Abstract
Embryonic hematopoiesis starts via the generation of primitive red blood cells (RBCs) that satisfy the embryo's immediate oxygen needs. Although primitive RBCs were thought to retain their nuclei, recent studies have shown that primitive RBCs in mice enucleate in the fetal liver. It has been unknown whether human primitive RBCs enucleate, and what hematopoietic site might support this process. Our data indicate that the terminal maturation and enucleation of human primitive RBCs occurs in first trimester placental villi. Extravascular ζ-globin+ primitive erythroid cells were found in placental villi between 5-7 weeks of development, at which time the frequency of enucleated RBCs was higher in the villous stroma than in circulation. RBC enucleation was further evidenced by the presence of primitive reticulocytes and pyrenocytes (ejected RBC nuclei) in the placenta. Extravascular RBCs were found to associate with placental macrophages, which contained ingested nuclei. Clonogenic macrophage progenitors of fetal origin were present in the chorionic plate of the placenta before the onset of fetoplacental circulation, after which macrophages had migrated to the villi. These findings indicate that placental macrophages may assist the enucleation process of primitive RBCs in placental villi, implying an unexpectedly broad role for the placenta in embryonic hematopoiesis.
Introduction
The hematopoietic system during embryonic development provides two important functions: rapid generation of terminally differentiated blood cells for the survival and growth of the embryo and establishment of a pool of undifferentiated hematopoietic stem cells (HSCs) for postnatal life. To achieve these goals, embryonic hematopoiesis is segregated into multiple waves that occur in several anatomical sites,1 a process that is broadly conserved in vertebrates.2,3 The yolk sac is the site of the first wave of embryonic hematopoiesis that generates both primitive red blood cells (RBCs) that deliver oxygen to the embryo and macrophages that assist in tissue remodeling and immune defense.4 The second wave of hematopoiesis also commences in the yolk sac with the production of a transient pool of erythromyeloid progenitors. Although they lack self-renewal ability and lymphoid potential, they have an important function in fetal hematopoiesis as they rapidly differentiate into mature definitive erythroid and myeloid cells after migration to the fetal liver.5,6 The third wave of hematopoiesis emerges in the major intra- and extraembryonic arteries, generating HSCs that can both self-renew and differentiate into all blood cell types, including lymphoid cells. HSCs subsequently colonize the fetal liver where they expand before eventually seeding the bone marrow. HSCs emerge in the AGM (aorta-gonad-mesonephros region) and attached vitelline and umbilical arteries,7-11 the yolk sac, and the placenta. The capacity of the placenta for generation12 and expansion13-15 of multipotential hematopoietic stem/progenitor cells has been described recently in both mouse and human,16-18 whereas its potential function as a primitive hematopoietic organ has not been evaluated.
The most important products of primitive hematopoiesis that are critical for the survival of the embryo are the primitive RBCs. Experimental evidence suggests that the yolk sac-derived primitive erythroid cells are specified directly from mesoderm with restricted hematopoietic potential, rather than from a multipotential HSC.19-22 Primitive red cells differ from definitive red cells not only in their developmental origin, but also in their larger size and distinct globin expression pattern.23 In mice, primitive RBCs can be identified by expression of ϵy-globin,24 which is absent from definitive red cells derived from the fetal liver and the adult bone marrow that express β-major globin.25 In human, primitive red cells uniquely express the α-like ζ-globin as well as the β-like ϵ-globin.26 Furthermore, primitive red cells differ from definitive red cells in that they enter circulation as nucleated erythroblasts, whereas the definitive erythroid cells complete maturation and enucleation in their site of origin, the fetal liver27 or bone marrow, before entering circulation (reviewed in Chasis28 ). It has been documented that this process occurs in “erythroblast islands” in association with macrophages, which digest the ejected RBC nuclei27 and serve other supportive functions.28 However, there is evidence that macrophages are not essential for RBC enucleation.29 Although the long-standing dogma asserts that primitive red cells, or erythroblasts, remain nucleated and never mature into enucleated erythrocytes, it has been recently documented that primitive erythroblasts in mouse embryos do enucleate. In the process, a transient population of free nuclei termed pyrenocytes is generated.30 The enucleation of primitive RBCs lay undiscovered for a century because it starts in mouse embryos at the same developmental time when definitive erythroid cells begin to enter circulation.31 Although the site of enucleation of primitive red cells was unknown, association of primitive red cells and macrophages in the fetal liver, and the ability of the liver macrophages to ingest the nuclei of primitive red cells in vitro, suggested that fetal liver macrophages can support maturation of both primitive and definitive erythroblasts during mouse development.30,32 In humans, the limited access to the early embryonic hematopoietic tissues has hindered our understanding of human developmental hematopoiesis. It is unknown if human primitive erythroblasts enucleate or which anatomical sites might support the enucleation process.
Here we show that human primitive RBCs do enucleate, segregating the nucleated erythroblast into a reticulocyte and a pyrenocyte. Our data reveal that primitive RBC enucleation occurs in the placental villous stroma in association with macrophages. The presence of macrophage progenitors in the chorionic plate of precirculation placentas suggests that the human placenta may autonomously generate the macrophages that migrate to the villi to assist the terminal maturation of primitive RBCs. These data indicate that the placenta has a broader role as a hematopoietic organ than previously appreciated, functioning as an integral component of both primitive and definitive hematopoiesis.
Methods
Tissue collection and procurement
Extra-embryonic and embryonic tissues were discarded material obtained from elective terminations of first and second trimester pregnancies performed by Family Planning Associates or University of California Los Angeles (UCLA) Medical Center. The gestational age of the specimen was determined by ultrasound. If this information was not available, the date from the last menstrual period and histologic features of the specimen were used to determine the duration of pregnancy. The age of the specimen is indicated in this work as developmental age, which is 2 weeks less than the gestational/clinical age. Tissues were harvested directly into sterile containers with phosphate-buffered saline (PBS) and transported on ice in PBS containing 5% fetal bovine serum (FBS; Hyclone), 0.1% ciprofloxacin HCl (10 μg/mL; Sigma-Aldrich), 1% amphotericin B (250 μg/mL; Invitrogen), and 1% penicillin (10 000 U/mL)–streptomycin (10 000 μg/mL; Invitrogen) and processed the same day.
Preparation of tissue sections
Tissues were fixed in 3.7% phosphate-buffered formalin (Protocol) for 12-16 hours, then rinsed with tap water and transferred to 70% ethanol for storage. Fixed specimens were embedded in paraffin and cut into 5-μm sections by the UCLA Translational Pathology Core Laboratory (TPCL). Hematoxylin/eosin (H&E) stainings were performed by TPCL.
Histologic analysis of erythroblast maturation
To assess RBC maturation, 5 random microscope fields were selected from each slide and photographed (Zeiss Axiovert 40 CFL with attached Canon Powershot G6 camera) at ×400 total magnification. RBCs were identified by an eosinophilic cytoplasm and designated as nucleated or enucleated (with or without a dark purple nucleus) and circulating or extravascular (inside or outside of blood vessels). Cells were annotated on printed photographs while viewed in the scope. All cells in each category were then summed across the fields for each slide. The enucleation ratios (ERs) were defined for each specimen by dividing the number of enucleated RBCs by the total number of RBCs counted. To compare the enucleation status of extravascular versus circulating RBCs, ERs were determined for both compartments for each specimen, and relative enrichment of enucleated cells in extravascular versus intravascular spaces was determined by dividing the ERs in the 2 compartments.
