3 days. Cytogenetics would not alter the initial attempt to achieve CR. As postremission therapy, she should then receive 1 cycle of consolidation therapy, using an attenuated high-dose cytarabine regimen. If an HLA-matched donor is available, the patient should be offered an RIC HCT in CR1, without any prior consolidation; an exception is for patients with unfavorable cytogenetics.
Patient 5
A 52-year-old woman presented with AML with the following chromosomal abnormalities: del(5q), del(7q), del(12p), and abn(11q;p3). The patient has a long history of prior chemotherapy given for diffuse large cell lymphoma. She was last treated with autologous transplantation 4 years previously and is now free of lymphoma.
Question: What is the appropriate induction and postremission therapy? How is this affected by cytogenetics?
The patient presents with therapy-related AML. In general, the management of this type of AML is fraught with uncertainty because, among other reasons, most early studies included small numbers of patients and were retrospective. There have been no prospective randomized studies specifically directed at the treatment of therapy-related leukemias. Furthermore, the published data often included patients with myelodysplastic syndromes. Finally, the data are confounded because of variable definitions. Until recently, the term “secondary leukemia” broadly included any AML with a history of prior malignancy as well as patients with any antecedent hematologic disorder and, in some series, any patient who presented with unfavorable cytogenetics. Among therapy-related AML patients, 70% present with abnormalities of chromosome 5 or 7, which is the most typical presentation after the exposure to alkylating agents and/or ionizing radiation.69 Another important group, recognized only in the 1990s and accounting for approximately 30% of therapy-related AMLs, are those that arise after treatment with topoisomerase-2 inhibitors.70
Historically, it was presumed that every patient with therapy-related leukemia had an adverse prognosis and that standard induction therapy was inappropriate; high-dose cytarabine was suggested in one report.71 However, there is no evidence that any induction therapy is superior to the standard 3 + 7 regimen. Among young adults, quite remarkably, prospective studies report an almost identical CR rate of 55% to 60% for patients treated with recognized unfavorable cytogenetics, and there are no reports that anything is better than this (Table 2). Therefore, this patient should be treated with standard induction therapy assuming, of course, that there are no comorbidities related to her prior therapies that preclude the administration of anthracyclines.
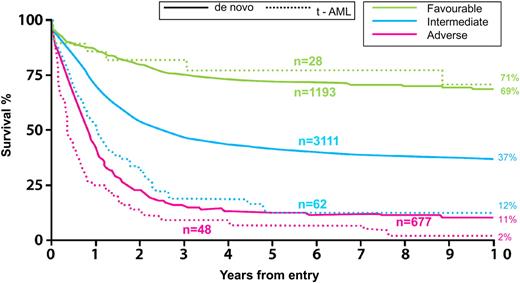
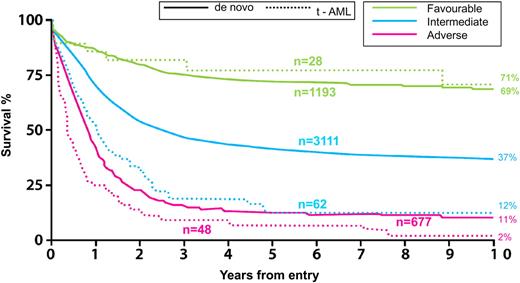
This presence of a complex karyotype classifies this patient in the unfavorable-risk category, irrespective of whether or not she has received prior therapy. It is still somewhat controversial whether therapy-related AML has a prognosis that is intrinsically worse than de novo AML, independent of cytogenetics. In a very large database, the National Cancer Research Institute (formerly MRC) in Great Britain reported a significantly worse outcome for therapy-related AML than de novo AML, within each cytogenetic risk group. This was based on an analysis from the MRC's AML 10, 12, and 15 trials10,72 (Figure 7). The caveat here is that the impact of prior therapy or organ damage is impossible to reliably ascertain. Furthermore, it is not known whether molecular determinants now recognized to be so crucially important in the prognosis of AML, such as FLT3-ITD, NPM1, and CEBPA, are more or less frequent in therapy-related AML and how this may account for differences between de novo and therapy-related AML.
AML in patients less than 60 years of age. Survival, by karyotype, of de novo and therapy-related (t-AML) AML. MRC/NCRI AML Trials: overall survival. Adapted from Grimwade and Hills10 with permission.
AML in patients less than 60 years of age. Survival, by karyotype, of de novo and therapy-related (t-AML) AML. MRC/NCRI AML Trials: overall survival. Adapted from Grimwade and Hills10 with permission.
