Abstract
In somatic cells, eroded telomeres can induce DNA double-strand break signaling, leading to a form of replicative senescence or apoptosis, both of which are barriers to tumorigenesis. However, cancer cells might display telomere dysfunctions which in conjunction with defects in DNA repair and apoptosis, enables them to circumvent these pathways. Chronic lymphocytic leukemia (CLL) cells exhibit telomere dysfunction, and a subset of these cells are resistant to DNA damage-induced apoptosis and display short telomeres. We show here that these cells exhibit significant resection of their protective telomeric 3′ single-stranded overhangs and an increased number of telomere-induced foci containing γH2AX and 53BP1. Chromatin immunoprecipitation and immunofluorescence experiments demonstrated increased levels of telomeric Ku70 and phospho-S2056-DNA-PKcs, 2 essential components of the mammalian nonhomologous end-joining DNA repair system. Notably, these CLL cells display deletions of telomeric signals on one or 2 chromatids in parallel with 11q22 deletions, or with 13q14 deletions associated with another chromosomal aberration or with a complex karyotype. Taken together, our results indicate that a subset of CLL cells from patients with an unfavorable clinical outcome harbor a novel type of chromosomal aberration resulting from telomere dysfunction.
Introduction
Telomeres are nucleoprotein complexes at the ends of eukaryotic chromosome that discriminate these ends from DNA double strand breaks (DSBs). The essential function of telomeres is to ensure genome stability by preventing chromosome end attrition and end-to-end fusions. This is accomplished by telomeric tandem repeat sequence structure (TTAGGG) and the combined action of several factors.1,2 The uncapping of telomeres resulting from a dysfunction of telomeric proteins or telomere shortening can disrupt their protective function and activate a DNA damage response.3,4 In this circumstance, the DNA damage signaling proteins γ-H2AX and 53BP1 localize at the telomeres, activate an irreversible cell growth arrest pathway known as senescence through the phosphorylation of Chk2, and/or induce genomic instability via the nonhomologous end joining (NHEJ) repair system.5-8 Using single-molecule approaches, it has been demonstrated that in human cells telomeric fusions events may contain less than 12.8 of no canonical telomere repeats at the fusion point. This distinct type of fusions of critically short telomeres may be generated through an error-prone alternative NHEJ.9 However, at the cell population level, it has been observed that a critical size of telomere would be less than 5 kb, when DNA damage response and cellular senescence were elicited.5,10 This discrepancy may be explained by the high heterogeneity of telomere length existing even in a single cell.11 In consequence, the presence of only a few dysfunctional telomeres might be sufficient to induce cell cycle arrest.12
In chronic lymphocytic leukemia (CLL), average telomere length has been reported as an emerging prognostic factor because cells of the more aggressive form of this disease have shorter telomeres than cells of the indolent form; a cutoff of between 4 kb and 6 kb can be used to discriminate between these 2 forms of disease.13-16 In addition, short telomeres may be associated with genetic complexity and a high risk of genomic aberrations.17 Because genomic complexity is implicated in disease outcome,18 it remains crucial to determine whether telomere dysfunction is causal for chromosomal aberrations in CLL cells. As suggested by Roos et al,17 short telomeres may induce complex chromosomal abnormalities before the onset of malignant transformation. However, it cannot yet be ruled out that short telomeres appear as a consequence of complex chromosomal abnormalities.19
We have recently demonstrated that telomere length can be used to discriminate between CLL cells resistant in vitro to DNA damage-induced apoptosis (CLL-R) and those that are sensitive (CLL-S). In CLL-R cells, the mean telomere length is less than 4 kb, whereas in CLL-S cells telomeric sequences are longer than 6 kb.20 Here, we evaluated whether these short telomeres in resistant cells might elicit DNA damage response by assessing a classic DSB signaling and an induction of telomere dysfunction-induced foci (TIF). This hypothesis has been supported in previous reports showing that CLL-R cells display deregulated NHEJ and, in particular, by evidence showing up-regulation of the DNA end-binding activity of the Ku80/Ku70 heterodimer in these cells.21,22 Both Ku80 and Ku70 have been identified in telomeric complexes, thus emphasizing the deregulation of these factors also at the telomeres in CLL-R cells. We show in the current study that CLL-R cells form TIF and display an increased telomeric concentration of 2 NHEJ factors, Ku70 and phospho-S2056-DNA-PKcs. Moreover, these cells display telomeric deletions at one or 2 chromatids. It is noteworthy that the manifestation of these telomeric abnormalities in CLL-R cells is concomitant with the appearance of the multiple chromosomal aberrations and complex karyotypes, which are known as faithful markers of a poor disease outcome.
Methods
Patient samples, isolation of CLL lymphocytes, and apoptosis assays
Peripheral blood samples were collected, after informed consent, from patients 53 to 91 years of age. Primary CD19+ B cells were isolated using the RosetteSep B cell enrichment kit (Stem Cell Technologies) according to the manufacturer's instructions. Apoptosis was assessed as previously described.21 For sensitive samples (CLL-S), the level of apoptosis increased from 8.2% plus or minus 6.2% (spontaneous apoptosis) to 75.8% plus or minus 18.7% after genotoxic stress (10 Gy irradiation or 1μM neocarzinostatin). For resistant samples (CLL-R), the apoptosis levels of the irradiated cells remained unchanged (6.1% ± 3.8% compared with 4.8% ± 2.4% of spontaneous apoptotic cells). Approval for this study was obtained from the institutional review boards of the Commissariat à l'Energie Atomique and the Pitié-Salpêtrière Hospital. Patient clinical and biologic behaviors are presented in Table 1.