Preparation of single–cell suspensions from hematopoietic tissues
Single-cell suspensions were prepared from fresh specimens for use in culture assays or flow cytometry. Tissues were first mechanically dissociated by mincing with scalpels, then subjected to enzymatic dissociation in 10 mL of enzyme solution per gram tissue (2.5 U dispase [Invitrogen], 90 mg collagenase [Sigma-Aldrich], 5% FBS [Hyclone], 1% amphotericin B [250 μg/mL; Invitrogen], 1% penicillin [10 000 U/mL]–streptomycin [10 000 μg/mL; Invitrogen] and 0.1% ciprofloxacin HCl [10 μg/mL; Sigma-Aldrich]) for 30 minutes with agitation at 37°C. After 30 minutes, DNase I (0.075 mg/g tissue; Sigma-Aldrich) was added, and samples were incubated for an additional 30 minutes. Dissociated cells were then filtered through 70-μm mesh, washed with PBS, and counted.
Flow cytometry and cell sorting
Fluorescence-activated cell sorting (FACS) analysis was performed using single-cell suspensions prepared as described above. Intracellular FACS of RBCs was performed essentially as described,33 with the following modifications. Cells were stained with antibodies against CD235 (1:100, fluorescein isothiocyanate or phycoerythrin [PE]; BD Biosciences) and CD71 (1:25, Alexa Fluor 647; Santa Cruz Biotechnology) before fixation by incubation with antibodies at 4°C for 20 minutes. After fixation and permeabilization, cells were stained with an anti–ζ-globin antibody (1:1000; a kind gift from T. Papayannapoulou, University of Washington Medical School) followed by an anti-mouse Alexa Fluor 488–conjugated secondary antibody (1:250; Invitrogen) or a fluorescein isothiocyanate–conjugated anti–ϵ-globin antibody (1:2000; Fitzgerald Industries). For ζ-globin analysis, cells were briefly postfixed with 1% formaldehyde. To detect erythroid cells at different stages of maturation, cells were stained with CD235 as above, and DRAQ5 (Biostatus Limited) was used at 5μM as directed. To detect hematopoietic progenitors, cells were stained with antibodies against CD43 PE (used at 1:25, mouse anti–human; Santa Cruz Biotechnology), and CD34 APC (1:50, mouse anti–human; BD Biosciences) diluted in 5% FBS (Hyclone). 7-Aminoactinomycin D (BD Biosciences) was added at a final dilution of 1:50 in all analyses of nonfixed cells. Stained cells were analyzed on a LSR II cytometer (BD Biosciences) and processed with FlowJo software Version 7.6.1 (TreeStar). Sorting for hematopoietic progenitors (CD34+CD43+) and RBC populations defined by DRAQ5 and CD235 was performed as above using a BD FACSAria II in the UCLA Broad Stem Cell Center Core facility.
In vitro erythroblast island formation assay
Island assays were performed essentially as described,30 with the following modifications. Macrophages were labeled with CD68 PE and isolated from placental cell suspensions (6 weeks) using anti-PE magnetic beads (Miltenyi Biotec) according to manufacturer's instructions. Isolated macrophages were plated at a density of 2.5 × 104 cells per well in fibronectin-coated 8-well chamber slides (BD). Primitive RBCs (CD235+CD71+) were sorted from pooled 4- to 6-week placental suspensions, labeled with CellTracker Green (Molecular Probes), and combined with macrophages in chamber slides at a density of 1 × 105 cells per well in “association media” (30% plasma-derived serum, Iscove modified Dulbecco medium, 2mM glutamine, 0.15mM monothioglycerol, 1 ng/mL macrophage colony-stimulating factor, 2 U/mL erythropoietin, 300 mg/mL transferrin, and 40 ng/mL insulin-like growth factor 1) and cultured for 48 hours. Wells were washed 3× with PBS, fixed in ice-cold methanol for 5 minutes, and then stained for FXIII and DAPI (4′,6-diamidino-2-phenylindole).
Immunohistochemistry
For immunohistochemistry (IHC), unstained sections were deparaffinized and rehydrated through a xylene/alcohol gradient. Antigen retrieval was performed in an antigen-dependent manner using a pressure cooker and a microwave. For CD235 (Santa Cruz Biotechnology), FXIII (Vector Laboratories), and CD68 (Santa Cruz Biotechnology) staining, slides were immersed in a 10mM Tris, 1mM EDTA (ethylenediaminetetraacetic acid), 0.05% Tween 20 solution, at pH 9.0, and microwaved on high for 8 minutes. For slides stained with ζ-globin and ϵ-globin (Fitzgerald Industries), microwave retrieval was performed in a 100mM Tris solution at pH 6.0. CD34 and cytokeratin (Vector Laboratories) staining was of equal quality with either retrieval technique. After cooling, endogenous peroxidases were quenched in a 0.9% solution of H2O2 in methanol for 20 minutes at room temperature. Slides were blocked in 5% normal horse serum in PBS, pH 7.4, containing 0.05% Tween 20 for 10 minutes. Primary antibodies were diluted in blocking buffer and incubated for 1 hour at room temperature (FXIII 1:50, CD235 1:75, cytokeratin 1:1200, and CD34 1:50) or overnight at 4°C (1:1500; ζ- and ϵ-globin). Biotinylated anti-rabbit (1:500; cytokeratin) or anti-mouse (1:500; all others) secondary antibodies were diluted in blocking buffer and incubated for 30 minutes at room temperature, then detected following manufacturer's instructions (Vector Laboratories). Nuclear counterstain (Fast Red; Vector Laboratories) was used as directed. Slides were dehydrated, mounted (Vectamount; Vector Laboratories), and imaged with an Olympus BX51 microscope and a DP72 camera.
Image acquisition information
For all brightfield micrographs, an Olympus BX51 microscope with an attached DP72 camera and DP72-BSW software (Version 2.2) were used to acquire images. For 100× images, a 10×/0.40 UPlanSApo objective was used, for 200× images, a 20×/0.75 UPlanSApo objective was used, and for 400× images a 40×/1.30 UPlanFLN oil objective was used. All slides were mounted with Vectamount (Vector Laboratories). ImageJ (Version 1.40g) was used to process images.
