Abstract
B-cell lymphoma is a clonal expansion of neoplastic cells that may result in fatal outcomes. Here, we report the in vivo targeting and growth inhibition of aggressive A20 murine B-cell lymphoma by idiotype-specific peptide pA20-36. pA20-36 was selected from random peptide libraries and bound specifically to the B-cell receptor (BCR) of A20 cells in mice engrafted with A20 lymphoma, as shown by histology and positron emission tomographic analysis. BCR cross-linking of A20 cells with pA20-36 resulted in massive apoptosis of targeted tumor cells and in an increased survival of the diseased animals without any detectable evidence of toxicity. The pA20-36 treatment reverted the immune suppression of the tumor microenvironment as shown by reduced expression of vascular endothelial growth factor, interleukin-10, and transforming growth factor-β cytokines together with a lower number of CD11b+Gr-1+ inhibitor myeloid-derived suppressor cells and Foxp3+CD4+ Treg cells. Furthermore, pA20-36 treatment was associated with an increased number of tumor-infiltrating, activated CD8+ T cells that exerted a tumor-specific cytolytic activity. These findings show that a short peptide that binds specifically to the complementarity-determining regions of the A20 BCR allows in vivo detection of neoplastic cells together with significant inhibition of tumor growth in vivo.
Introduction
Therapeutic advance in the clinical treatment of B-cell lymphoma has taken advantage from the development of targeted therapy based on monoclonal antibodies (MAbs), including CD20, CD22, and CD52, which target cell-surface receptors of neoplastic cells.1 Therapeutic MAbs have been shown to be effective either alone or in combination with chemotherapeutics, and as vehicle to deliver radionuclides or toxins to neoplastic cells. However, the effectiveness of therapeutic MAbs is limited by their extended half-life and toxicity as a consequence of nonspecific binding to the reticule-endothelium and to unaffected organs, including the bone marrow.2 This drawback is critical in the case of antibodies used as a vehicle to deliver radionuclides, cytotoxic drugs, or toxins to tumor sites. Hence, although the introduction of therapeutic MAbs has broadened the treatment options for subjects with B-cell lymphoma, a substantial number of patients experience a clinical relapse as a consequence of a lack or poor response to the treatments. This grim picture calls for new strategies to improve the in vivo detection and treatment of neoplastic B cells.
Compared with antibodies, peptides are attractive alternatives for cell targeting and in vivo cancer imaging and therapy. Indeed, peptides, which are considerably smaller than antibodies and antibody fragments, do not bind to the reticule-endothelium and do not elicit a significant immune response on repeated inoculation.3 Peptides with blocked N- or C-terminus may acquire a cyclic, constrained conformation and may include unnatural and D-amino acids that allow an extended half-life in vivo. In addition, peptides are endowed with several functions, including multivalence for target moieties and cargo delivery, including radionuclides, cytotoxic drugs, and toxins that target cell surface receptors of cancer cells. Examples of tumor-specific peptides include somatostatin4 and bombesin/gastrin-releasing peptide,5 which exhibit high affinities for the cognate cell-surface receptors. Peptides also facilitate the transport of cytotoxic compounds and stretches of nucleic acid into specific tumor tissues.6
Random peptide libraries (RPLs) allow the selection of therapeutic peptides for tumor cell-surface receptors.7 RPLs express a large collection of peptide sequences (≥ 108) that mimic both linear and conformational epitopes of folded protein domains, which are displayed in multiple copies fused to phage coat proteins.8,9 In addition, the linear or conformational epitopes displayed on phages can be translated in short cyclic peptides with little or no loss of binding specificity.10,11
The hypervariable regions (idiotypes) of the surface immunoglobulin (Ig) B-cell receptor (Ig-BCR) expressed by lymphoma cells result from the rearrangement of the immunoglobulin genes and are unique for a given clonal B-cell population. In this settings, the idiotypic determinants of the Ig-BCR expressed by lymphoma cells function as a specific tumor antigen that may be exploited for cell-specific targeted therapy.12 Previous studies using RPLs identified peptides (Id-peptide) that exhibited antitumor activity against human and murine B lymphoma by triggering apoptosis of target cells in vitro when administered as dimers or tetramers.13,14
In this study, we evaluated the tumor targeting properties of an Id-peptide, which was selected from RPLs by using as a bait the Ig-BCR of the highly aggressive murine A20 B lymphoma.15,16 The RPL-selected Id-peptide specifically bound the A20 cells both in cell cultures and in A20-engrafted syngenic immune competent mice, as shown by fluorescence-activated cell sorting, confocal microscopy, and positron emission tomography (PET). Cross-linking of the A20 BCR with Id-peptide resulted in the massive apoptosis of targeted cells in vitro by a caspase-dependent mechanism. Consistently, inoculation of the Id-peptide in A20-engrafted mice caused apoptosis of tumor cells and resulted in tumor growth inhibition and extended survival of diseased animals. Because apoptotic tumor cells are a source of antigens for the immune system,17 we also performed an extensive analysis of tumor microenvironment to evaluate the effect of Id-peptide treatment on tumor immunity. Indeed, the Id-treatment reverted the immunosuppressive characteristics of the tumor microenvironment and stimulated a tumor-specific CD8+ T-cell activity that contributed to the peptide-driven antitumor activity.