Genomic DNA isolation and analysis of G-rich 3′ overhangs
CLL cells (5 × 106) were resuspended in 1 mL of phosphate-buffered saline (PBS) and lysed with 10 mL of lysis buffer (10mM Tris, pH 7.8, 10mM ethylenediaminetetraacetic acid, 0.5% sodium dodecyl sulfate, vol/vol) containing 100 μg/mL proteinase K (Sigma-Aldrich) at 37°C overnight. Proteins were then precipitated using NaCl solution (6M). After centrifugation (3300g for 10 minutes at 4°C), the supernatant was collected in a new tube and DNA was precipitated with isopropanol. The DNA concentration was determined from the absorption of the solution at 260 nm.
The relative lengths of the 3′ overhangs in CLL cells were measured using a telomeric-oligonucleotide ligation assay (T-OLA) as previously described,23 with minor modifications. Briefly, nondenatured genomic DNA was hybridized with a radioactively labeled oligonucleotide probe (CCCTAA)8. This probe hybridizes to the naturally accessible single-strand 3′ overhang at the extreme end of the telomere and incubation with a Taq DNA ligase and then joins adjacent oligonucleotides with no gap between them. The DNA fragments obtained are denatured and separated on an acrylamide gel under denaturing conditions in which a ladder ranging from 48 nt (free probes) to a maximum, which was considered to correlate with the 3′ overhang length (maximum number of hybridized probes). Hybridizations were conducted using a mixture containing γ-32P radiolabeled (CCCTAA)8 complementary to the G-rich single-strand 3′ overhang and 5 μg of undenatured genomic DNA. Identical experiments using oligonucleotides complementary to the C rich telomeric strand (TTAGGG)8 were performed as a control. A ligation step using 2 U of thermostable Taq ligase (BioLabs) was then carried out. The genomic DNA and ligated products were purified by phenol-chloroform extraction and ethanol precipitation and resuspended in 6 μL of Tris-EDTA buffer. The samples were denatured at 90°C with 8 μL of formamide dye, immediately quenched on ice, and resolved in 6% acrylamide, 8M urea with a buffer gradient at 300 V for 3 hours. After fixation and drying, the gel was exposed to a PhosphorImager screen (GE Healthcare). Images were acquired using Typhoon 9400 (GE Healthcare). The migration distance of the highest band was determined with ImageQuant software (GE Healthcare), and the 3′ overhang length was then deduced using the low range DNA ladder (Fermentas) as the molecular weight standards. As a control, hybridizations were conducted in parallel with a γ-32P radiolabeled (TTAGGG)8 oligonucleotide.
Immunofluorescence and FISH analysis of interphase CLL cells
Purified CLL cells were cytospun for 10 minutes at 600g at room temperature and permeabilized for 10 minutes in a permeabilization buffer containing 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.9, 50mM NaCl, 3mM MgCl2, 300 mM sucrose, and 5% Triton X-100 (vol/vol). The cells were then fixed for 10 minutes in solution containing formaldehyde 4% (vol/vol) and sucrose 2% (wt/vol) in PBS. Cells were repermeabilized in the same permeabilization buffer for 10 minutes and incubated for 10 minutes in blocking solution I (PBS, 0.5% Triton [vol/vol], 1% bovine serum albumin [BSA, wt/vol], and 5% fetal bovine serum [vol/vol]), followed by incubation for 2 hours at 37°C with primary antibodies in blocking solution II (PBS, 0.1% Triton, 1% BSA). Antibodies used at the appropriate dilutions were mouse anti-TRF2 (Imgenex, IMG-124A), rabbit anti-TRF1 (Santa Cruz Biotechnology, sc-5596), anti–γ-H2AX (rabbit polyclonal antibody from Cell Signaling, 9718L or mouse monoclonal antibody from Upstate Biotechnology, 05–636), rabbit anti-53BP1 (Novus Biologicals), and rabbit antiphospho-S2056-DNA-PKcs (Abcam). Cells were rinsed 3 times in PBS, 0.1% Triton for 5 minutes, and incubated for 1 hour at room temperature with secondary antibodies, including Alexa Fluor 488 goat anti–mouse IgG and Alexa Fluor 568 goat anti–rabbit IgG (to visualize TRF2 and γ-H2AX, 53BP1, or P-DNA-PKcs) or Alexa Fluor 488 goat anti–rabbit IgG and Alexa Fluor 594 goat anti–mouse IgG (to visualize TRF1 and γ-H2AX; Invitrogen), all at a 1:1000 dilution. Cells were rinsed 3 times in PBS, 0.1% Triton, and nuclear DNA was counterstained with 1 μg/mL 4′,6′-diamidino-2-phenylindole (DAPI). Slides were then mounted in glycerol with 0.1% p-phenylenediamine (wt/vol; Sigma-Aldrich). As a control, telomere labeling was performed in parallel by fluorescence in situ hybridization (FISH) with a telomere-specific peptide nucleic acid (FISH-PNA) probe, as previously described.24 Image acquisition was performed using a Leica SPE confocal microscope, using ACS AAPO 40×/1.15 numeric aperture (NA) or 63×/1.30 NA oil objectives. Image treatment and analysis were performed using Leica and ImageJ 1.43u (NIH) software.
ChIP
Chromatin immunoprecipitation (ChIP) assays were performed as described.20 A mouse monoclonal anti-Ku70 antibody (clone N3H10; Thermo Fisher Scientific) was used at 5 μg per immunoprecipitation reaction.