Immunofluorescence and confocal microscopy
For immunofluorescence, unstained sections were deparaffinized and rehydrated through a xylene/alcohol gradient. Antigen retrieval was performed at 95°C for 30 minutes each sequentially in 10mM Tris, 1mM EDTA, 0.05% Tween 20 solution, pH 9.0, followed by 10mM citrate solution in PBS, pH 6.0. After cooling, endogenous peroxidases were quenched in a 0.9% solution of H2O2 in methanol for 20 minutes at room temperature. Sections were permeabilized with 1% Triton X-100 in PBS for 1 hour, then blocked in tyramide blocking solution (Invitrogen) plus 1% Triton X-100 (TBST) for 1 hour. Slides were incubated with primary antibodies overnight at 4°C (1:1000, CD43 MT1, Santa Cruz Biotechnology; 1:1000, CD34 QBEnd/10, Vector Laboratories; 1:1000, CD235 11E4B7.6, Santa Cruz Biotechnology; 1:750, CD31 0.N.100, Santa Cruz Biotechnology; 1:1500, Flk1 A-3, Santa Cruz Biotechnology; 1:500, FXIII E980.1, Vector Laboratories; 1:2500, CD68 KP1, Santa Cruz Biotechnology; 1:1000, phosphatidylserine 1H6, Millipore). Slides were washed with 0.1% Tween in PBS (PBST) followed by incubation with biotinylated secondary antibodies (1:500; Vector Laboratories) in TBST for 30 minutes at room temperature. Slides were washed 3× with PBST followed by incubation with streptavidin–horseradish peroxidase (1:500 in TBST; Invitrogen) for 30 minutes at room temperature. Slides were washed 3× in PBST, then tyramide biotin-XX amplification was performed for 7.5 minutes as per manufacturer's instructions (Invitrogen). Slides were washed in PBST followed by incubation with ABC Elite (Vector Laboratories) for 30 minutes at room temperature. Slides were washed in PBST and incubated with tyramide-fluorophore for 7.5 minutes prepared as directed (Invitrogen). Slides were washed in PBS and incubated with 0.05M HCl for 20 minutes at room temperature to quench peroxidases. Slides were washed 3× in PBST, then blocked, and incubated with primary antibodies as above. After the final tyramide-fluorophore step, slides were washed in PBS then incubated with DAPI solution (5 μg/mL in PBS) for 5 minutes at room temperature. Slides were washed 3× with PBS, then mounted in ProLong Gold mounting medium (Molecular Probes), and stored at 4°C. TUNEL stains were performed by first deparaffinizing slides and performing antigen retrieval as above, then using the In Situ Cell Death Detection Kit AP (Roche) as directed. Subsequent stains were then performed using the same immunofluorescent protocol. Confocal images were obtained on a Zeiss LSM 510 equipped with 405, 488, 543, and 633 nm lasers. Images were processed with ImageJ software Version 1.40g (National Institutes of Health).
Electron microscopy
Individual or small groups of villi were dissected away from fresh placental samples and immersed in electron microscopy (EM) fixative (2% glutaraldehyde and 1% paraformaldehyde in PBS) at 4°C overnight. Second-trimester fetal liver tissues were used as controls. Ultrathin sections were cut and viewed in a JEOL 100CX transmission electron microscope (JEOL USA). Images were captured on a Gatan UltraScan 2k*2k camera (Gatan), developed, and scanned at 1200 dots per inch.
Clonogenic progenitor assays
Single-cell suspensions were prepared as described above. Cell populations sorted based on CD34 and CD43 expression were plated in MethoCult GF+ H4435 methylcellulose (StemCell Technologies) containing stem cell factor, granulocyte macrophage colony-stimulating factor, interleukin-3, and erythropoietin supplemented with 1:2000 thrombopoietin (10 μg/mL; Peprotech), 1% penicillin-streptomycin (Invitrogen), 1% amphotericin B (Invitrogen), and 0.1% ciprofloxacin HCl (Sigma-Adrich). Cultures were incubated at 37°C and 5% CO2 for 14 days, and colonies were scored based on classical morphologic characteristics. Cytospins of individual colonies were performed using a Shandon Cytospin 4 (Thermo Electron Corporation) followed by May-Grunwald Giemsa staining (Sigma-Aldrich).
PCR for fetal-maternal identity
To verify the fetal origin of hematopoietic colonies derived from placental samples, colonies were picked from methylcellulose assays, lysed, and tested first for the 2 sex chromosomes: the SRY gene on the Y chromosome and the HTR2C gene on the X chromosome by polymerase chain reaction (PCR) amplification. The primers used were SRY-F (5′-TAATACGACTCACTATAGGGAGAATAAGTATCGACCTCGTCGGAA-3′), and SRY-R (5′-AATTAACCCTCACTAAAGGGAGACACTTCGCTGCAGAGTACCGA-3′), and HTR2C-F (5′-TAATACGACTCACTATAGGGAGAGTGGTTTCAGATCGCAGTAA-3′), HTR2C-R (5′-AATTAACCCTCACTAAAGGGAGAATATCCATCACGTAGATGAGAA-3′). Both SRY and HTR2C primers were combined in one PCR, which was performed as follows: the initial denaturation was done at 95°C for 15 minutes, followed by 15 cycles of amplification, which included a 30-second denaturation step at 95°C, a 64°C annealing step for 30 seconds, and a 1-minute extension step at 68°C. For the first 15 cycles, the temperature was decreased by 0.5°C per cycle. This was followed by 24 cycles done as follows: a 30-second denaturation step at 95°C, a 56°C annealing step for 30 seconds, and an extension for 1 minute at 68°C. The final extension was done at 72°C for 10 minutes. In the absence of a signal from the Y chromosome, microsatellite PCR was used to verify that placental colonies differed in their genotype from the maternal decidua. For the MTC118 microsatellite PCR, the primers used were MCT118A (5′-GTCTTGTTGGAGATGCACGTGCCCCCTTGC-3′) and MCT118B (5′-GAAACTGGCCTCCAAACACTGCCCGCCG-3′). The PCR conditions were as follows: the initial denaturation was done at 95°C for 15 minutes and was followed by 31 cycles, which consisted of a 1-minute denaturation step at 94°C, a 62°C annealing step for 1 minute, and a 1-minute extension step at 72°C. As above, the final extension was done at 72°C for 10 minutes. All PCRs were completed in a total volume of 25 μL, and HotStar Taq DNA polymerase was used. Gels were imaged and digitized using a BioDoc-It Imaging System (UVP).
Results
Enucleated primitive RBCs populate the placental villous stroma during the first trimester
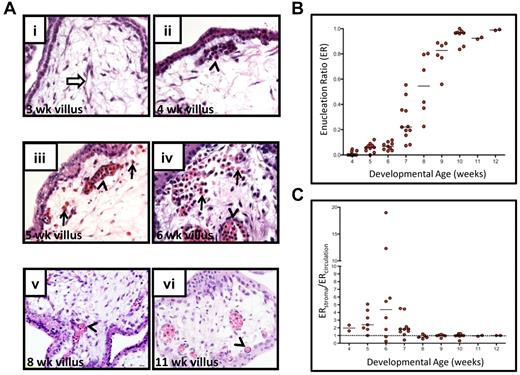
To determine whether human primitive RBCs enucleate, we assessed the enucleation status of RBCs in the placenta, which is an accessible fetal tissue that can be used to investigate circulating blood cells from the early first trimester onward. The status of fetoplacental circulation was determined by flow cytometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) by identifying precirculation specimens based on the absence of CD235+CD71+ fetal erythroblasts. H&E stained sections from placentas were used for quantitative analysis of RBC enucleation and localization status at different ages (Figure 1). As expected, erythroblasts were detectable in the placental vasculature by 4 weeks of developmental age (corresponding to 6 weeks clinical age). By 5 weeks of developmental age, a fraction of the RBCs had lost their nucleus. Interestingly, at this stage some of the erythroid cells were found in the stroma of the placental villi rather than contained within placental vessels (Figure 1Aiii). The frequency of extravascular RBCs in the villous stroma was highest between 6 and 7 weeks, coincident with an increase in the overall frequency of enucleated RBCs in the placenta. When both the enucleation status and localization (circulating or extravascular) of RBCs in the placenta was scored, an enrichment of enucleated RBCs in the placental extravascular stroma in comparison to blood vessels was observed (ie, ER was higher) at 5-7 weeks of development, with the peak at 6 weeks (Figure 1C). Toward the end of the first trimester, most RBCs were contained in the vessels, and very few RBCs with a nucleus were present. The enrichment of enucleated RBCs in the placental stroma at the time when enucleation of RBCs starts raised the hypothesis that circulating erythroblasts may extravasate into the placental stroma for the purpose of terminal maturation and enucleation.
