Abstract
Engagement of T cells with antigen-presenting cells requires T-cell receptor (TCR) stimulation at the immune synapse. We previously reported that TCR stimulation induces the release of cellular adenosine-5′-triphosphate (ATP) that regulates T-cell activation. Here we tested the roles of pannexin-1 hemichannels, which have been implicated in ATP release, and of various P2X receptors, which serve as ATP-gated Ca2+ channels, in events that control T-cell activation. TCR stimulation results in the translocation of P2X1 and P2X4 receptors and pannexin-1 hemichannels to the immune synapse, while P2X7 receptors remain uniformly distributed on the cell surface. Removal of extracellular ATP or inhibition, mutation, or silencing of P2X1 and P2X4 receptors inhibits Ca2+ entry, nuclear factors of activated T cells (NFAT) activation, and induction of interleukin-2 synthesis. Inhibition of pannexin-1 hemichannels suppresses TCR-induced ATP release, Ca2+ entry, and T-cell activation. We conclude that pannexin-1 hemichannels and P2X1 and P2X4 receptors facilitate ATP release and autocrine feedback mechanisms that control Ca2+ entry and T-cell activa-tion at the immune synapse.
Introduction
T-cell activation requires a sustained elevation of intracellular Ca2+ levels, which is accomplished by Ca2+ entry through calcium release-activated calcium (CRAC) channels that are composed of stromal interaction molecule 1 (STIM1) and Orai1 proteins.1-3 Both proteins translocate to the immune synapse upon T-cell activation, where they mediate localized influx of extracellular Ca2+.4 Ca2+ entry contributes to the activation of nuclear factors of activated T cells (NFATs) that induce interleukin-2 (IL-2) gene expression and subsequent signaling events that lead to T-cell proliferation.5-7
Recent studies have shown that extracellular adenosine-5′-triphosphate (ATP) regulates T-cell activation.8-10 T cells release ATP in a controlled manner, as do other leukocytes, thereby facilitating intercellular communication and autocrine feedback regulation of cell function.8-13 Stimulation of T cells by T-cell receptor (TCR) ligation, mechanical stimulation, membrane deformation, or osmotic stress induces the release of cellular ATP.9,10,13-16 T cells express the gap junction hemichannels pannexin-1, which can mediate ATP release and T-cell activation.8,10 T-cell activation has been shown to involve P2X receptor subtypes.8,10 The 7 mammalian P2X receptor family members (P2X1-7) are ATP-gated ion channels.17,18 All these receptors, with the exception of P2X5, can facilitate entry of Ca2+ in response to stimulation by extracellular ATP,18-20 thus suggesting that P2X receptors regulate T-cell activation by mediating Ca2+ entry.
T-cell activation during antigen recognition requires the formation of an immune synapse between T cells and antigen-presenting cells. The immune synapse is a complex structure with a limited number of TCRs, implying that TCR stimulation requires signal amplification.21 Here we examined the role of ATP release and P2X receptor activation at the immune synapse. Our findings reveal that pannexin-1 and P2X1 and P2X4 receptors translocate to the immune synapse, where they contribute to Ca2+ entry and T-cell activation. The results thus indicate that pannexin-1–induced ATP release and autocrine activation of P2X1 and P2X4 receptors have important functions in TCR signal amplification and intercellular communication between T cells and antigen-presenting cells at the immune synapse.
Methods
Cells
Jurkat cells (E6-1 clone, ATCC) were maintained and human CD4+ T cells were isolated as previously described.14 All human and animal studies were approved by the Institutional Review Board and Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Real-time PCR and ATP release
Real-time polymerase chain reaction (PCR), performed as previously described (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article),12,14 was used to assess the gene expression of P2X1 and P2X4 receptors in resting or stimulated Jurkat and/or human CD4+ T cells. IL-2 gene expression was measured in human peripheral blood mononuclear cells (PBMCs) and mouse splenocytes (BALB/c) after stimulation with anti-CD3/CD28 coated Dynabeads for 4 hours in the presence or absence of antagonists (carbenoxolone, A438079,10panx-1, NF023, TNP-ATP, and suramin). IL-2 primers were purchased from QIAGEN. IL-2 mRNA expression was also measured in CD3/CD28-bead stimulated Jurkat cells after siRNA silencing of P2X1 and P2X4 receptors (described in “P2X receptor silencing by siRNA”). ATP release upon stimulation with anti-CD3/CD28-coated Dynabeads was assessed using the ATP Bioluminescence Assay Kit HSII (Roche), as previously described.8
Immunocytochemistry
Immunocytochemistry of Jurkat cells and human CD4+ T cells with goat anti–pannexin-1 (Santa Cruz Biotechnology), rabbit anti-P2X1 or anti-P2X4 receptor antibody (Alomone Labs) was performed as described.8 For receptor redistribution experiments, primary CD4+ T cells were stimulated with anti-CD3/CD28 coated Dynabeads for 0-30 minutes in complete medium (RPMI 1640, 10% fetal bovine serum [FBS], 100 U/mL penicillin, and 100 μg/mL streptomycin] before fixation. Immunofluorescence and brightfield images were captured using a confocal laser scanning microscope Zeiss LSM510 META.