FISH analysis of CLL cells in metaphase
B cells were separated on a Ficoll gradient (Abcys SA), washed once in PBS buffer, and cultured in a 5% CO2 atmosphere in RPMI Glutamax medium (Invitrogen), supplemented with 20% BSA (wt/vol), 5000 U/mL heparin, 125 U/mL penicillin, and 31 U/mL streptomycin (Sigma-Aldrich). To this medium was added CpG oligonucleotide at 10 nmol/mL (Tib Molecular Biology) or 12-O-tetradecanoyl-phorbol-13-acetate (TPA) in addition to interleukin 2 at 20 U/mL (Roche Biotechnology) for 72 hours. The cell cycle was arrested in metaphase by the addition of 1 μg/mL colchicine (Sigma-Aldrich). Cells were resuspended in a hypotonic buffer containing KCl (5.6 g/L). After incubation for 20 minutes at 37°C, cells were washed and fixed several times with acetic acid/methanol (1:3 vol/vol) and deposited on a slide. Slides were fixed with 3.7% formaldehyde (vol/vol) for 2 minutes, washed twice in washing buffer (Cytology FISH accessory kit; Dako North America) for 5 minutes, and then dehydrated in an ethanol series (70%, 90%, and 100% for 2 minutes each at room temperature). Telomeres were labeled with a (C3TA2)3 probe (conjugated to cyanine-3 and diluted in hybridization buffer [70% formamide, 10mM Tris, pH 7.2, 1% BSA]) by denaturing for 3 minutes at 80°C and hybridizing for 2 hours at room temperature. Slides were then washed twice in hybridization buffer for 15 minutes and 3 times in 50mM Tris, pH 7.2, 150mM NaCl, and 0.05% Tween 20 for 5 minutes and counterstained with DAPI for chromosome identification.
After image acquisition, the slides were washed for 20 minutes in a dehybridizing stringent buffer (Cytology FISH accessory kit; Dako), washed twice with wash buffer for 3 minutes, and hybridized de novo using Vysis LSI p53/LSI ataxia telangiectasia mutated (ATM) and LSI D13S319/LSI 13q34/CEP 12 set probes and CEP11, CEP17 probes (Abbott, 05J83001, 6J3611, and 6J3717) in accordance with the manufacturer's instructions. The set labeling enabled the detection of chromosomal deletions 17p13, 11q22, 13q14, and trisomy 12, respectively, which are the most frequent abnormalities observed in CLL. Only metaphase spreads with 13q14, 11q22, or 17p13 deletions or trisomy 12 were further analyzed for telomeric aberrations (except for 3 healthy donors used as controls). Image acquisition was performed with a motorized reflected BX61 Olympus fluorescence microscope with filters enabling the separate detection of each fluorochrome used. Image treatment and analysis were undertaken with Genikon and ImageJ 1.43u software.
Statistical analysis
The Mann-Whitney U test was used to compare the values obtained in each experiment between the 2 subgroups of patients. A P value less than .05 was considered significant.
Results
Shortened G-rich strand 3′ overhangs in CLL-R compared with CLL-S
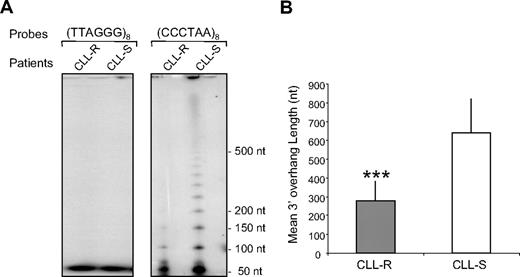
Telomere shortening has been reported to be proportional to the size of the single-stranded telomeric 3′ overhangs.25 Moreover, when telomeres become dysfunctional, NHEJ activation can result in a 3′ overhang resection.8,26 We therefore examined whether changes in the 3′ overhang are associated with decreased telomere length. To measure single-stranded 3′ overhangs, we used a T-OLA and analyzed samples from 20 CLL patients (10 CLL-R and 10 CLL-S; Figure 1). The results showed that the telomeric 3′ overhangs are significantly (U test; P < .001) shorter in CLL-R compared with CLL-S cells (279 ± 104 vs 638 ± 182 nt; Figure 1B).
Assay of G-rich 3′ overhang length in CLL cells according to their in vitro sensitivity to apoptosis. G-rich 3′ overhang lengths are expressed as nucleotides (nt). (A) Representative example of a 3′ overhang length measurement using the T-OLA analysis. As indicated, 2 probes were tested: one complementary to the 3′ overhang (CCCTAA)8 and one homologous to the 3′ overhang sequence (negative control; TTAGGG)8. (B) Comparison of the 3′ overhang lengths between CLL-R and CLL-S. Values are the mean 3′ overhang length (nt) ± SD (n = 10 for both CLL-R and CLL-S). The Mann-Whitney U test was used to assess statistical significance: ***P < .001.
Assay of G-rich 3′ overhang length in CLL cells according to their in vitro sensitivity to apoptosis. G-rich 3′ overhang lengths are expressed as nucleotides (nt). (A) Representative example of a 3′ overhang length measurement using the T-OLA analysis. As indicated, 2 probes were tested: one complementary to the 3′ overhang (CCCTAA)8 and one homologous to the 3′ overhang sequence (negative control; TTAGGG)8. (B) Comparison of the 3′ overhang lengths between CLL-R and CLL-S. Values are the mean 3′ overhang length (nt) ± SD (n = 10 for both CLL-R and CLL-S). The Mann-Whitney U test was used to assess statistical significance: ***P < .001.
Short telomeres are recognized as DNA DSBs in resistant CLL cells
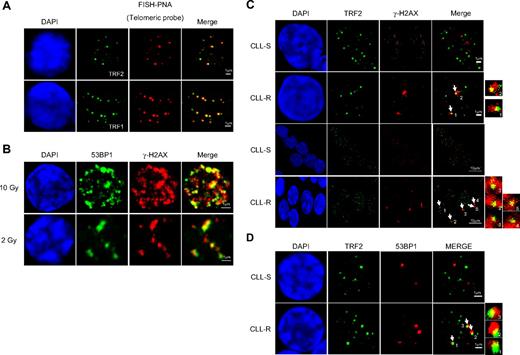
In a recent study, we showed that the mean telomere length in CLL-R cells reaches a critical value less than 4 kb,20 which in other human cells could induce DNA damage signals and senescence.5,10 We thus addressed whether short telomeres may signal DSBs in CLL-R cells. We first established experimental conditions for FISH-PNA whereby anti-TRF2 and anti-TRF1 antibodies colocalized with telomeric sequences when using (C3TA2)3–Cy3-labeled as the hybridization probe. We observed a perfect colocalization of both TRF1 and TRF2 with telomeric sequences, indicating that the labeling of these proteins could be used as marker of telomeres in nuclei (Figure 2A).