The first trimester placental stroma becomes populated by extravascular RBCs that enucleate. (A) Localization and enucleation status of erythroid cells in the placenta was determined by H&E staining. At 3 weeks of developmental age (i), placental blood vessels had started to form and were devoid of RBCs (white arrow). By 4 weeks (ii), nucleated RBCs had appeared in the placental vasculature (arrowhead). Between 5-7 weeks of developmental age, RBCs were also found in the villous stroma (arrows) with increasing frequency (iii-iv), and many were enucleated, whereas most circulating RBCs still retained their nuclei (arrowheads). Toward the end of first trimester, RBCs were found mainly in circulation (arrowheads in v-vi). All images shown were acquired at 400× original magnification. (B) Calculation of ERs (number of enucleated RBCs divided by the total number of RBCs counted, see “Histologic analysis of erythroblast maturation”) for the placental RBC pool for each week of development revealed a marked change in their maturation state between 4 and 8 weeks. Bars represent median for each age. (C) Analysis of enucleation ratios of RBCs in circulation (inside blood vessels) or extravascular (in placental stroma) revealed an enrichment of enucleated RBCs in the extravascular stroma during 5-7 weeks of development. Dotted line at 1 indicates equal representation of enucleated cells in both compartments, and bars represent the median at each age.
The first trimester placental stroma becomes populated by extravascular RBCs that enucleate. (A) Localization and enucleation status of erythroid cells in the placenta was determined by H&E staining. At 3 weeks of developmental age (i), placental blood vessels had started to form and were devoid of RBCs (white arrow). By 4 weeks (ii), nucleated RBCs had appeared in the placental vasculature (arrowhead). Between 5-7 weeks of developmental age, RBCs were also found in the villous stroma (arrows) with increasing frequency (iii-iv), and many were enucleated, whereas most circulating RBCs still retained their nuclei (arrowheads). Toward the end of first trimester, RBCs were found mainly in circulation (arrowheads in v-vi). All images shown were acquired at 400× original magnification. (B) Calculation of ERs (number of enucleated RBCs divided by the total number of RBCs counted, see “Histologic analysis of erythroblast maturation”) for the placental RBC pool for each week of development revealed a marked change in their maturation state between 4 and 8 weeks. Bars represent median for each age. (C) Analysis of enucleation ratios of RBCs in circulation (inside blood vessels) or extravascular (in placental stroma) revealed an enrichment of enucleated RBCs in the extravascular stroma during 5-7 weeks of development. Dotted line at 1 indicates equal representation of enucleated cells in both compartments, and bars represent the median at each age.
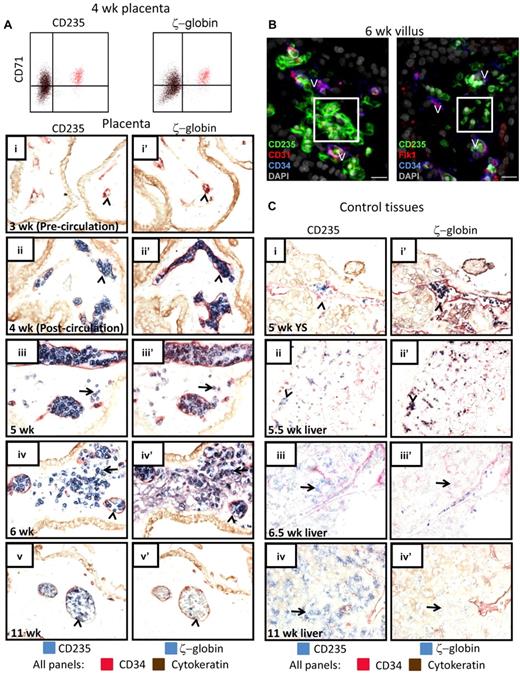
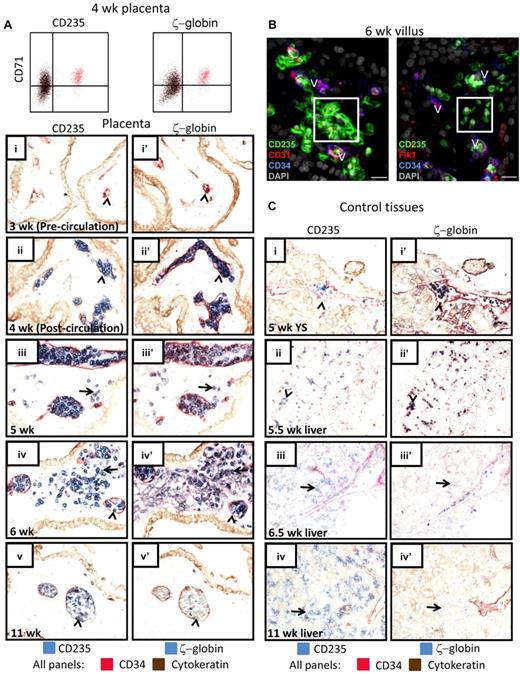
Based on the developmental timing when extravascular RBCs are found in the placenta and the fact that these RBCs initially possess a nucleus, we hypothesized that they represent the primitive erythroid lineage. To test this, an antibody against ζ-globin, which is characteristic for primitive RBCs, was used along with CD235 (Figure 2A,C) to mark primitive erythroid cells. Flow cytometry for ζ-globin in combination with CD71 and CD235 confirmed that the earliest RBCs in the placenta express ζ-globin and are of the primitive lineage (Figure 2A). To investigate the localization of primitive RBCs in hematopoietic tissues, adjacent sections were stained for ζ-globin and CD235 to identify erythroid cells, while CD34 staining was used to delineate endothelial cells that separate vascular lumens from stromal regions (Figure 2A,C). From the time when RBCs first appear in the placental vessels through 6 weeks of age, when many of these cells were found in the extravascular stroma and had started to enucleate, essentially all RBCs in the placenta expressed ζ-globin (Figure 2A) and ϵ-globin (supplemental Figure 2A). Furthermore, flow cytometry for ϵ-globin confirmed that ϵ-globin+ primitive RBCs enucleate (supplemental Figure 2B). Staining with 2 other endothelial markers, CD31 and Flk1, confirmed the lack of blood vessels surrounding primitive RBCs in the villous stroma (Figure 2B). In contrast, all CD235+ cells in the yolk sac and the fetal liver at 5-5.5 weeks were contained within the vasculature (Figure 2C), whereas the first CD235+ RBCs found in the liver parenchyma at 6.5 weeks were ζ-globinneg definitive erythroid cells (Figure 2Ciii,iiii′). These data suggest that at the time when primitive erythroid cells start to enucleate, they are highly enriched in the placental villous stroma compared with the liver parenchyma. By 11 weeks, most RBCs in the placenta were enucleated, contained in vessels, and no longer expressed ζ-globin (Figure 2Av,v′), whereas most of the RBCs in the fetal liver were devoid of ζ-globin expression and resided in the liver parenchyma (Figure 2Biv,iv′). Importantly, TUNEL staining implied that there was no significant cell death among the extravascular RBCs in the placenta (supplemental Figure 3A). Moreover, staining for phosphatidylserine (PTDS), a lipid exposed both on the surface of apoptotic cells and on the nuclear membrane surrounding ejected RBC nuclei,34 verified that there was little apoptosis among the extravascular primitive RBCs and that some of the RBCs had begun to expose PTDS on the nuclear membrane (supplemental Figure 3B). Altogether, these data suggested that human primitive erythroid cells may enucleate in the placental villous stroma.