Immunoblotting
Jurkat cells (5 × 107) were stimulated with phytohemagglutinin (PHA; 50 ng/mL) and phorbol 12-myristate 13-acetate (PMA; 5 ng/mL) for various times, resuspended in low salt buffer, sonicated on ice, and centrifuged. Bicinchoninic acid protein assays (Pierce) were performed. Samples were prepared in Novex 2× Tris glycine sodium dodecyl sulfate loading buffer (Invitrogen) containing 100μM dithiothreitol and boiled. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Western blotting was performed using standard procedures and rabbit anti-P2X1 or anti-P2X4 receptor antibodies (1:200, Alomone Labs).
Jurkat cell transfection
Transfections were carried out using an Eppendorf multiporator with cells suspended in hypo-osmolar electroporation buffer. Electroporation was performed with 10 μg of the respective plasmid using the following settings: 260 V, 70 microseconds, and 2-mm path length. After transfer to complete media, cells were cultured for 24 hours.
Plasmids
Plasmids containing cDNAs of the wild-type P2X1 and P2X4 receptor were purchased from Origene. The NFAT-luciferase reporter plasmid was a gift from Dr A. Altman (La Jolla Institute of Allergy and Immunology), and the β-galactosidase control plasmid was purchased from Roche. STIM1-mCFP and Orai1-mYFP constructs were kindly provided by Dr L. E. Samelson (Laboratory of Cellular and Molecular Biology, Center for Cancer Research, National Institutes of Health). Mutated and fluorescent P2X1 and P2X4 receptor fusion constructs were generated as described in the following section.
P2X1 and P2X4 receptor-EGFP fusion proteins
Enhanced green fluorescent protein (EGFP)–tagged P2X1 and P2X4 receptors were synthesized as follows: the P2X1 and P2X4 receptor cDNAs (Origene) were amplified by PCR using the following primers: P2X1 sense: 5′ATATAGAAGCTTGCCACCATGGCACGGCGGTTCCAGGAG3′ and antisense: 5′ATATGAATTCTAGCGTAGTCTGGGACGTCGTATGGGTAGGATGTCCTCATGTTCTC3′. P2X4 sense: 5′ATATAGAAGCTTGCCACCATGGCGGGCTGCTGCGCCGCG3′ and antisense: 5′ATATGAATTCTAGCGTAGTCTGGGACGTCGTATGGGTACTGGTCCAGCTCACTAGC3′. The primers introduce a HindIII restriction site upstream of the start codon, remove the stop codon, and add an in-frame EcoRI restriction site at the 3′ end. The PCR products and the pEGFP-N1 vector (Clontech) were digested, purified and then ligated together. Plasmids were transformed into competent DHα Escherichia coli cells, screened, and sequences were confirmed by DNA sequencing (Seqxcel).
Design and synthesis of P2X1 T18A and P2X4 L352W receptor constructs
The T18A and L352W mutations, which impair receptor function, were introduced into the P2X1, and P2X4 receptors, respectively.22,23 Site-directed mutagenesis was performed with the Quick-Change mutagenesis kit (Stratagene), according to the manufacturer's instructions, using the following primers: P2X1 sense: 5′ CTTCGAGTATGACGCTCCCCGCATGGTGC 3′; and antisense: 5′ GCACCATGCGGGGAGCGTCATACTCGAAG 3′, or P2X4 sense: 5′GGCATGGCGACCGTGTGGTGTGACATCATAGTC 3′; and antisense: 5′ GACTATGATGTCACACCACACGGTCGCCATGCC 3′. Successful introduction of the mutations was confirmed by DNA sequencing.
NFAT-luciferase reporter gene assay
The NFAT-luciferase reporter gene assay was performed as described.8,24 To examine the effect of P2X1 and P2X4 receptor silencing on NFAT activation, cells were electroporated with 6 μg of the NFAT-luciferase reporter plasmid, 8 μg of the β-galactosidase reporter plasmid and 3 μg of P2X1 or P2X4 receptor siRNA. After 72 hours, cells were stimulated with anti-CD3/CD28 antibody-loaded Dynabeads. Luciferase and β-galactosidase activity were measured 8 hours later.
P2X receptor silencing by siRNA
siRNA constructs targeting the mRNA of P2X1 (sense: 5′GGCCGAGAACUUCACUCUUtt3′; antisense: 5′AAGAGUGAAGUUCUCGGCCtc3′), P2X4 (sense: 5′GUACUACAGAGACCUGGCUtt3′; antisense: 5′AGCCAGGUCUCUGUAGUACtt3′) and P2X7 (sense: 5′GGUGAAAGAGGAGAUCGUGtt3′; antisense: 5′CACGAUCUCCUCUUUCACCtc3′) receptors were purchased from Ambion and a nonsense siRNA control was purchased from QIAGEN. Silencing efficiency was tested and Jurkat cells were transfected with 3 μg siRNA by electroporation as described (supplemental Figure 1C-F).8 Triple silencing was carried out using one-third of the siRNA per gene compared with silencing of individual genes.