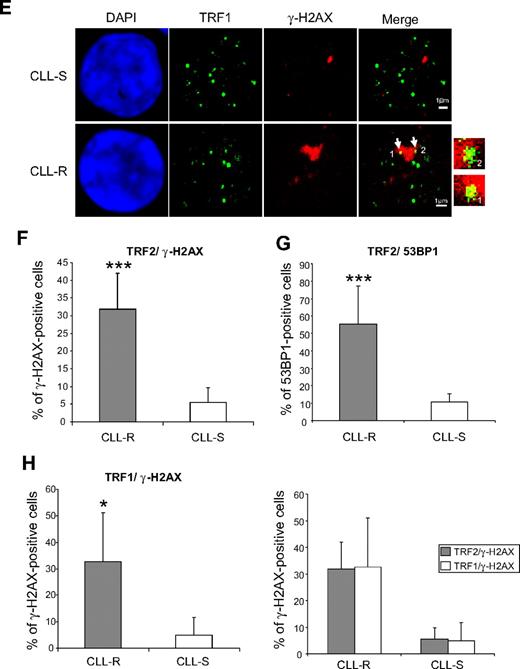
Quantification of TIF in CLL cells according to their in vitro sensitivity to apoptosis. (A) FISH with a telomere-specific (C3TA2)3–Cy3-labeled peptide nucleic acid probe (PNA; red fluorescence) and immunostaining of TRF2 or TRF1 used to control for the telomere position in the interphase nuclei of CLL cells. (B) Double immunostaining of γ-H2AX and 53BP1 in CLL cells after 2 Gy or 10 Gy irradiations used to label DNA DSBs in interphase nuclei. (C) Double immunostaining of γ-H2AX and TRF2 in CLL-R and CLL-S cells. Arrows indicate the γ-H2AX/TRF2 colocalizations. (D) Double immunostaining of 53BP1 and TRF2. Their colocalization is indicated by arrows. (E) Double immunostaining of γ-H2AX and TRF1. Their colocalization is indicated by arrows. (F) Comparisons of γ-H2AX/TRF2-positive cells between CLL-S and CLL-R. Positivity was assigned when more than or equal to 50% γ-H2AX foci colocalized with TRF2 in one cell. Bars represent the mean percentage ± SD. (G) Comparison of 53BP1/TRF2-positive cells between CLL-S and CLL-R. Bars represent the mean percentage ± SD. (H) Comparison of γ-H2AX/TRF1–positive cells between CLL-S and CLL-R and comparison of γ-H2AX–positive cells between γ-H2AX/TRF2 and γ-H2AX/TRF1 in CLL-S and CLL-R. Bars represent the mean percentage ± SD. The Mann-Whitney U test was used to assess statistical significance: *P < .01; ***P < .001
Quantification of TIF in CLL cells according to their in vitro sensitivity to apoptosis. (A) FISH with a telomere-specific (C3TA2)3–Cy3-labeled peptide nucleic acid probe (PNA; red fluorescence) and immunostaining of TRF2 or TRF1 used to control for the telomere position in the interphase nuclei of CLL cells. (B) Double immunostaining of γ-H2AX and 53BP1 in CLL cells after 2 Gy or 10 Gy irradiations used to label DNA DSBs in interphase nuclei. (C) Double immunostaining of γ-H2AX and TRF2 in CLL-R and CLL-S cells. Arrows indicate the γ-H2AX/TRF2 colocalizations. (D) Double immunostaining of 53BP1 and TRF2. Their colocalization is indicated by arrows. (E) Double immunostaining of γ-H2AX and TRF1. Their colocalization is indicated by arrows. (F) Comparisons of γ-H2AX/TRF2-positive cells between CLL-S and CLL-R. Positivity was assigned when more than or equal to 50% γ-H2AX foci colocalized with TRF2 in one cell. Bars represent the mean percentage ± SD. (G) Comparison of 53BP1/TRF2-positive cells between CLL-S and CLL-R. Bars represent the mean percentage ± SD. (H) Comparison of γ-H2AX/TRF1–positive cells between CLL-S and CLL-R and comparison of γ-H2AX–positive cells between γ-H2AX/TRF2 and γ-H2AX/TRF1 in CLL-S and CLL-R. Bars represent the mean percentage ± SD. The Mann-Whitney U test was used to assess statistical significance: *P < .01; ***P < .001
We next sought whether telomeres could activate DSB signaling in colocalization experiments with TRF2 and γ-H2AX or 53BP1 in cell samples from 10 CLL-R and 10 CLL-S patients. Cells were considered to be positive when 50% or more of the γ-H2AX or 53BP1 foci colocalized with TRF2. We first verified the anti–γ-H2AX and anti-53BP1 colocalizations at DSBs in nuclei of CLL cells irradiated at different doses. We observed colocalizations between 53BP1 and γ-H2AX, as well as a significant increase of the numbers of foci in a radiation dose-dependent manner (Figure 2B). We then analyzed a mean of 50 cells per patient and observed a significant increase (U test, P < .05) in TIF-positive cells in CLL-R compared with CLL-S (32.8% ± 10% in CLL-R vs 5.5% ± 4% in CLL-S for γ-H2AX/TRF2-positive cells and 55.6% ± 21% in CLL-R vs 10.6% ± 4% for 53BP1/TRF2-positive cells), indicating that short telomeres are recognized as DSBs in CLL-R (Figure 2C-D,F-G). It has been reported that a marked discrepancy in TRF1 and TRF2 colocalizations with γ-H2AX may occur in senescent human fibroblasts.6 We did not observe this in CLL-R cells, however, where the γ-H2AX/TRF1 and γ-H2AX/TRF2 colocalization patterns were found to be identical (32.6% ± 18% γ-H2AX/TRF1 vs 32.8% ± 10% γ-H2AX/TRF2-positive cells in CLL-R and 4.8% ± 6% γ-H2AX/TRF1 vs 5.5% ± 4% γ-H2AX/TRF2-positive cells in CLL-S), further indicating that the results obtained for TRF2/γ-H2AX colocalization analyses are representative of the recognition of telomeres in CLL-R cells as DNA damage (Figure 2E,H).