RBCs in the placental villous stroma are of the primitive erythroid lineage. (A) Intracellular flow cytometry confirmed that the CD235+CD71+ erythroid cells in the early first trimester placenta were ζ-globin expressing primitive RBCs (colored cells are the same in both FACS plots). IHC of adjacent tissue sections demonstrated that the RBCs observed in the placental villous stroma were of the primitive lineage. In precirculation placentas (i-i′), no ζ-globin+CD235+ cells were found in placental blood vessels (arrowheads). The first cells in the vasculature (arrowheads) were CD235+ (II) and ζ-globin+ (ii′). At 5 weeks of age, all RBCs in the placenta expressed ζ-globin (iii vs iii′) and some were localized to the placental stroma (arrows). By 6 weeks many more primitive RBCs were observed in the villous stroma (arrows in iv-iv′). At 11 weeks, the placental RBC pool comprised mostly ζ-globin− RBCs that were contained in blood vessels (arrowheads in v-v′). Images were acquired at 400× original magnification. (B) Immunofluorescence using 3 different endothelial markers, CD31, Flk1, and CD34, confirmed the extravascular localization of placental RBCs (white boxes). V indicates blood vessels; scale bars, 20 μm. (C) Staining of yolk sac and fetal liver at the developmental stages when RBC extravasation was prominent in the placenta did not reveal major populations of ζ-globin+ extravascular primitive RBCs (i-iii). By 11 weeks, the liver had generated ζ-globin− definitive RBCs (iv-iv′). Arrowheads mark circulating RBCs; arrows mark RBCs in the fetal liver parenchyma. Images were acquired at 200× original magnification.
RBCs in the placental villous stroma are of the primitive erythroid lineage. (A) Intracellular flow cytometry confirmed that the CD235+CD71+ erythroid cells in the early first trimester placenta were ζ-globin expressing primitive RBCs (colored cells are the same in both FACS plots). IHC of adjacent tissue sections demonstrated that the RBCs observed in the placental villous stroma were of the primitive lineage. In precirculation placentas (i-i′), no ζ-globin+CD235+ cells were found in placental blood vessels (arrowheads). The first cells in the vasculature (arrowheads) were CD235+ (II) and ζ-globin+ (ii′). At 5 weeks of age, all RBCs in the placenta expressed ζ-globin (iii vs iii′) and some were localized to the placental stroma (arrows). By 6 weeks many more primitive RBCs were observed in the villous stroma (arrows in iv-iv′). At 11 weeks, the placental RBC pool comprised mostly ζ-globin− RBCs that were contained in blood vessels (arrowheads in v-v′). Images were acquired at 400× original magnification. (B) Immunofluorescence using 3 different endothelial markers, CD31, Flk1, and CD34, confirmed the extravascular localization of placental RBCs (white boxes). V indicates blood vessels; scale bars, 20 μm. (C) Staining of yolk sac and fetal liver at the developmental stages when RBC extravasation was prominent in the placenta did not reveal major populations of ζ-globin+ extravascular primitive RBCs (i-iii). By 11 weeks, the liver had generated ζ-globin− definitive RBCs (iv-iv′). Arrowheads mark circulating RBCs; arrows mark RBCs in the fetal liver parenchyma. Images were acquired at 200× original magnification.
Enucleation of human primitive RBCs in the placental villous stroma generates a transient population of pyrenocytes
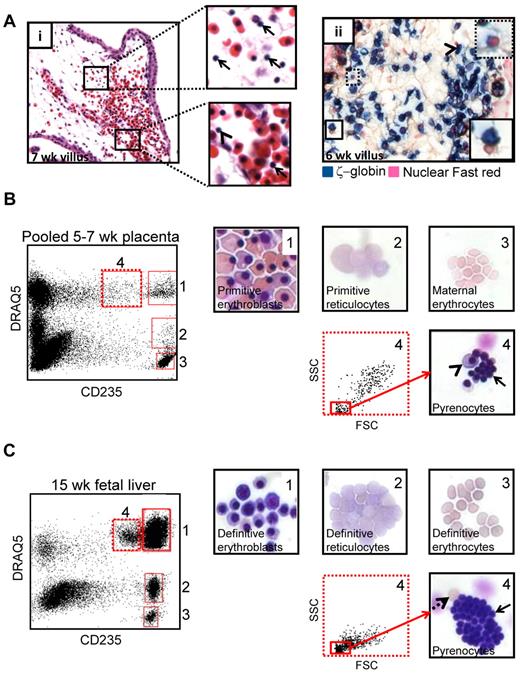
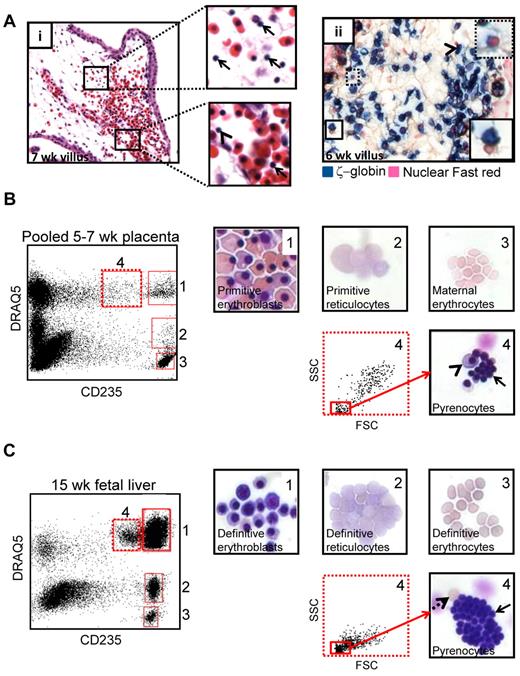
During enucleation, RBCs segregate their contents into 2 compartments: a reticulocyte and a pyrenocyte. Analysis of H&E stained placental sections revealed that the placental villous stroma contained a large number of what appeared to be free red cell nuclei (Figure 3Ai top inset) at the time when primitive RBC enucleation was occurring. Occasional free nuclei were also found both in placental (Figure 3Ai bottom inset) and systemic (supplemental Figure 4) circulation. IHC for ζ-globin followed by a nuclear counterstain suggested that the free red cell nuclei in the placenta were primitive pyrenocytes due to their close association with ζ-globin+ RBCs (Figure 3Aii). To confirm the presence of pyrenocytes in the placenta, FACS was combined with May-Grunwald Giemsa staining. Analysis of dissociated placental specimens from 5-7 weeks of developmental age, the time of maximal primitive RBC enucleation, based on CD235 and the nucleic acid dye DRAQ5 allowed the identification and sorting of distinct stages of primitive RBC maturation (Figure 3B): CD235+DRAQ5+ erythroblasts (gate 1), CD235+DRAQ5lo reticulocytes (gate 2), and CD235medDRAQ5+ pyrenocytes (gate 4). At this developmental stage no definitive fetal RBCs have circulated to the placenta, and CD235+DRAQ5neg maternal erythrocytes (gate 3) are the only enucleated RBCs that lack both DNA and RNA. As a control, the same sorting strategy was applied to second trimester fetal liver, which is an active hematopoietic site with ongoing definitive erythroid maturation (Figure 3C). A similar maturation profile, with the addition of fully mature fetal erythrocytes, was observed in the fetal liver.