Methods
Cell lines, immunoglobulin purification, and peptides
The A20 and 5T33MM cell line growth conditions are reported in supplemental Materials and Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The A20-Ig was purified from the culture supernatants by using the Mab Trap antibody purification Kit (GE Healthcare). All peptides were purchased from Caslo Laboratory ApS. The amino acid sequence of the control scrambled peptide (pCNT) was DQEWCKTISFEPCLEN.
Generation and screening of RPLs
We generated 2 RPLs consisting of nona-peptides (f88-C7C) and dodeca-peptides (f88-12) displayed on the N-terminus of pVIII major coat protein of filamentous bacteriophage f88-tet, as previously described.9,18 A third f88-Cys6 RPL was a kind gift of G. P. Smith (University of Missouri–Columbia).18 Screening was performed by using A20-Ig purified from culture supernatants. Details for constructions and screening of RPLs, as well as enzyme-linked immunoabsorbent assay (ELISA) procedures are reported in the supplemental data.
Peptide binding
Binding of peptides to purified immunoglobulins, mice sera, or cells, and competitive peptide binding inhibition were evaluated by ELISA and described in the supplemental data.
Cell viability, cell cycle, and apoptosis
A20 cells (2 × 107/mL) were incubated with biotinylated peptides (20 μg/mL). For peptide tetramerization, streptavidin (100 μg/mL) was added to the cell cultures. Cell viability was determined by trypan blue dye exclusion; apoptosis was measured with annexin V–fluorescein isothiocyanate (FITC) staining (Apoptosis Kit; Miltenyi Biotec), and cell cycle was analyzed with the use of the CycleTest Plus DNA Reagent Kit (BD Biosciences). Caspase activities were measured by luminometric assay (Promega).
Histologic analysis
Detailed procedures for histologic evaluation of metastasis, apoptosis, and intratumoral microvessel density are reported in the supplemental data.
In vivo studies
The Institutional Animal Care and Use Committee of the University “Magna Græcia” Catanzaro approved the animal protocols, according to the guidelines of the National Institute of Health, Italy. Detailed procedures for in vivo toxicity, MicroPET imaging, and tumor growth inhibition analysis are reported in supplemental data.
Analysis of tumor-infiltrating leukocytes
Analysis of tumor-infiltrating leukocytes was performed by flow cytometry. Experimental details are reported in supplemental data.
T-cell functional assays
CD8+ T cells were isolated from tumor-infiltrating lymphocytes with the use of an indirect magnetic labeling system (magnetic cell sorting; Miltenyi Biotec), according to the manufacturer's instruction. Purified CD8+ T cells were labeled with 5,6-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes) and resuspended in RPMI 1640 supplemented with 5% fetal calf serum. Purified CD8+ T cells (5 × 104 cells/well) were mixed with cultured A20 or 5T33MM cells (2 × 105 cells/well) and seeded into a 96-well plate. After 1 week of incubation, CFSE-labeled cells were analyzed by flow cytometry. To assess the tumor-specific cytolytic activity of the tumor-infiltrating CD8+ cells, a time-resolved fluorometric assay (DELFIA; PerkinElmer) was performed according to the manufacturer's instruction.