Short telomeres are recognized by Ku70 and phospho-S2056-DNA-PKcs, 2 essential components during the first-step recognition and processing of DSBs by the NHEJ pathway
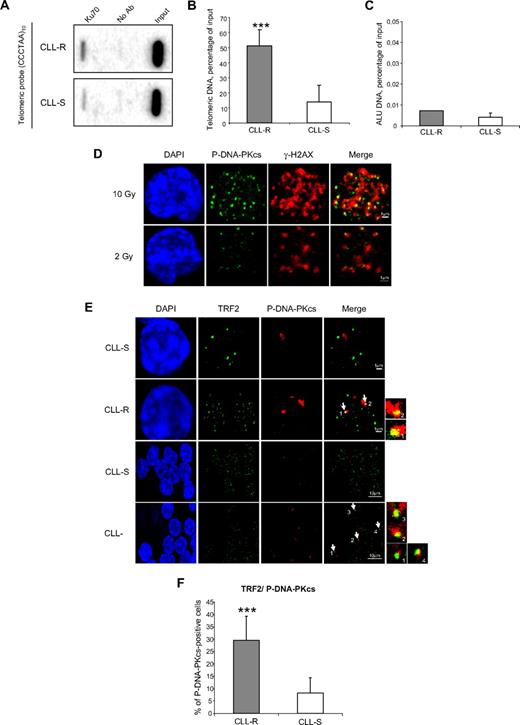
The main threat posed by dysfunctional mammalian telomeres is their possible recognition by DNA repair proteins, leading to NHEJ-mediated processing of 3′ overhangs and the generation of chromosomal abnormalities.8 DNA repair by the NHEJ system is dependent on the formation of the DNA-PK complex containing the Ku70/Ku80 heterodimer and DNA-PK catalytic subunit (DNA-PKcs). Ku70 has been shown to stimulate the end-to-end fusion of dysfunctional telomeres in mouse cells.27 We quantified the presence of Ku70 at telomeric chromatin (expressed as a percentage of the total telomeric DNA) by ChIP assay in 13 CLL patients (5 CLL-R and 8 CLL-S; Figure 3A). A significant increase (U test; P < .05) in Ku70 binding was observed in CLL-R compared with CLL-S cells (51% ± 10% vs 14% ± 11%; Figure 3B-C).
Ku70 and phospho-S2056-DNA-PKcs localize at dysfunctional telomeres in CLL-R cells. (A) Ku70 protein levels at telomeric chromatin in CLL cells. ChIP assays were carried out on 5 CLL-R and 8 CLL-S samples. To exclude nonspecific binding between beads and crosslinked chromatin, a ChIP assay was performed in the absence of any antibody (No Ab). Input represents the total DNA used in each assay. (B) Quantification of the telomeric signals expressed as a percentage of the total telomeric DNA. Bars represent the mean percentage ± SD. (C) Alu probe control analysis. The quantification of the telomeric signal is expressed as described for the telomeric probe. (D) Double immunostaining of γ-H2AX and of phospho-S2056-DNA-PKcs in CLL cells after 2 Gy and 10 Gy irradiations was used as a nuclear labeling control for DNA DSBs. (E) Double immunostaining of phospho-S2056-DNA-PKcs and TRF2. Colocalizations are indicated by arrows. (F) Comparisons of phospho-DNA-PKcs-positive cells between CLL-S and CLL-R samples. Bars represent the mean percentage ± SD. The Mann-Whitney U test was used to assess statistical significance: ***P < .001
Ku70 and phospho-S2056-DNA-PKcs localize at dysfunctional telomeres in CLL-R cells. (A) Ku70 protein levels at telomeric chromatin in CLL cells. ChIP assays were carried out on 5 CLL-R and 8 CLL-S samples. To exclude nonspecific binding between beads and crosslinked chromatin, a ChIP assay was performed in the absence of any antibody (No Ab). Input represents the total DNA used in each assay. (B) Quantification of the telomeric signals expressed as a percentage of the total telomeric DNA. Bars represent the mean percentage ± SD. (C) Alu probe control analysis. The quantification of the telomeric signal is expressed as described for the telomeric probe. (D) Double immunostaining of γ-H2AX and of phospho-S2056-DNA-PKcs in CLL cells after 2 Gy and 10 Gy irradiations was used as a nuclear labeling control for DNA DSBs. (E) Double immunostaining of phospho-S2056-DNA-PKcs and TRF2. Colocalizations are indicated by arrows. (F) Comparisons of phospho-DNA-PKcs-positive cells between CLL-S and CLL-R samples. Bars represent the mean percentage ± SD. The Mann-Whitney U test was used to assess statistical significance: ***P < .001
Autophosphorylation of DNA-PKcs at serine 2056 occurs after irradiation-induced DNA damage and is required for the rejoining of DNA DSBs.28,29 This phosphorylated form of DNA-PKcs may therefore reflect the first step in NHEJ activation. Accordingly, we measured the percentage of cells from 20 CLL patient samples displaying phospho-S2056-DNA-PKcs/TRF2 colocalizations (10 CLL-R and 10 CLL-S). As control, we first verified that phospho-S2056-DNA-PKcs alone was a good marker for DSBs in nuclei of CLL cells by double staining of irradiated cells with anti–γ-H2AX and antiphospho-S2056-DNA-PKcs. We observed a colocalization between these 2 signals, as well as an increase of both signals' intensity in a dose-dependent manner (Figure 3D). We then analyzed a mean of 50 cells per patient and observed a significant (U test, P < .05) increase in TIF-positive cells in the CLL-R compared with CLL-S cases (29.5% ± 9% vs 8.1% ± 6%; Figure 3E-F). Together with the results of our ChiP experiments showing an increased level of Ku70, these results may be indicative of NHEJ activation at short telomeres in CLL-R cells.