Intracellular Ca2+ measurements
Ca2+ signaling was assessed before and after P2X receptor silencing of Jurkat cells using the Ca2+ indicator Fluo-3-AM (Molecular Probes), and of Fluo-4-AM (Invitrogen)–loaded primary CD4+ T cells after treatment with P2X antagonists: 10μM NF023, 30μM 2′,3′-O-(2,4,6-Trinitrophenyl)adenosine-5′-triphosphate tetra(triethylammonium) salt (TNP-ATP), 200μM suramin, or 200μM or 400μM of the pannexin-1 mimetic inhibitory peptide 10panx-1 or the scrambled control peptide scpanx-1 (TOCRIS), and a FACSCalibur flow cytometry system (Becton Dickinson). Changes in intracellular Ca2+ levels in response to stimulation with anti-CD3 antibodies (0.5 μg/mL) were assessed as previously described.8 We observed donor-to-donor differences in the response of PBMC preparations to CD3 stimulation compared with controls treated with ionomycin. However, each PBMC preparation showed consistent patterns of inhibition of Ca2+ signaling by P2X receptor antagonists and by 10panx-1. Data representative of at least 3 individual experiments are shown.
For microscopy experiments measuring Ca2+ influx, 5 × 106 cells were washed in pre-warmed Ca2+ -free Hanks balanced salt solution (Thermo Scientific), and incubated with 4μM Fluo-4 (Invitrogen) and 10μM EGTA (ethyleneglycoltetraacetic acid) for 1 hour. Cells were washed in Ca2+ -free Hanks balanced salt solution, seeded on fibronectin-coated glass bottom dishes (MatTek), and incubated with anti-CD3/CD28–coated beads for 30 minutes. Ca2+ was added to a final concentration of 2mM. Cells were scanned with a Zeiss LSM 510 META in 200-millisecond intervals.
Translocation of P2X1and P2X4 receptors to the immunologic synapse
Jurkat cells were transfected with EGFP–tagged P2X1 and P2X4 receptor constructs, placed into sterile fibronectin-coated glass bottom dishes, and stimulated with anti-CD3/CD28 antibody–coated beads. Brightfield and time-lapse images were captured using a Zeiss LSM 510 META.
Localization of P2X1and P2X4 receptors and STIM1 and Orai1
Jurkat cells were transfected with 10 μg of STIM1-mCFP or Orai1-mYFP plasmids, and 5 μg of EGFP-tagged P2X-receptor constructs, and cultured for 24 hours. Cells were seeded on fibronectin-coated glass bottom dishes, and stimulated with anti-CD3/CD28 coated Dynabeads. Brightfield and confocal fluorescence images were captured using a Zeiss LSM 510 META.
Statistical analysis
Unless otherwise stated, all data are expressed as mean ± SEM of ≥ 4 experiments performed in triplicate. Statistical analyses were performed with GraphPad Prism 4.0 software program (GraphPad). For comparisons between groups, 2-tailed unpaired Student t tests were used. A P value of ≤ .05 was considered significant.
Results
T cells express P2X1, P2X4, P2X5, and P2X7 receptors
We have previously shown that human lymphocytes express P2X7 receptors.8 Real-time reverse transcription PCR analysis revealed that Jurkat T cells and human primary CD4+ T cells also express P2X1, P2X4, and P2X5 receptors (Figure 1A). Previous studies have demonstrated lower Ca2+ permeation for P2X5 receptors compared with P2X1 and P2X4 receptors.25,26 Thus, we focused our study on P2X1 and P2X4 receptors. Immunocytochemistry indicated that Jurkat cells and human CD4+ T cells express P2X1 and P2X4 receptors on the cell surface (Figure 1B). T-cell activation with PHA and PMA alters the expression of these receptors, increasing P2X1 receptor mRNA expression by approximately 15-fold and decreasing P2X4 mRNA expression by 50% within 4 hours after cell stimulation (Figure 1C). Immunoblots show corresponding changes in expression of P2X1 and P2X4 protein (Figure 1D). T-cell stimulation induces ATP release and extracellular ATP enhances IL-2 production of activated T cells.15 Such findings and the current data implicate P2X1 and P2X4 receptors in mediating T-cell activation in response to extracellular ATP.
Expression of P2X receptors by human T cells. (A) P2X receptor mRNA expression in Jurkat cells and human peripheral CD4+ T cells determined by real-time reverse transcriptase PCR analysis. (B) Immunocytochemical assessment of P2X1 and P2X4 receptor expression of Jurkat cells and human primary CD4+ T cells evaluated by fluorescence microscopy. (C) P2X1 and P2X4 receptor mRNA expression levels of Jurkat cells in response to stimulation by PHA (50 ng/mL) and PMA (5 ng/mL). (D) Immunoblotting of P2X1 and P2X4 receptors before and after stimulation of Jurkat cells with PHA (50 ng/mL) and PMA (5 ng/mL). Data represent means ± SEM from triplicate experiments.