The management of patients with therapy-related AML should be guided by the cytogenetic and molecular features. Although there is a perception that any patient with therapy-related AML should be considered at high risk and referred to an allogeneic transplantation, there is no evidence that the long-term outcome for patients who present with a favorable karyotype, with no adverse molecular features, is different from patients with de novo AML. Thus, such patients should not be referred to an allogeneic transplantation in CR1.
Proposed treatment: This patient should be treated with standard induction therapy. Postremission therapy should be guided by cytogenetic and molecular determinants. Patients with favorable cytogenetics and no adverse molecular features should not undergo an allogeneic transplantation in CR1.
Patient 6
A 48-year-old man presents with relapsed AML. He receives induction therapy and chemotherapy consolidation with high-dose cytarabine. Fifteen months after achieving CR1, his complete blood count was normal apart from a platelet count of 92 000/μL, but his bone marrow has 18% blasts. The patient has an HLA-matched sibling.
Question: Should this patient undergo an immediate allogeneic transplantation without an attempt at reinduction? If induction is used, what are the best drugs?
Several authors have attempted to define the prognosis of relapsed AML patients.20,73-75 Nevertheless, the only cure for an adult with relapsed AML is a transplantation, and it is clear that this patient will be referred for an allogeneic transplantation from his HLA-compatible sibling.
However, this patient raises 3 important questions. The first is whether this patient should receive a transplantation in untreated relapse rather than undergo reinduction therapy. Although a transplantation can be performed safely in early relapse with an outcome that is probably not significantly inferior to performing this in CR2,76,77 in this particular patient, given the long duration of CR1, there is a greater than 50% chance of achieving CR2.78-81 Because it is always preferable to undergo a transplantation while in CR2, our own choice in this patient would be to attempt reinduction before transplantation. If, on the other hand, the duration of CR1 would be less than 6 months, where the likelihood of achieving CR2 is no greater than 20%,74,78,79,82 the equation will change such that, given the immediate availability of an HLA-compatible sibling, we would elect to proceed to an allogeneic transplantation in an untreated first relapse. The issue becomes more complex for older individuals, more than or equal to 60 to 65 years, in whom RIC is the preferred option for an allogeneic transplantation. There are absolutely no prospective data or established guidelines in such a scenario, and clinical practice varies enormously. Despite some hesitation, given the low likelihood of a cure when transplanting a patient with 18% blasts with RIC, our own preference in this case would be to administer one cycle of induction therapy in an attempt to obtain a better control of the disease before transplantation.
The second issue relates to the choice of regimen to use for reinduction. There is no evidence that any given regimen is superior and much of standard practice is guided by unsubstantiated opinion. Although in theory the use of a non–cross-resistant agent has intuitive appeal, there is no evidence that the efficacy of high-dose cytarabine as a salvage regimen is lessened by the prior use of this agent in consolidation, particularly after a long CR1.83 Furthermore, although commonly used with or without anthracyclines, etoposide, mitoxantrone, fludarabine, amsacrine, or asparaginase, there is no information collected prospectively to indicate that this is more efficacious than high-dose cytarabine alone.83-87 Somewhat lower doses of cytarabine may be equally effective.88 Regimens that do not include cytarabine are equally effective for relapsed patients, and the use of mitoxantrone with etoposide is a well-tolerated regimen with published data that are at least as good as cytarabine used alone or in combination.79,89
Our preference is to use mitoxantrone with etoposide. With this regimen, close to 60% of patients with a long CR1 can expect to achieve CR2,79 although similar results can be achieved with cytarabine-containing regimens.21
The third issue is as follows: once the patient has achieved CR2, should consolidation be administered before transplantation. For a patient in CR2, some investigators would add consolidation before an allogeneic transplantation if the patient is medically fit, even if this is not the practice in CR1. Our own practice would be, also in CR2, to proceed directly to transplantation, with the primary consideration being to reduce transplantation-related toxicity.
Proposed treatment: This patient should be reinduced with mitoxantrone and etoposide. After achievement of CR2, this patient should be referred for an allogeneic transplantation without any additional consolidation. If a sibling were not available, an alternative donor transplantation in CR2 is recommended.
Conclusion
New insights into the pathogenesis of AML have demonstrated that we are treating patients with ever-increasing disease heterogeneity with different clinical manifestations, genetic abnormalities, and outcome with current therapies. New treatment strategies generate genuine excitement about the future. The care of patients with AML has become increasingly complicated but remains remarkably gratifying.
Authorship
Contribution: J.M.R. and M.S.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacob M. Rowe, Department of Hematology and Bone Marrow Transplantation, Rambam Health Care Campus and, Technion, Israel Institute of Technology, Haifa 31096, Israel; e-mail: rowe@jimmy.harvard.edu.