Short telomeres are associated with telomere deletions and with cytogenetic markers of a poor prognosis in CLL-R cells
Telomere dysfunction involving aberrant recognition by DNA repair systems inevitably leads to genomic instability. The chromosomal aberrations that are typical of telomeric dysfunction can take different forms (ie, end-to-end fusion, chromosomes lacking a telomere signal on one or both sister chromatids, referred as one telomere loss and terminal deletion, respectively); extrachromosomal telomere signals; telomere doublets on 1 or 2 sister chromatid ends (referred as one telomere doublet and terminal duplication, respectively); sister chromatid fusion; and telomeric DNA-containing double-minute chromosome.30 We assayed for these types of chromosomal aberrations in CLL cells in our current study to assess the impact of telomeric dysfunction on genomic integrity. We performed FISH analysis of telomeric sequences in metaphase CLL cells from 29 patients (13 CLL-R, 11 CLL-S, and 5 CLL cases for whom the DNA damage-induced apoptosis sensitivity levels had not been determined) and 3 healthy donors. In all cases, metaphase stimulation with CpG oligonucleotides in addition to interleukin-2 or TPA had occurred. This allowed us to analyze 20 metaphases per patient sample as recommended in a European consensus protocol.18
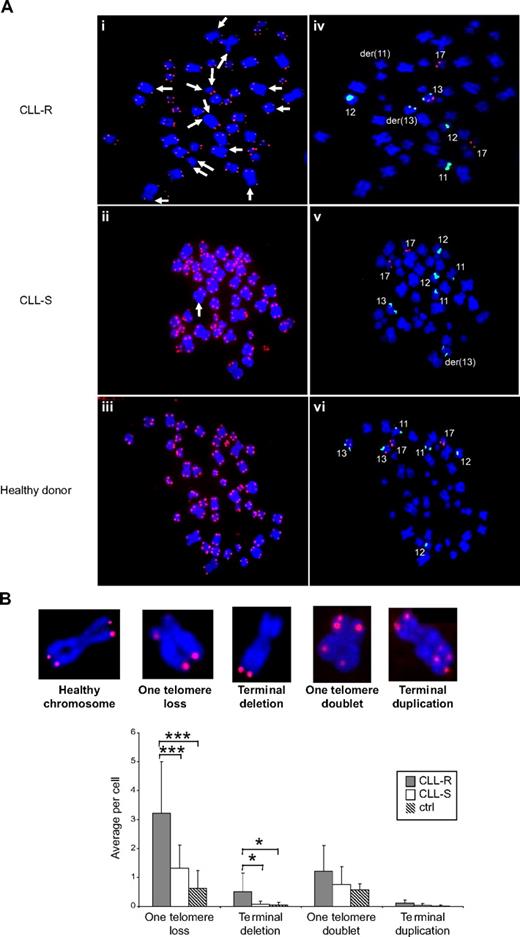
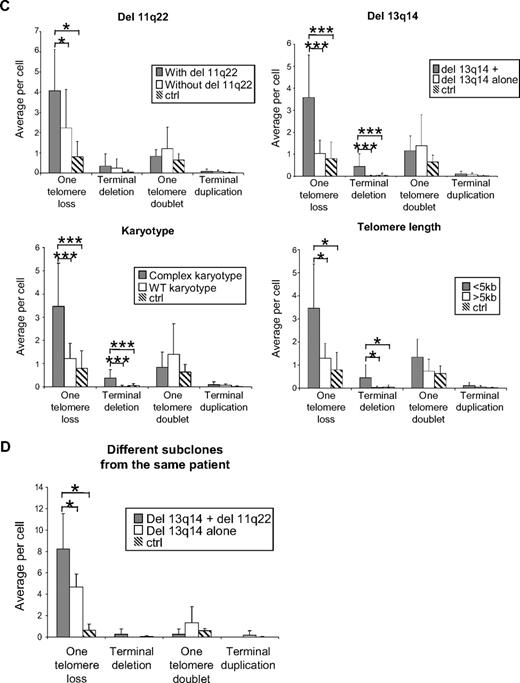
We did not observe any dicentric chromosomes or telomeric DNA-containing double-minute chromosome (Figure 4A). As shown in Figure 4A-B, although no significant differences were observed in terms of telomere duplication, we noted a significant increase (P < .01) in telomere loss on one or both sister chromatids in CLL-R compared with CLL-S (a mean of 3.5 ± 2 single telomere losses per metaphase in CLL-R vs 1.4 ± 0.9 in CLL-S and 0.5 ± 0.6 terminal deletions per metaphase in CLL-R vs 0.07 ± 0.1 in CLL-S). We did not observe any differences between CpG oligonucleotide and TPA stimulation of cells from a same patient (data not shown), suggesting that these experiments can be directly compared. Telomere losses on one or both sister chromatids are associated with the presence of cytogenetic aberrations, which are also markers of poor disease outcomes. These include del13q14 associated with del 11q22 or 17p13, complex karyotypes (≥ 3 abnormalities), short telomeres, and deletion 11q22 (associated with an increase in one telomere losses only) (Figure 4C). Moreover, we compared 2 subclones from the same patient (CLL-12): one with a del 13q14 only and the other with a del 13q14 associated with del 11q22. We observed a significant (U test; P < .05) increase in telomere losses on one sister chromatid in the subclone with a del 13q14 associated with a del 11q22 compared with the subclone showing a del 13q14 alone (Figure 4D).