Expression of P2X receptors by human T cells. (A) P2X receptor mRNA expression in Jurkat cells and human peripheral CD4+ T cells determined by real-time reverse transcriptase PCR analysis. (B) Immunocytochemical assessment of P2X1 and P2X4 receptor expression of Jurkat cells and human primary CD4+ T cells evaluated by fluorescence microscopy. (C) P2X1 and P2X4 receptor mRNA expression levels of Jurkat cells in response to stimulation by PHA (50 ng/mL) and PMA (5 ng/mL). (D) Immunoblotting of P2X1 and P2X4 receptors before and after stimulation of Jurkat cells with PHA (50 ng/mL) and PMA (5 ng/mL). Data represent means ± SEM from triplicate experiments.
P2X1 and P2X4 receptors translocate to the immune synapse
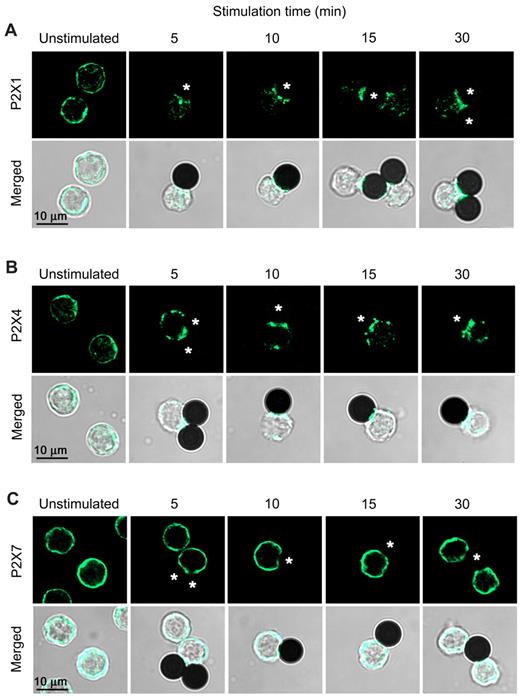
To study the potential role of P2X1 and P2X4 receptors in T-cell activation, we expressed EGFP-tagged receptor constructs in Jurkat cells. Upon stimulation of cells with microbeads bearing antibodies to CD3 and CD28 coreceptors, EGFP-tagged P2X1 and P2X4 receptors aggregate in clusters and translocate to the contact sites with the microbeads. A large portion of P2X4 receptors on the cell surface translocate to these contact sites within 2 minutes after cell stimulation (Figure 2). Cell stimulation reduced the amount of fluorescent submembranous vesicles, suggesting the integration of receptor-loaded vesicles into the cell membrane (Figure 2). While some P2X1 receptor redistribution was evident at 2-6 minutes (Figure 2), this translocation process is less rapid than that of P2X4 and requires ≥ 15 minutes for significant receptor accumulation at the contact site (Figure 3). Although P2X1 and P2X4 receptors differ in their kinetics of translocation to the immune synapse, both receptor subtypes remain closely associated with the synapse for at least 1 hour after cell stimulation (Figure 2). P2X1 and P2X4 receptors also translocate to the immune synapse of primary T cells with kinetics of redistribution similar to those observed in Jurkat cells (Figure 3A-B). By contrast, P2X7 receptors do not translocate to the immune synapse (Figure 3C).
Translocation of P2X1 and P2X4 receptors to the immune synapse of activated T cells. Time-lapse confocal live-cell microscopy images showing the expression of EGFP-tagged P2X1 (A) and P2X4 receptors (B) in Jurkat cells stimulated with anti-CD3/CD28 antibody-loaded beads (asterisks). Localization of receptors persists at least 1 hour after stimulation (Right panels: fluorescent microscopy images 60 minutes after stimulation).
Translocation of P2X1 and P2X4 receptors to the immune synapse of activated T cells. Time-lapse confocal live-cell microscopy images showing the expression of EGFP-tagged P2X1 (A) and P2X4 receptors (B) in Jurkat cells stimulated with anti-CD3/CD28 antibody-loaded beads (asterisks). Localization of receptors persists at least 1 hour after stimulation (Right panels: fluorescent microscopy images 60 minutes after stimulation).
P2X1 and P2X4, but not P2X7, receptors translocate to the immune synapse of T cells. Laser scanning microscopy images of primary human T cells activated with anti-CD3/CD28 antibody–loaded beads (asterisks). Cells were fixed after the indicated stimulation time and stained with anti-P2X1 (A), P2X4 (B), or P2X7 (C) receptor antibodies. While P2X4 receptors rapidly translocate to the immune synapse, P2X1 receptors first aggregate in clusters, and then translocate to the synapse after 15-30 minutes. P2X7 receptors do not redistribute upon TCR/CD28 stimulation and maintain their uniform distribution on the cell surface.