Results
Identification of peptide ligands of the A20 monoclonal immunoglobulin
To identify peptide ligands of surface IgG (sA20-Ig) of A20 B-lymphoma cell line,15,16 we screened 3 f88 phage-displayed RPLs using as bait purified A20-Ig, as previously reported.9,19 After 3 rounds of panning with A20-Ig, 22 phage clones, which tested positive for A20-Ig binding in ELISA, were selected (Table 1); among these, A20-1, A20-6, and A20-36 showed the highest binding affinities to A20-Ig (Figure 1A left). Synthetic peptides pA20-1, pA20-6, and pA20-36 corresponding to the insert peptides of A20-1, A20-6, and A20-36 phage clones, respectively, showed a concentration-dependent binding to A20-Ig (Figure 1A middle), whereas testing was negative for mouse polyclonal immunoglobulins (Figure 1A right).
Peptide ligands isolated from phage-display libraries
| Phage clones . | Sequence . | Absorbance* . |
|---|---|---|
| f88-C7C library | ||
| A20-2 | CSSLVWYRC | 0.45 |
| A20-5 | CKSSSPIIC | 0.88 |
| A20-6 | CKSSSPIVC | 1.20 |
| A20-12 | CTLTPWYMC | 0.55 |
| A20-28 | CHTQKASVC | 0.38 |
| A20-60 | CQPWYSGLC | 0.39 |
| f88-12 library | ||
| A20-1 | QVYKQSGSPVYF | 1.15 |
| A20-3 | HYCTSPVVASYA | 0.66 |
| A20-9 | QVYKQSGSPVYF | 1.05 |
| A20-13 | STLPWYQQLSAP | 0.35 |
| A20-15 | NFPIRAWYQDVS | 0.43 |
| A20-41 | QVYKQSGPSVYF | 0.58 |
| A20-42 | QVYKQSGPSVYF | 0.55 |
| A20-43 | QVYKQSGPSVYF | 0.55 |
| f88-Cys6 | ||
| A20-7 | ENPICELSKQECDFWT | 0.73 |
| A20-17 | DRGGCKLSVNGCVGNG | 0.69 |
| A20-36 | EYVNCDNLVGNCVIRG | 1.88 |
| A20-37 | EYVNCDNLVGNCVIRG | 1.65 |
| A20-44 | EYVNCDNLVGNCVIRG | 1.78 |
| A20-45 | EYVNCDNLVGNCVIRG | 1.80 |
| A20-46 | EYVNCDNLVGNCVIRG | 1.78 |
| A20-52 | EYVNCDNLVGNCVIRG | 1.80 |
| Wild-type | 0.04 |
| Phage clones . | Sequence . | Absorbance* . |
|---|---|---|
| f88-C7C library | ||
| A20-2 | CSSLVWYRC | 0.45 |
| A20-5 | CKSSSPIIC | 0.88 |
| A20-6 | CKSSSPIVC | 1.20 |
| A20-12 | CTLTPWYMC | 0.55 |
| A20-28 | CHTQKASVC | 0.38 |
| A20-60 | CQPWYSGLC | 0.39 |
| f88-12 library | ||
| A20-1 | QVYKQSGSPVYF | 1.15 |
| A20-3 | HYCTSPVVASYA | 0.66 |
| A20-9 | QVYKQSGSPVYF | 1.05 |
| A20-13 | STLPWYQQLSAP | 0.35 |
| A20-15 | NFPIRAWYQDVS | 0.43 |
| A20-41 | QVYKQSGPSVYF | 0.58 |
| A20-42 | QVYKQSGPSVYF | 0.55 |
| A20-43 | QVYKQSGPSVYF | 0.55 |
| f88-Cys6 | ||
| A20-7 | ENPICELSKQECDFWT | 0.73 |
| A20-17 | DRGGCKLSVNGCVGNG | 0.69 |
| A20-36 | EYVNCDNLVGNCVIRG | 1.88 |
| A20-37 | EYVNCDNLVGNCVIRG | 1.65 |
| A20-44 | EYVNCDNLVGNCVIRG | 1.78 |
| A20-45 | EYVNCDNLVGNCVIRG | 1.80 |
| A20-46 | EYVNCDNLVGNCVIRG | 1.78 |
| A20-52 | EYVNCDNLVGNCVIRG | 1.80 |
| Wild-type | 0.04 |
Peptide insert sequences of phage clones isolated from 3 RPLs by affinity purification with A20-Ig.