Chromosomal aberrations and telomere dysfunction in CLL cells according to their in vitro sensitivity to apoptosis. (Ai-iii) Representative examples of CLL cells in metaphase from CLL-R (Ai), CLL-S (Aii), and healthy B cells from healthy donors (Aiii). Telomeres are labeled with a telomere-specific (C3TA2)3–Cy3-labeled peptide nucleic acid probe (PNA; red fluorescence). Chromosomes are counterstained with DAPI (blue). Arrows indicate the sites of genomic aberrations resulting from telomere dysfunction. (Aiv-vi) De novo labeling of the metaphase cells shown in panels Ai-iii with CLL multicolor probes to detect chromosomes 11, 12, 13, and 17 and specific chromosomal aberrations therein. Chromosome 11 is labeled green on region 11q22–23. Chromosome 12 is labeled green on centromeric sequences. Chromosome 13 is labeled aqua on region 13q34 and red on region 13q14. Chromosome 17 is labeled red on region 17p13. der indicates abnormal chromosome (derivated). In these cases, this mark shows events of deletions. (B) Representative examples of chromosomal aberrations at the telomeres in CLL cells. Comparison of the average number of telomeric aberrations between CLL-R, CLL-S, and B cell samples from healthy donor controls. Bars represent the mean ± SD. (C) Comparison of the average number of telomeric aberrations and other defined chromosomal aberrations in CLL cell samples. Ctrl indicates control (healthy donors); del 11q22, 11q22 deletion; del 13q14+, 13q14 deletion associated with del 11q22 or del 17p13 detected by FISH; and complex karyotype, karyotype with more than or equal to 3 chromosomal aberrations. Bars represent the mean ± SD. (D) Comparison of average number of telomeric aberrations between 2 CLL cell subclones from the same patient (CLL-12). One subclone has a 13q14 deletion alone, whereas the second has both a 13q14 and 11q22 deletion. ctrl indicates cells from healthy donors. Bars represent the mean ± SD. The Mann-Whitney U test was used to assess statistical significance: *P < .01; ***P < .001
Chromosomal aberrations and telomere dysfunction in CLL cells according to their in vitro sensitivity to apoptosis. (Ai-iii) Representative examples of CLL cells in metaphase from CLL-R (Ai), CLL-S (Aii), and healthy B cells from healthy donors (Aiii). Telomeres are labeled with a telomere-specific (C3TA2)3–Cy3-labeled peptide nucleic acid probe (PNA; red fluorescence). Chromosomes are counterstained with DAPI (blue). Arrows indicate the sites of genomic aberrations resulting from telomere dysfunction. (Aiv-vi) De novo labeling of the metaphase cells shown in panels Ai-iii with CLL multicolor probes to detect chromosomes 11, 12, 13, and 17 and specific chromosomal aberrations therein. Chromosome 11 is labeled green on region 11q22–23. Chromosome 12 is labeled green on centromeric sequences. Chromosome 13 is labeled aqua on region 13q34 and red on region 13q14. Chromosome 17 is labeled red on region 17p13. der indicates abnormal chromosome (derivated). In these cases, this mark shows events of deletions. (B) Representative examples of chromosomal aberrations at the telomeres in CLL cells. Comparison of the average number of telomeric aberrations between CLL-R, CLL-S, and B cell samples from healthy donor controls. Bars represent the mean ± SD. (C) Comparison of the average number of telomeric aberrations and other defined chromosomal aberrations in CLL cell samples. Ctrl indicates control (healthy donors); del 11q22, 11q22 deletion; del 13q14+, 13q14 deletion associated with del 11q22 or del 17p13 detected by FISH; and complex karyotype, karyotype with more than or equal to 3 chromosomal aberrations. Bars represent the mean ± SD. (D) Comparison of average number of telomeric aberrations between 2 CLL cell subclones from the same patient (CLL-12). One subclone has a 13q14 deletion alone, whereas the second has both a 13q14 and 11q22 deletion. ctrl indicates cells from healthy donors. Bars represent the mean ± SD. The Mann-Whitney U test was used to assess statistical significance: *P < .01; ***P < .001
Discussion
One of the crucial parameters for telomere function and maintenance is the presence of a G-rich single-strand 3′ overhang, which maintains chromosome end into an enclosed structure through the formation of the T-loop or via G-quadruplex formation.31-33 The T-loop structure should prevent telomeres from being recognized as DNA DSBs and thus from activating cell cycle checkpoints, inappropriate DNA repair, and cell death. The loss of a 3′ overhang could thus be critical in terms of telomere maintenance and genomic stability. We observe that telomere shortening is associated with a significant loss of 3′ overhangs in CLL-R compared with CLL-S (Figure 1). We then hypothesize that short telomeres may be limited in their ability to adopt an enclosed structure in resistant cells and thus could be recognized as DSBs by the DNA signaling and repair systems. To test this hypothesis, we evaluated TIF formation by measuring the percentage of cells showing telomeric localization of 53BP1 and γ-H2AX. We observed a significant increase in the proportion of cells positive for TRF2/53BP1 and TRF2/γ-H2AX colocalizations in CLL-R cells (Figure 2C-D,F-G). This indicates that short telomeres should be uncapped, thus allowing their recognition as DSBs. The depletion of TRF2 at dysfunctional telomeres could induce a bias in TRF2/γ-H2AX colocalization compared with TRF1/γ-H2AX or telomeric DNA sequence/γ-H2AX.6 This is unlikely in CLL cells, however, as we have already shown previously17 that there is no depletion of TRF2 at short telomeres in CLL-R cells. In addition, in our current experiments, we did not observe any differences between TRF1/γ-H2AX and TRF2/γ-H2AX colocalizations in either CLL-R or CLL-S cells (Figure 2E,H).