P2X1 and P2X4, but not P2X7, receptors translocate to the immune synapse of T cells. Laser scanning microscopy images of primary human T cells activated with anti-CD3/CD28 antibody–loaded beads (asterisks). Cells were fixed after the indicated stimulation time and stained with anti-P2X1 (A), P2X4 (B), or P2X7 (C) receptor antibodies. While P2X4 receptors rapidly translocate to the immune synapse, P2X1 receptors first aggregate in clusters, and then translocate to the synapse after 15-30 minutes. P2X7 receptors do not redistribute upon TCR/CD28 stimulation and maintain their uniform distribution on the cell surface.
These results suggest that translocation of P2X1 and P2X4 receptors to the immune synapse facilitates autocrine/paracrine signaling through these receptors. Thus, P2X1 and P2X4 receptors may amplify TCR signaling in response to ATP released at the synapse by T cells or antigen-presenting cells. By contrast, P2X7 receptors, which do not accumulate at the immune synapse, appear less restricted in terms of the source of ATP to which they respond.
P2X1 and P2X4 receptors are expressed with STIM1 and Orai1 at the immune synapse
Ca2+ entry is a key event in T-cell activation.5-7 We suspended Fluo-4-loaded Jurkat cells in Ca2+-free medium and then stimulated the cells for 30 minutes with CD3/CD28-antibody–bearing beads to allow synapse formation. After returning extracellular Ca2+ to the culture medium, we observed Ca2+ entry that originates at the immune synapses (supplemental Figure 2). STIM1 and Orai1 are components of CRAC channels that play a critical role in T-cell activation.1,27,28 STIM1 and Orai1 translocate to the immune synapse where they facilitate localized Ca2+ entry in response to TCR stimulation.4,29 Using confocal laser scanning microscopy, we found that STIM1 and Orai1 reside with P2X1 and P2X4 receptors at the immune synapse of Jurkat cells (Figure 4). These findings suggest that P2X1 and P2X4 receptors along with STIM1 and Orai1 contribute to localized Ca2+ entry at the immune synapse.
P2X1 and P2X4 receptors are expressed with Orai1 and STIM1 at the immune synapse. Confocal live-cell images of Jurkat cells coexpressing EGFP-tagged P2X1 or P2X4 receptors and either Orai1-EYFP (A,C) or STIM1-ECFP (B,D). Unstimulated cells show uniform Orai1, STIM1, and P2X1 and P2X4 receptor distributions (top panels) that localize at the immune synapse within 30 minutes of stimulation with anti-CD3/28 antibody-loaded beads (marked with asterisks in bottom panels). Scale bars represent 10 μm.
P2X1 and P2X4 receptors are expressed with Orai1 and STIM1 at the immune synapse. Confocal live-cell images of Jurkat cells coexpressing EGFP-tagged P2X1 or P2X4 receptors and either Orai1-EYFP (A,C) or STIM1-ECFP (B,D). Unstimulated cells show uniform Orai1, STIM1, and P2X1 and P2X4 receptor distributions (top panels) that localize at the immune synapse within 30 minutes of stimulation with anti-CD3/28 antibody-loaded beads (marked with asterisks in bottom panels). Scale bars represent 10 μm.
P2X1 and P2X4 receptors contribute to Ca2+ influx after T-cell stimulation
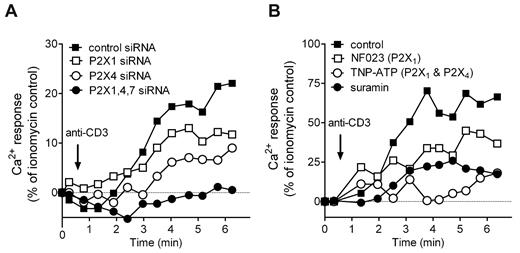
While the roles of STIM1 and Orai1 in Ca2+ entry and T-cell activation are well established, the possible involvement of P2X receptors is less clear.1-4,7 We found that silencing of P2X1, P2X4, and P2X7 receptors inhibits Ca2+ signaling in response to TCR stimulation (Figure 5A), thus implicating all 3 P2X receptor subtypes in Ca2+ entry during T-cell activation.
P2X1 and P2X4 receptors regulate Ca2+ signaling in response to TCR stimulation. Effects of silencing P2X receptors in Jurkat cells (A) or pharmacologic P2X receptor inhibition of human primary CD4+ T cells (PBMCs; B) on Ca2+ signaling. Cells were stimulated by TCR activation (0.5 μg/mL anti-CD3 antibody). The response was assessed by flow cytometry using Fluo-3 as a Ca2+ indicator. Triple silencing was carried out using one-third of siRNA per gene compared with silencing of individual genes. Graphs depict representative results from at least 3 experiments performed with cells from different donors.