Primitive RBCs eject their nuclei in the placental villous stroma. (A) H&E staining of 7-week placenta (i) suggested the presence of free RBC nuclei (pyrenocytes) in the extravascular stroma of placental villi (top inset arrow) as well as less frequent pyrenocytes and primitive RBCs with pyknotic nuclei in circulation (bottom inset, arrow and arrowhead, respectively). Staining for ζ-globin and the nuclear counterstain Fast red (ii) demonstrated that pyrenocytes in the villous stroma were associated with ζ-globin+ primitive RBCs (solid boxed inset arrowhead) or contained a small amount of ζ-globin (dotted boxed inset). Images were acquired at 400× original magnification. (B) The presence of primitive pyrenocytes in the placenta was further verified by cell sorting using the nucleic acid dye DRAQ5 in conjunction with CD235. Subgating for small cells in the CD235medDRAQ5+ (dotted gate 4) fraction permitted purification of primitive pyrenocytes from the placenta. Arrow marks pyrenocytes; arrowhead points to an erythroblast that cosorted with pyrenocytes. Images were acquired at 400× original magnification. (C) The same sorting strategy allowed the isolation of definitive pyrenocytes from the fetal liver. Solid arrow marks pyrenocytes, and dashed arrow indicates an enucleated erythrocyte that cosorted with the pyrenocytes. Images were acquired at 400× original magnification.
Primitive RBCs eject their nuclei in the placental villous stroma. (A) H&E staining of 7-week placenta (i) suggested the presence of free RBC nuclei (pyrenocytes) in the extravascular stroma of placental villi (top inset arrow) as well as less frequent pyrenocytes and primitive RBCs with pyknotic nuclei in circulation (bottom inset, arrow and arrowhead, respectively). Staining for ζ-globin and the nuclear counterstain Fast red (ii) demonstrated that pyrenocytes in the villous stroma were associated with ζ-globin+ primitive RBCs (solid boxed inset arrowhead) or contained a small amount of ζ-globin (dotted boxed inset). Images were acquired at 400× original magnification. (B) The presence of primitive pyrenocytes in the placenta was further verified by cell sorting using the nucleic acid dye DRAQ5 in conjunction with CD235. Subgating for small cells in the CD235medDRAQ5+ (dotted gate 4) fraction permitted purification of primitive pyrenocytes from the placenta. Arrow marks pyrenocytes; arrowhead points to an erythroblast that cosorted with pyrenocytes. Images were acquired at 400× original magnification. (C) The same sorting strategy allowed the isolation of definitive pyrenocytes from the fetal liver. Solid arrow marks pyrenocytes, and dashed arrow indicates an enucleated erythrocyte that cosorted with the pyrenocytes. Images were acquired at 400× original magnification.
Placental macrophages associate with extravascular primitive RBCs
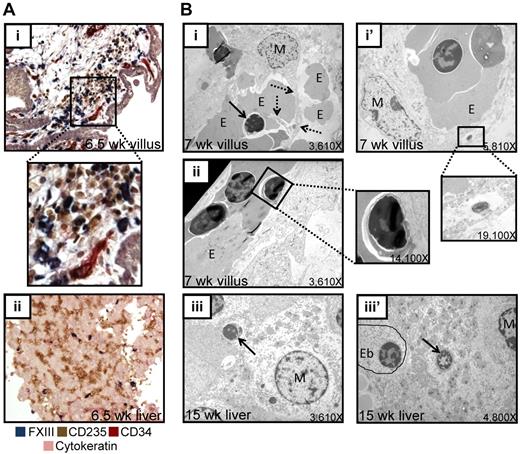
As macrophages are known to associate with erythroblasts and digest enucleated RBC nuclei during definitive erythropoiesis in the fetal liver27 and bone marrow, we investigated whether the enucleation of primitive erythroblasts in the human placenta also occurs in physical association with macrophages. IHC revealed a close association between placental macrophages (identified by factor XIII [FXIII]) and RBCs (CD235; Figure 4Ai) in the extra vascular stroma of the placental villi in vivo. Furthermore, sorted placental macrophages were able to bind primitive erythroblasts in vitro (supplemental Figure 5). Macrophage–red cell clusters in the placental stroma were prominent at 5-7 weeks of developmental age. At 6.5 weeks, few macrophages were present in the fetal liver (Figure 4Aii). At 5.5 weeks, when enucleated primitive RBCs had already appeared in the placental stroma, there were rare, if any, FXIII+ or CD68+ macrophages in the fetal liver, whereas the placenta was readily populated by these cells (supplemental Figure 6). EM of placental villi demonstrated cytoplasmic projections from macrophages contacting extravascular erythroblasts (Figure 4Bi), forming structures reminiscent of erythroblast islands in the fetal liver (Figure 4Biii). Furthermore, both placental and fetal liver macrophages were observed to both engulf and digest nuclei (Figure 4Bi′,iii′). Free red cell nuclei were also detected by EM both in the placenta villous stroma (Figure 4Bi) and placental circulation (Figure 4Bii) as well as the fetal liver (Figure 4Biii). These data suggest that placental macrophages may function in primitive erythropoiesis to digest ejected primitive RBC nuclei.
Primitive RBCs enucleate in the placental stroma in close association with placental macrophages. (A) Immunohistochemical staining of 6.5-week placental sections for macrophages (FXIII, blue), RBCs (CD235, brown), endothelial cells (CD34, red), and trophoblast (cytokeratin, purple) demonstrated the congregation of macrophages and RBCs in the extravascular stroma in placental villi (i; a higher magnification inset of the boxed area is also shown), whereas at the same developmental age very few macrophages were found in the fetal liver (ii). Images were acquired at 200× original magnification. (B) EM performed on 7-week placental villi demonstrated both direct physical interactions between placental macrophages and erythroblasts (i). Dashed arrows indicate macrophage cytoplasmic projection, solid arrow denotes ejected nucleus; M, macrophage; E, erythroid cell. as well as macrophages containing ingested nuclei (i′ inset) and pyrenocytes in circulation (ii inset). These macrophage-RBC associations were reminiscent of RBC maturation during definitive erythropoiesis in the fetal liver, where both free pyrenocytes (iii arrow) and engulfed nuclei (iii′ arrow; Eb, erythroblast) were present. All original magnifications for EM micrographs are as indicated.
Primitive RBCs enucleate in the placental stroma in close association with placental macrophages. (A) Immunohistochemical staining of 6.5-week placental sections for macrophages (FXIII, blue), RBCs (CD235, brown), endothelial cells (CD34, red), and trophoblast (cytokeratin, purple) demonstrated the congregation of macrophages and RBCs in the extravascular stroma in placental villi (i; a higher magnification inset of the boxed area is also shown), whereas at the same developmental age very few macrophages were found in the fetal liver (ii). Images were acquired at 200× original magnification. (B) EM performed on 7-week placental villi demonstrated both direct physical interactions between placental macrophages and erythroblasts (i). Dashed arrows indicate macrophage cytoplasmic projection, solid arrow denotes ejected nucleus; M, macrophage; E, erythroid cell. as well as macrophages containing ingested nuclei (i′ inset) and pyrenocytes in circulation (ii inset). These macrophage-RBC associations were reminiscent of RBC maturation during definitive erythropoiesis in the fetal liver, where both free pyrenocytes (iii arrow) and engulfed nuclei (iii′ arrow; Eb, erythroblast) were present. All original magnifications for EM micrographs are as indicated.