We also demonstrate in the present analyses that there is a significant increase in Ku70 and phospho-S2056-DNA-PKcs (2 proteins implicated in the NHEJ repair system) at the telomeres in CLL-R cells (Figure 3). Whereas the phosphorylation on S2056 of DNA-PKcs is a hallmark of its activation in response to a DSB, the significance of the Ku70 increase we observed seems to be more complex because of its multiple functions at the telomeres (ie, telomere protection, telomere length regulation, and end-to-end fusion).22 Indeed, any increase in Ku70 could reflect an increase in local heterochromatin concentration through the interaction of this protein with HP1α.34 It could also be speculated that, in CLL-R cells, Ku70 should function as a DNA repair protein as critically short telomere lengths have been shown to activate DNA repair systems,6,35 and a 3′ overhang loss could be the result of NHEJ activation.8,26,36 This possibility is further supported by the telomeric localization of 53BP1 and γ-H2AX, 2 hallmarks of DSB signaling. Increases in the DNA end-binding activity of the Ku70/80 heterodimer, which have been previously observed in CLL-R cells,21 may therefore be the result of a permanent activation by critically short telomeres mimicking DNA strand breaks. In addition, DNA-PKcs seems to contribute to drug resistance in CLL as the use of specific inhibitors sensitizes in vitro CLL cells to drug-induced DNA damage.21,37,38 Consistent with our results, critically short telomeres may activate autophosphorylation of DNA-PKcs, thus providing an explanation for its activation in diseases with poor outcomes.
Malignant CLL cells display chromosomal aberrations in more than 80% of cases, and these abnormalities may evolve in the course of the disease and be indicative of its aggressiveness.39-41 Roos et al have observed that short telomeres are associated with genetic complexity and high-risk chromosomal aberrations17 and speculated a cause-and-effect link between these 2 phenomena in the poor prognosis CLL subgroup. Consistently, short telomeres may be involved in genomic instability because of telomeric dysfunction.8,27 In the current study, we observed an activation of the DNA damage response by short telomeres through the induction of DNA damage signaling and the first steps in the NHEJ process. We then evaluated the potential presence of chromosomal aberrations, which may appear as a consequence of telomere dysfunction in CLL. Classically, uncapped telomeres may activate NHEJ and result in chromosomal end-to-end fusions, but these aberrations have never been reported to occur in CLL. We show from present experiments, however, that telomeric deletions and duplications, 2 other hallmarks of telomeric dysfunction, occur in CLL-R cells with short telomeres (Figure 4A-B). We found a significant increase in telomeric deletions on one or both sister chromatids in CLL-R compared with CLL-S (Figure 1B). We cannot exclude the possibility in these cases that a small part of the telomere is still present but remains undetectable by FISH. However, the fact that only one or 2 telomeres are undetectable on the same chromosome is indicative of a loss of genetic material and thus can be considered a deletion event.
Terminal deletions occurring in cells with increased telomeric localizations of phospho-S2056-DNA-PKcs and Ku70 are probably mediated through an NHEJ pathway, already shown to be deregulated in resistant CLL cells.21,22 In consequence, we could expect that proteins implicated in DNA damage signalization should localize with faint or undetectable telomeric signals. However, we have observed good colocalizations between telomeric and DNA damage signaling proteins. One explanation could be that some of ultrashort and dysfunctional telomeres may form the clusters, which still bind TRF1 and TRF2.42 Furthermore, clustering of telomeres could also favor recombination events and explain rapid losses of telomeric sequences and losses of telomeric signals.43 This hypothesis implies Ku70 in a new type of interactions with ultrashort telomeric clusters in resistant CLL cells, which warrant further characterizations.
Finally, we observed that terminal deletions also occur in cells with a 13q14 deletion associated with del 11q22 and/or del 17p53 detected by FISH, complex karyotype, short telomeres, and deletion 11q22, which are markers of a poor prognosis in CLL (Figure 4C)
It is noteworthy that, in one CLL patient (CLL-12), we observed a significant (U test; P < .05) increase in telomere loss on one sister chromatid in a subclone with a del 13q14 associated with del 11q22, which was not observed in another subclone with a del 13q14 alone derived from this person (Figure 4D). As del 13q14 alone is a marker of a good prognosis and del 13q14 associated with a del 11q22 is a marker of a bad prognosis, we hypothesize that the subclone with del 13q14 associated with a del 11q22 is the more aggressive. This result further emphasizes the likelihood that telomere losses are associated with disease aggressiveness and may be clinically relevant in terms of distinguishing CLL subclones from an individual patient. Telomere loss and ATM deficiency have been previously reported to occur in ataxia-telangiectasia patients.44 It is thus tempting to speculate that a similar mechanism could occur in CLL patients with ATM deletion.
Hence, it should be considered that telomere dysfunction in CLL causes a novel type of chromosomal abnormality at the ends of chromosomes, which is associated with multiple cytogenetic aberrations and shorter mean telomere length, both known indicators of an aggressive form of CLL and a poor prognosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the leukemia patients and healthy blood donors who voluntarily participated in this study.
This work was supported by Association pour la Recherche contre le Cancer and Commissariat à l'Energie Atomique. T.B. was suppored by Commissariat à l'Energie Atomique and Association pour la Recherche contre le Cancer fellowship.
Authorship
Contribution: T.B., F.N.-K., and J.D. designed research and interpreted data; T.B. performed research and analyzed data; A.G. contributed vital analytical tools; and T.B., H.M.-B., and J.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jozo Delic, Laboratoire d'Onco-Hématologie, iRCM, Direction des Sciences du Vivant, Commissariat à l'Energie Atomique, 18 Av du Panorama, Fontenay aux Roses, 92265 Paris, France; e-mail: jozo.delic@cea.fr.