P2X1 and P2X4 receptors regulate Ca2+ signaling in response to TCR stimulation. Effects of silencing P2X receptors in Jurkat cells (A) or pharmacologic P2X receptor inhibition of human primary CD4+ T cells (PBMCs; B) on Ca2+ signaling. Cells were stimulated by TCR activation (0.5 μg/mL anti-CD3 antibody). The response was assessed by flow cytometry using Fluo-3 as a Ca2+ indicator. Triple silencing was carried out using one-third of siRNA per gene compared with silencing of individual genes. Graphs depict representative results from at least 3 experiments performed with cells from different donors.
We used pharmacologic inhibitors to evaluate the roles of P2X1 and P2X4 receptors in Ca2+ signaling of human primary CD4+ T cells. Similar to our findings with Jurkat T cells, we observed that receptor blockade reduces Ca2+ influx in response to TCR stimulation (Figure 5B). TNP-ATP, an antagonist of P2X1 and P2X4 receptors, suramin, a general P2 receptor antagonist, and NF023, a P2X1 receptor antagonist, block Ca2+ influx in a manner that suggests a predominant role for P2X4 receptors in facilitating Ca2+ influx in primary CD4+ T cells.
Taken together, these findings indicate that P2X1, P2X4, and P2X7 receptors contribute to Ca2+ signaling in T cells. While P2X4 and P2X1 receptors associate with the immune synapse, P2X7 receptors remain uniformly distributed (Figure 3), which suggests that the cellular localization of these receptor subtypes helps determine their roles in T-cell activation.
P2X1 and P2X4 receptors contribute to NFAT activation and IL-2 expression
Sustained intracellular Ca2+ levels lead to the activation of NFAT family signaling molecules that control cytokine expression.6 Using NFAT-luciferase reporter assays,24 we found that overexpression of P2X1 receptors augments NFAT activation in response to TCR/CD28 stimulation (Figure 6A). Conversely, overexpression of mutated P2X1 or P2X4 receptors, which were rendered dysfunctional by point mutations at T18A and L352W, respectively, or silencing of P2X1 or P2X4 receptors, inhibits NFAT activation (Figure 6B-C). NFAT is required to induce IL-2 gene expression and to promote T-cell activation events.6 Silencing of both P2X1 and P2X4 receptors decreased IL-2 mRNA transcription (Figure 6D). Because the effect of silencing of P2X4 receptors was more pronounced than that of P2X1 receptors, P2X4 receptors may have a more important role in activation processes induced by TCR stimulation at the immune synapse. We previously showed that silencing of P2X7 receptors inhibits Ca2+ influx and IL-2 gene transcription.8,10 Here we show that the combined silencing of P2X1, P2X4, and P2X7 receptors results in a stronger suppression of IL-2 transcription than silencing of either receptor subtype alone (Figure 6D-E), which suggests that all 3 P2X receptor subtypes contribute to TCR induced T-cell activation.
P2X1 and P2X4 receptors contribute to NFAT activation and IL-2 expression of T cells. NFAT activation of Jurkat cells overexpressing wild-type (A) or mutated P2X1 or P2X4 receptors (B). NFAT activation (C) and IL-2 mRNA expression (D) of Jurkat cells after silencing of P2X1 or P2X4 receptors. Cell responses were assessed after stimulation with anti-CD3/CD28 antibody-coated beads for 8 hours. (E) IL-2 mRNA expression in Jurkat cells after combined silencing of P2X1, P2X4, and P2X7 receptors. (F) IL-2 mRNA expression in human PBMCs and mouse splenocytes stimulated for 4 hours in the presence or absence of P2X1 receptor–selective (NF023, 10μM), P2X1 and P2X4 receptor–selective (TNP-ATP, 30μM), P2X7 selective (A438079, 10μM), or nonselective (suramin, 100μM) P2 receptor antagonists. #P ≤ .05 compared with resting cells, *P ≤ .05, **P ≤ .01 compared with CD3/CD28- stimulated controls.
P2X1 and P2X4 receptors contribute to NFAT activation and IL-2 expression of T cells. NFAT activation of Jurkat cells overexpressing wild-type (A) or mutated P2X1 or P2X4 receptors (B). NFAT activation (C) and IL-2 mRNA expression (D) of Jurkat cells after silencing of P2X1 or P2X4 receptors. Cell responses were assessed after stimulation with anti-CD3/CD28 antibody-coated beads for 8 hours. (E) IL-2 mRNA expression in Jurkat cells after combined silencing of P2X1, P2X4, and P2X7 receptors. (F) IL-2 mRNA expression in human PBMCs and mouse splenocytes stimulated for 4 hours in the presence or absence of P2X1 receptor–selective (NF023, 10μM), P2X1 and P2X4 receptor–selective (TNP-ATP, 30μM), P2X7 selective (A438079, 10μM), or nonselective (suramin, 100μM) P2 receptor antagonists. #P ≤ .05 compared with resting cells, *P ≤ .05, **P ≤ .01 compared with CD3/CD28- stimulated controls.