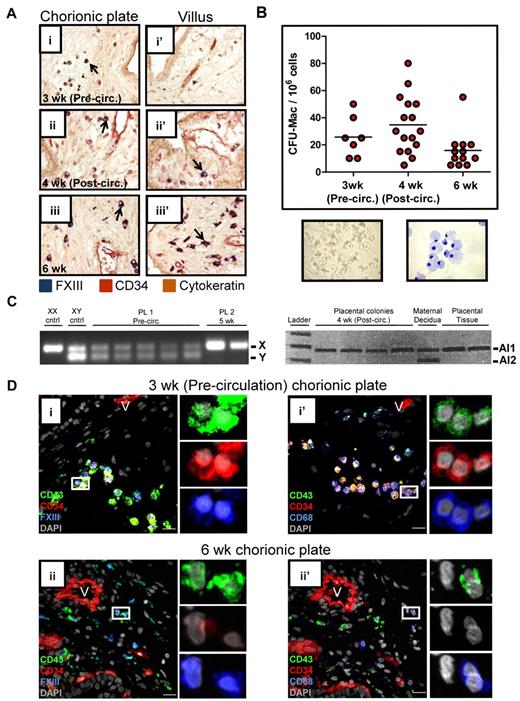
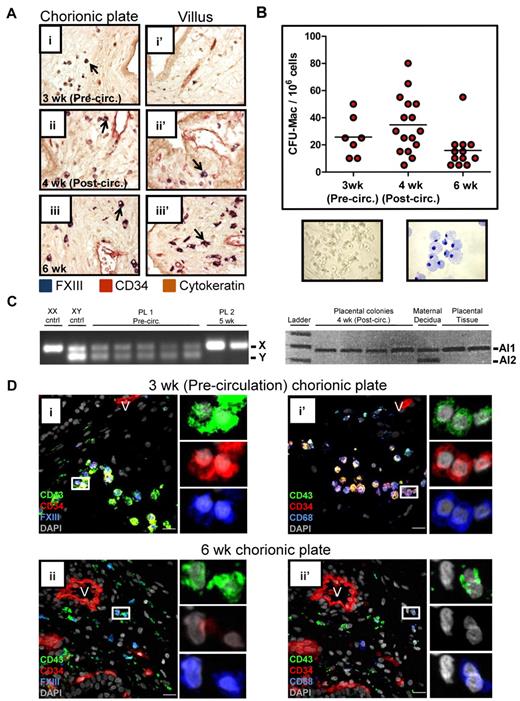
Fetal macrophage progenitors are found in the precirculation placenta
Given the association of placental macrophages with primitive RBC enucleation, we next asked at what developmental stage macrophages entered the placenta. IHC for FXIII demonstrated that rare macrophages were present already in precirculation specimens in the chorionic plate (Figure 5Ai). With increasing developmental age, macrophages became more numerous and populated both the chorionic plate (Figure 5Aii-iii) and the villi (Figure 5Aii′-iii′). To determine whether macrophage progenitors were present in the placenta, colony-forming assays were performed (Figure 5B). This analysis revealed that clonogenic macrophage progenitors are present in the placenta even before the onset of fetoplacental circulation. XY and microsatellite PCR confirmed that these progenitors were fetal in origin (Figure 5C). FACS and methylcellulose colony assays demonstrated that only CD34+CD43+ cells were capable of giving rise to a hematopoietic colony (supplemental Figure 7A). Flow cytometry confirmed that CD34+CD43+ progenitors are present in the placenta already in precirculation specimens (supplemental Figure 7B). Immunofluorescence revealed putative macrophage-committed progenitors expressing CD34, CD43, and either FXIII or CD68, in the chorionic plate of precirculation specimens (Figure 5Di-i′), suggesting that macrophages are generated de novo in the placenta or migrate there as progenitors through the extraembryonic mesoderm. By 6 weeks, most macrophages in the chorionic plate and villi no longer expressed CD34 (Figure 5Dii-ii′), and the frequency of macrophage progenitors, as determined by colony-forming assays, decreased (Figure 5B and supplemental Figure 7C). In summary, these results imply that macrophage progenitors populate the chorionic plate of the placenta before the onset of fetoplacental circulation, and their progeny migrate into the villous stroma and participate in the terminal stages of the enucleation process of primitive RBCs.
The human placenta contains macrophage progenitors before the onset of fetoplacental circulation. (A) IHC of placental sections revealed the progressive migration of FXIII+ macrophages (arrows) from the chorionic plate (i) into the villi (ii′-iii′). Images were acquired at 200× original magnification. (B) Methylcellulose colony- forming assays documented the presence of clonogenic macrophage progenitors in the chorionic plate of the human placenta before the onset of fetoplacental circulation. A representative macrophage colony (original magnification, ×100) and May-Grunwald Giemsa–stained cytospin of a macrophage colony (original magnification, ×400) are shown. (C) PCR performed on single colonies from methylcellulose for the Y chromosome was able to demonstrate the fetal origin of placental colonies from male (sample 1, XY) but not female (sample 2, XX) specimens. In the case of an XX specimen, informative microsatellites, defined by differences in the number of repeats contained in alleles (Al1 and Al2) inherited from each parent could be used to verify the fetal origin of placental progenitors. Of 176 colonies picked and analyzed (ranging from precirculation to 8 weeks developmental age), none was found to be maternal (data not shown). (D) Immunofluorescent staining of placental sections demonstrated the presence of macrophage-committed progenitors (CD34+CD43+FXIII+ and CD34+CD43+CD68+ cells; i and i′ insets, respectively) in the chorionic plate of precirculation specimens. Later in development, placental macrophages no longer expressed CD34. V indicates blood vessels; scale bars, 20 μm.
The human placenta contains macrophage progenitors before the onset of fetoplacental circulation. (A) IHC of placental sections revealed the progressive migration of FXIII+ macrophages (arrows) from the chorionic plate (i) into the villi (ii′-iii′). Images were acquired at 200× original magnification. (B) Methylcellulose colony- forming assays documented the presence of clonogenic macrophage progenitors in the chorionic plate of the human placenta before the onset of fetoplacental circulation. A representative macrophage colony (original magnification, ×100) and May-Grunwald Giemsa–stained cytospin of a macrophage colony (original magnification, ×400) are shown. (C) PCR performed on single colonies from methylcellulose for the Y chromosome was able to demonstrate the fetal origin of placental colonies from male (sample 1, XY) but not female (sample 2, XX) specimens. In the case of an XX specimen, informative microsatellites, defined by differences in the number of repeats contained in alleles (Al1 and Al2) inherited from each parent could be used to verify the fetal origin of placental progenitors. Of 176 colonies picked and analyzed (ranging from precirculation to 8 weeks developmental age), none was found to be maternal (data not shown). (D) Immunofluorescent staining of placental sections demonstrated the presence of macrophage-committed progenitors (CD34+CD43+FXIII+ and CD34+CD43+CD68+ cells; i and i′ insets, respectively) in the chorionic plate of precirculation specimens. Later in development, placental macrophages no longer expressed CD34. V indicates blood vessels; scale bars, 20 μm.