ELISA absorbance values of single phage clones were expressed as the difference between OD405nm and OD620nm.
In vitro–specific targeting of A20-Ig peptide ligands. (A) ELISA binding analysis of selected phages clones (left) or phage derived N-biotinylated synthetic peptides (middle) to purified A20-Ig. Peptide binding to polyclonal mouse immunoglobulins is also shown (right). Absorbance was calculated as the difference between OD405nm and OD620nm. Mean absorbance values ± SEMs of 4 independent experiments are shown; RU indicates relative units. The wild-type f88-4 phage, or a scrambled peptide (pCNT) was used as a control for the binding analysis of the selected phage clones or peptides, respectively. (B) Binding of FITC-conjugated peptides to A20 target cells by flow cytometry. Values are the mean fluorescent intensities (MFIs), representative of 2 independent experiments (n = 4). (C) Surface plasmon resonance analysis of the binding of the pA20-36 to A20-Ig. (D) Colocalization of FITC-conjugated pA20-36 peptide with the surface A20-Ig, as shown by confocal microscopy. Scale bar = 10 μm. In the diagram, the intensity profile for the pA20-36 and A20-Ig channel along a line scan through a representative cell is shown. Pictures were captured with a Leica TCS SP2 confocal microscope with a HCX PL APO 63.0×/1.40 oil UV objective (NA1.40) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS.
In vitro–specific targeting of A20-Ig peptide ligands. (A) ELISA binding analysis of selected phages clones (left) or phage derived N-biotinylated synthetic peptides (middle) to purified A20-Ig. Peptide binding to polyclonal mouse immunoglobulins is also shown (right). Absorbance was calculated as the difference between OD405nm and OD620nm. Mean absorbance values ± SEMs of 4 independent experiments are shown; RU indicates relative units. The wild-type f88-4 phage, or a scrambled peptide (pCNT) was used as a control for the binding analysis of the selected phage clones or peptides, respectively. (B) Binding of FITC-conjugated peptides to A20 target cells by flow cytometry. Values are the mean fluorescent intensities (MFIs), representative of 2 independent experiments (n = 4). (C) Surface plasmon resonance analysis of the binding of the pA20-36 to A20-Ig. (D) Colocalization of FITC-conjugated pA20-36 peptide with the surface A20-Ig, as shown by confocal microscopy. Scale bar = 10 μm. In the diagram, the intensity profile for the pA20-36 and A20-Ig channel along a line scan through a representative cell is shown. Pictures were captured with a Leica TCS SP2 confocal microscope with a HCX PL APO 63.0×/1.40 oil UV objective (NA1.40) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS.
Competition of pA20-1, pA20-6, and pA20-36 for the same binding site of A20-Ig BCR was assessed by an inhibition assay (supplemental Figure 1C). In this assay, the binding of N-biotinylated pA20-36 to A20-Ig was inhibited by increasing amounts of either unbiotinylated pA20-1 or pA20-6, albeit to a less extent compared with the inhibition of unbiotinylated pA20-36, indicating that pA20-36, pA20-1, and pA20-6 bound to the same binding site with different affinities (supplemental Figure 1C). The scrambled pCNT did not inhibit the binding of pA20-36 to A20-Ig (supplemental Figure 1C).
Flow cytometric analysis with the use of FITC-conjugated peptides indicated that pA20-1, pA20-6, and pA20-36 bound specifically to A20 cells in a dose-dependent manner (Figure 1B; supplemental Figure 1A-B). Because of the high-affinity binding to A20 cells, the pA20-36 peptide was selected for further evaluation.