To test the involvement of P2X1 and P2X4 receptors in the activation of primary T cells, we used P2 receptor antagonists in experiments with human and mouse lymphocytes. We found that the P2X1/P2X4 receptor antagonist TNP-ATP and the non-specific P2X receptor antagonist suramin inhibit IL-2 mRNA expression of TCR/CD28-stimulated primary human T cells by approximately 50%, while P2X1- and P2X7-selective antagonists (NF023 and A438079, respectively) produce an approximately 25% inhibition (Figure 6F). All 4 antagonists strongly suppressed IL-2 mRNA expression of mouse T cells stimulated with microbeads carrying anti–mouse CD3/CD28 antibodies. These findings implicate autocrine stimulation of P2X1, P2X4, and P2X7 receptors in T-cell activation. The differences in the responses of mouse versus human T cells to these P2 antagonists suggest inter-species variation in P2X receptor expression patterns of mouse and human T cells or differences in the inhibitory efficiency of the purinergic receptor antagonists.18,30-32 Moreover, P2X1, P2X4, and P2X7 receptors form heteromeric structures that have different combinations of the individual P2X receptor subunits and the composition of these heteromeric ion channels may differ in mouse versus human T cells.32,33
Pannexin-1 translocates to the immune synapse and is involved in T-cell activation
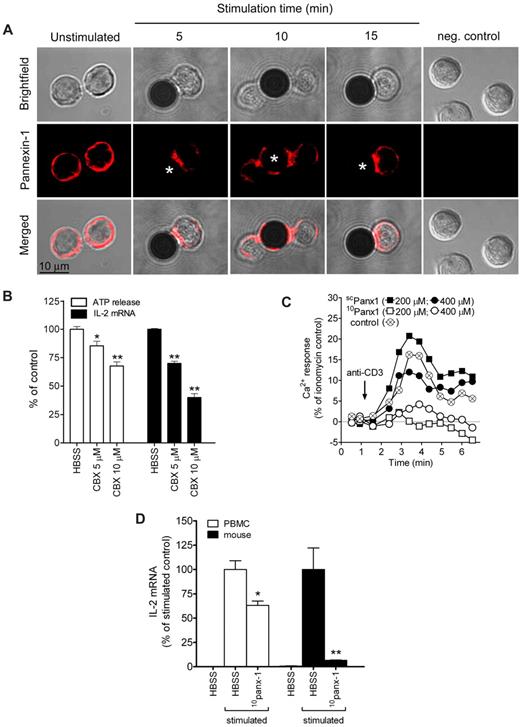
Pannexin-1 hemichannels have been identified as gap junction molecules involved in the release of ATP from activated T cells.10,34 We found that pannexin-1 is uniformly distributed across the cell surface of unstimulated primary T cells but translocates to the immune synapse within 5 minutes in response to TCR/CD28 stimulation (Figure 7A). This rapid translocation suggests that pannexin-1 may facilitate ATP release and contribute to autocrine feedback of TCR stimulation at the immune synapse. Indeed, inhibition of pannexin-1 with the gap junction inhibitor carbenoxolone blocks TCR-mediated ATP release and IL-2 gene transcription (Figure 7B). Treatment of T cells with a more specific pannexin-1 hemichannel blocker, 10panx-1, reduces Ca2+ entry in response to TCR stimulation (Figure 7C) and inhibits IL-2 transcription of human and mouse primary T cells (Figure 7D), while treatment with the scrambled control peptide scpanx-1 had no significant effect on Ca2+ entry. Pannexin-1 hemichannels thus appear to contribute to ATP release at the immune synapse.
Pannexin-1 hemichannels facilitate ATP release at the immune synapse. Stimulation of the TCR leads to redistribution of pannexin-1 to the immune synapse (A). Treatment with the gap channel inhibitor carbenoxolone reduces ATP release and IL-2 gene transcription upon CD3/CD28 stimulation. (B). The pannexin-1 specific inhibitor 10panx-1 reduced Ca2+ entry compared with the control peptide (scpanx-1), or to CD3 stimulation without any peptide (control) in human PBMCs (C) and IL-2 transcription in human PBMCs and mouse splenocytes (D). *P ≤ .05, **P ≤ .01 compared with CD3/CD28-stimulated controls.
Pannexin-1 hemichannels facilitate ATP release at the immune synapse. Stimulation of the TCR leads to redistribution of pannexin-1 to the immune synapse (A). Treatment with the gap channel inhibitor carbenoxolone reduces ATP release and IL-2 gene transcription upon CD3/CD28 stimulation. (B). The pannexin-1 specific inhibitor 10panx-1 reduced Ca2+ entry compared with the control peptide (scpanx-1), or to CD3 stimulation without any peptide (control) in human PBMCs (C) and IL-2 transcription in human PBMCs and mouse splenocytes (D). *P ≤ .05, **P ≤ .01 compared with CD3/CD28-stimulated controls.