Discussion
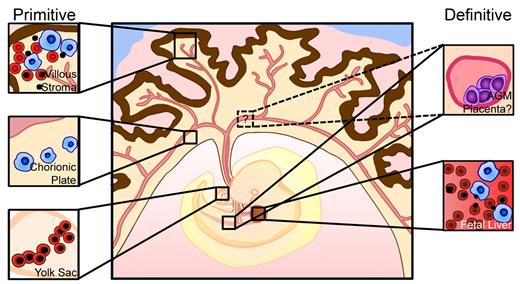
The identification of the human placenta as a hematopoietic organ has provided an accessible tissue to permit the study of early human embryonic hematopoiesis. In addition to being a source of definitive hematopoietic stem/progenitor cells,16-18 the placenta contains a large pool of primitive RBCs, making the study of RBC enucleation and embryonic globin regulation in vivo feasible.35 Here we show that the first trimester human placenta has an unexpected role in primitive erythroblast maturation. Our data reveal that circulating primitive erythroblasts extravasate into the stroma of placental villi and enucleate there in association with macrophages. The presence of clonogenic macrophage progenitors in the chorionic plate of precirculation placentas raises the possibility that macrophages may be generated de novo in the placenta and mature there, after which they migrate to the villi to physically interact with primitive RBCs and ingest primitive pyrenocytes. These data suggest that the first trimester human placenta, in addition to its newly identified function in definitive hematopoiesis, also has an integral role in primitive hematopoiesis (Figure 6).
Updated model of human embryonic hematopoiesis. Primitive erythroid cells are generated in the yolk sac (bottom left) and enter circulation with an intact nucleus. Macrophage progenitors appear in the chorionic plate of the placenta (middle left) and generate mature macrophages that migrate to the placental villi. Primitive erythroblasts and macrophages convene in the extravascular stroma of the placental villi to facilitate terminal maturation and enucleation of primitive RBCs and clearance of ejected nuclei (top left), in a similar manner to definitive RBC maturation in the fetal liver (bottom right). Concurrently, definitive HSCs are generated in the dorsal aorta, umbilical and vitelline arteries, and potentially in the placenta, and colonize the fetal liver for expansion and differentiation.
Updated model of human embryonic hematopoiesis. Primitive erythroid cells are generated in the yolk sac (bottom left) and enter circulation with an intact nucleus. Macrophage progenitors appear in the chorionic plate of the placenta (middle left) and generate mature macrophages that migrate to the placental villi. Primitive erythroblasts and macrophages convene in the extravascular stroma of the placental villi to facilitate terminal maturation and enucleation of primitive RBCs and clearance of ejected nuclei (top left), in a similar manner to definitive RBC maturation in the fetal liver (bottom right). Concurrently, definitive HSCs are generated in the dorsal aorta, umbilical and vitelline arteries, and potentially in the placenta, and colonize the fetal liver for expansion and differentiation.
Our study shows that human primitive erythroid cells, like their murine counterparts, ultimately enucleate.30-32,36 This suggests strong parallels between primitive erythroid maturation in human and mice, albeit with possible differences in the site of enucleation. Previous work with mouse embryos has suggested that enucleation of primitive RBCs may take place in the bloodstream31 or the fetal liver.30,32,36 Our data revealed that during human development, the placenta is a major, and possibly the first, site of primitive erythroid enucleation based on the enrichment of enucleated RBCs in the placental stroma (Figure 1) and the lack of extravascular RBCs in the early fetal liver (Figure 2). Furthermore, the relative paucity of macrophages in the fetal liver at the time when enucleation of primitive red cells is already occurring (Figure 4 and supplemental Figure 6) suggests that the human fetal liver may not be able to assist erythroblast enucleation until later in development. Despite overt differences in placental architecture37 in mice and humans, it is possible that the murine placenta also supports enucleation of primitive RBCs. Although this possibility was not examined in previous work, our data suggest that the murine placenta harbors major populations of macrophages and pyrenocytes at the time when enucleation of primitive RBCs occurs (E12.5-E16.5) and may also support primitive erythroblast maturation (A. Chhabra, A. Lechner, B.V.H., M. Tallquist, H.K.A.M., manuscript in preparation).
The presence of macrophages (Hofbauer cells) in the human placenta has been recognized for a long time,38,39 but their origin has been unknown. Our data revealed the presence of macrophages and macrophage committed progenitors in the chorionic plate of the placenta even before fetoplacental circulation has started, suggesting that the macrophages may be generated de novo in the placenta and later migrate to the villi to promote the terminal maturation and enucleation of primitive RBCs. Placental Hofbauer cells are thought to have multiple functions, including the promotion of vascularization via the production of vascular endothelial growth factor40 and angiopoietin-2.41 These factors may also affect definitive hematopoietic stem/progenitor cells in the placenta, either directly or indirectly via the vascular niche. In addition, the localization of placental macrophages at the site of fetal-maternal exchange and their documented expression of iron transport and storage proteins42 is suggestive of a potential role for placental macrophages in early iron transport to the embryo, but investigation of this hypothesis is beyond the scope of the present work. Together, these data indicate that Hofbauer cells may be generated in situ in the placenta and highlight their dynamic roles in early embryonic hematopoiesis and development.
Although the placenta has not been traditionally regarded as a hematopoietic organ, these findings together with the recent reports of the human placenta as a source of definitive hematopoietic stem/progenitor cells imply an important and much broader role for the human placenta in development beyond its well-established function in RBC oxygenation and iron transport. These findings have opened up new avenues to investigate the mechanisms that direct hematopoietic specification, hematopoietic stem/progenitor development and expansion, as well as terminal erythroid maturation during early human development and may help to understand the etiology of pregnancy complications that originate from defects in placental development and function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James Palis for insightful discussions about experimental design and data as well as critical reading of the manuscript and Thalia Papayannapoulou for the gift of the anti-ζ-globin antibody. The authors also thank the staff of the UCLA Translational Pathology Core Laboratory for assistance in specimen preparation and Marianne Cilluffo for expert assistance with EM.
This work was supported by a California Institute for Regenerative Medicine (CIRM) New Faculty Award (RN1-00 557-1) and an NIH/National Heart, Lung, and Blood Institute (NHLBI) RO1 (HL097766-01) to H.K.A.M. B.V.H was supported by the NIH Ruth Kirschstein National Research Service Award (GM007185), and S.L.P. was supported by the Howard Hughes Medical Institute Gilliam fellowship. M.M. was supported by the Swedish Research Council and the Tegger Foundation.
National Institutes of Health
Authorship
Contribution: B.V.H. designed and performed research and wrote the manuscript; S.L.P. designed and performed research; N. H.-K., A.H., M.M., B.A., and E.H. performed research; A.C. provided vital reagents; and H.K.A.M. designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: B.V.H., A.H., and M.M. are cofounders of and own significant financial stakes in Novogenix Laboratories, LLC. The remaining authors declare no competing financial interests.
Correspondence: Hanna Mikkola, 621 Charles E. Young Dr S, LSB 2204, Los Angeles, CA 90095; e-mail: hmikkola@mcdb.ucla.edu.
References
Author notes
S.L.P. and N.H.-K. contributed equally to this work.