The molecular form of pA20-36 was assessed by mass spectrometry. Analysis by matrix-assisted laser desorption ionization mass spectrometry showed that the peptide was present in its monomeric form, bearing an intramolecular disulfide bridge (supplemental Figure 2).
Functional outcome of pA20-36 peptide binding to sA20-Ig
Next, we tested whether the pA20-36 binding to sA20-Ig could induce calcium mobilization on BCR signaling. The pA20-36 induced calcium fluxes in A20 cells, which were significantly increased by a tetrameric form of streptavidin-linked pA20-36 (supplemental Figure 3A); scrambled pCNT peptide was ineffective.
Because Ig-BCR engagement results in antigen uptake by BCR-mediated endocytosis,20 we tested whether the binding of pA20-36 peptide to A20 cells could result in peptide internalization. The incubation of A20 cells with pA20-36 peptide resulted in peptide internalization on 1 hour of incubation, as shown by confocal microscopy (supplemental Figure 3B). The kinetics of BCR-mediated internalization were similar in the case of anti–mouse IgG F(ab)′2 and pA20-36 peptide (supplemental Figure 3C). The degree of internalization was measured as the percentage of cells no longer expressing surface peptides, assuming T50 as the time at which we observed 50% of BCR-IgG internalization. Despite a slight delay of pA20-36 internalization compared with the F(ab)′2 fragments (T50 ∼ 50 minutes vs T50 ∼ 30 minutes), similar kinetics and a complete internalization at 3 hours after stimulation were observed in the case of the F(ab)′2 fragments and the pA20-36 peptide (supplemental Figure 3C). As previously reported,20 the whole antibody was not internalized (supplemental Figure 3C).
Because cross-linking of the Ig-BCR results in growth-inhibiting signals that induced cell-cycle arrest and apoptosis in B-cell lymphoma cells,21 we tested whether the pA20-36 binding to A20 cells could affect cell division and apoptosis. To cross-link surface Ig-BCR, we used biotinylated peptides bound to tetravalent streptavidin.13,14 Cell viability was strongly impaired by in vitro incubation of A20 cells with either streptavidin-linked pA20-36 or anti-IgG F(ab)′2 antibodies up to 72 hours, whereas it was not significantly affected by monomeric pA20-36, pCNT, or streptavidin-linked pCNT peptide (Figure 2A). The cell-cycle analysis showed that the monomeric pA20-36 treatment reduced the G2 population after 24 to 48 hours and significantly increased the sub-G1 cell population (apoptotic cells; Figure 2B; supplemental Figure 4A). Moreover, the tetrameric pA20-36 treatment resulted either in a more consistent reduction of G2 population after 24 to48 hours and in 4- to 5-fold increase of the sub-G1 cell population at 48 hours, compared with the monomeric pA20-36. Consistently, the monomeric pA20-36 treatment resulted in a slight but significant increase of apoptosis, as assessed by annexin V binding, whereas it did not occur on pCNT peptide treatment (Figure 2C), although the tetrameric pA20-36 treatment resulted in a 4- to 5-fold increase of apoptotic cells compared with the monomeric pA20-36. Both monomeric and tetrameric pA20-36 treatments did not affect the growth of the 5T33MM cells (supplemental Figure 4B), a surface IgG-positive B-cell line unable to bind to pA20-36 peptide (supplemental Figure 1B), thus ruling out that the apoptotic death triggered by pA20-36 was because of a nonspecific toxic effect. The activity of the initiator caspase-9 activity was induced by pA20-36 at 24 hours of treatment followed by the activation of effector caspase-3/7 at 48 hours, whereas caspase-8 activity was unaffected; these caspases were not activated by the scrambled pCNT peptide (Figure 2D). Altogether, these results underscored that A20 tumor cells treated with pA20-36 peptide were driven toward a selective arrest of cell cycle at the G2 phase and a caspase-dependent apoptosis.