Discussion
Extracellular ATP can enhance IL-2 production of activated T cells via P2X7 receptors and ATP release from T cells plays an important role in T-cell activation.8-10 This study demonstrates novel roles for the ATP release channel pannexin-1 and for P2X1 and P2X4 receptors in events that control T-cell activation at the immune synapse, a membrane domain that facilitates T cell–accessory cell communication and antigen recognition.35-38 We find that P2X1, P2X4, and P2X7 receptors contribute to calcium influx and that pannexin-1, and P2X1 and P2X4, but not P2X7, receptors rapidly redistribute to the immune synapse upon TCR stimulation. Antagonists of pannexin-1 or of P2X1 and P2X4 receptors inhibit IL-2 mRNA expression of activated T cells. Taken together with the release of ATP upon T-cell activation,8,9,13 the current results indicate that pannexin-1–mediated ATP release and autocrine feedback through P2X1 and P2X4 receptors contribute to T-cell activation at the immune synapse. Furthermore, T-cell activation markedly changes P2X1 and P2X4 receptor expression, thus implicating such changes in receptor expression as additional mechanisms that may produce fine-tuning of T-cell responses.
The translocation of P2X1 and P2X4, but not P2X7 receptors to the immune synapse may relate to their localization in lipid rafts.39-41 Lipid rafts in T cells form the backbone of the immune synapse37,38 where, as implied by data here, P2X1 and P2X4 receptors and their ligand, ATP, contribute to Ca2+ entry and T-cell activation. The kinetics of translocation of pannexin-1 and P2X4 receptors to the immune synapse imply that these molecules are involved in T-cell activation processes that take place in the first few minutes after cell stimulation, while the slower translocation of P2X1 receptors suggest that these receptors contribute to subsequent activation events. These purinergic activation events may help amplify TCR-induced signals through localized autocrine and paracrine feedback mechanisms. Our findings thus provide an explanation as to how the limited number of TCR molecules at the synapse can trigger robust downstream signaling responses that are needed for T-cell activation.
Our results indicate that P2X1 and P2X4 receptors are redistributed to the immune synapse along with STIM1 and Orai1, critical components of CRAC channels that facilitate Ca2+ entry and activation of T cells.1,27,28,42-44 In addition to Orai1 and STIM1, additional proteins such as transient receptor potential–like channels, may also be involved in Ca2+ entry.7,42,45,46 Orai1 and STIM1 can translocate to the immune synapse, where they facilitate localized Ca2+ entry.4,29 Our results here indicate that P2X1 and P2X4 receptors also contribute to Ca2+ entry at the immune synapse, with P2X4 receptors contributing to the early phase of this process. Inhibition of purinergic response at the immune synapse, (by blocking pannexin-1–induced ATP release or targeting P2X1 or P2X4 receptors) inhibits Ca2+ entry. Combined silencing of P2X1, P2X4, and P2X7 receptors suppresses Ca2+ entry and downstream activation events to a greater extent than that of any one receptor alone, suggesting that all 3 P2X receptors regulate T-cell activation. Perhaps, as in other cell types, P2 receptor heteromers composed of P2X1, P2X4, and P2X7 receptor subunits contribute to Ca2+ entry at the T-cell immune synapse.18,33,47-49
We find that TCR stimulation induces translocation of pannexin-1 hemichannels to the immune synapse and that inhibition of pannexin-1 hemichannels suppresses TCR-induced ATP release, Ca2+ entry, and IL-2 expression. These findings imply that pannexin-1 along with P2X1 and P2X4 receptors form a purinergic feedback system that promotes T-cell activation at the immune synapse. This conclusion is supported by results indicating that removal of extracellular ATP by addition of apyrase and other enzymes that hydrolyze extracellular ATP blocks TCR-induced Ca2+ signaling and IL-2 production.8
The current results thus emphasize a key role for purinergic signaling, and pannexin-1 and P2X4 receptors in particular, in modulating TCR signaling at the immune synapse. These purinergic signaling components may serve to amplify TCR-induced activation signals at the immune synapse, thereby facilitating efficient T-cell responses despite the limited number of TCR molecules that are engaged by antigen-presenting cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by National Institutes of Health grants R01 GM-51 477, GM-60 475, AI-072287, and R01 AI-080582 (W.G.J.), and Department of Defense Congressionally Directed Medical Research Programs grant PR043034 (W.G.J.), GM-66 232, and a grant from the Leukemia & Lymphoma Society (P.A.I.), and National Institutes of Health General Clinical Research Center grant 5MO1-RR-00 827-25 (University of California San Diego, San Diego, CA).
National Institutes of Health
Authorship
Contribution: T.W. performed laser scanning microscopy, immuno-cytochemistry, and functional assays; L.Y. was responsible for generation of P2X receptor plasmids and functional assays; A.E., Y.S., Y.C., and Y.Y. performed functional assays and provided advice; P.A.I. provided advice and assistance with certain experiments and with manuscript preparation; and W.G.J. provided overall direction, supervised project planning and execution, and finalized the manuscript for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang G. Junger, Beth Israel Deaconess Medical Center, Harvard Medical School, Department of Surgery, 330 Brookline Ave, Dana 811, Boston, MA 02215; e-mail: wjunger@bidmc.harvard.edu.
References
Author notes
T.W. and L.Y. contributed equally to this work.