The pA20-36 peptide induces apoptosis of A20 cells in a caspase-dependent manner. A20 cells (2 × 107/mL) were incubated with monomeric pA20-36 or pCNT peptide (20 μg/mL), or with the streptavidin-coated pA20-36 or pCNT peptide, and analyzed for viability, cell-cycle profile, and apoptosis. As positive control of apoptosis, cells were cultured in the presence of goat anti–mouse IgG F(ab)′2 (20 μg/mL). (A) Cell viability is strongly affected by streptavidin-coated pA20-36. Number of viable A20 cells was measured by trypan blue. Values are the mean ± SEM of 3 independent experiments; statistical analysis was performed by Student t test. (B) The pA20-36 treatment increases sub-G1 (apoptotic) cell population. Histograms for cell-cycle profiles of 3 independent experiments with similar results, as measured by propidium iodide staining and flow cytometry. (C) The pA20-36 treatment induces apoptosis of A20 target cells. Dot plots for annexin V binding of 3 independent experiments with similar results. Numbers in the quadrants indicate the percentage of cells. (D) The pA20-36 peptide induces activation of caspases. The activity of caspase-8, caspase-9, and effector caspase 3/7 was analyzed by luminometric assay of A20 cell extracts. Values are the mean ± SEM of 3 independent experiments.
The pA20-36 peptide induces apoptosis of A20 cells in a caspase-dependent manner. A20 cells (2 × 107/mL) were incubated with monomeric pA20-36 or pCNT peptide (20 μg/mL), or with the streptavidin-coated pA20-36 or pCNT peptide, and analyzed for viability, cell-cycle profile, and apoptosis. As positive control of apoptosis, cells were cultured in the presence of goat anti–mouse IgG F(ab)′2 (20 μg/mL). (A) Cell viability is strongly affected by streptavidin-coated pA20-36. Number of viable A20 cells was measured by trypan blue. Values are the mean ± SEM of 3 independent experiments; statistical analysis was performed by Student t test. (B) The pA20-36 treatment increases sub-G1 (apoptotic) cell population. Histograms for cell-cycle profiles of 3 independent experiments with similar results, as measured by propidium iodide staining and flow cytometry. (C) The pA20-36 treatment induces apoptosis of A20 target cells. Dot plots for annexin V binding of 3 independent experiments with similar results. Numbers in the quadrants indicate the percentage of cells. (D) The pA20-36 peptide induces activation of caspases. The activity of caspase-8, caspase-9, and effector caspase 3/7 was analyzed by luminometric assay of A20 cell extracts. Values are the mean ± SEM of 3 independent experiments.
A20 B-lymphoma targeting in vivo by pA20-36
To test the in vivo targeting ability of pA20-36, we used the A20 syngenic model of murine lymphoma.15,16 BALB/c mice were subcutaneously injected with A20 cells, and at day 12 (tumor volume 63 ± 39 mm3) single-cell suspensions from tumor at the site of injection were analyzed for binding to the FITC-conjugated pA20-36 or control peptides; 80% of B220+ B cells from tumor were positive for pA20-36–FITC binding, whereas only 3.2% of them tested positive for the pCNT-FITC binding (Figure 3A top). To investigate the ability of pA20-36–FITC to detect metastatic A20 B cells, single cell suspensions from spleens of A20 tumor-bearing mice were analyzed; the pA20-36–FITC peptide specifically bound to 3.6% of B220+ spleen cells compared with 0.6% of B220+ spleen cells bound by the control peptide (Figure 3A middle); as expected, the pA20-36–FITC and pCNT-FITC peptides bound to 0.1% to 0.2% of B220+ spleen cells from tumor-free mice (Figure 3A bottom). Confocal microscopy confirmed the property of pA20-36–FITC to detect niches of metastatic cells in spleen of tumor-bearing mice (Figure 3B; supplemental Figure 5). The tumor targeting of pA20-36 peptide was also assessed by MicroPET with [18F]-radiolabeled peptides as a tracer.22 Because A20 tumor cells release the A20-Ig paraprotein, we first determined the concentration of serum A20-Ig, which might bind to pA20-36 peptide and prevent its binding to A20 cells. To this end, we developed a sensitive ELISA, which detected serum A20-Ig concentrations up to 0.1 ng/mL (supplemental Figure 6A). In A20 tumor-engrafted mice, serum A20-Ig levels ranged between 8 and 17 ng/mL at day 7 (tumors undetectable by palpation; n = 3), and 3.5 to 15.1 μg/mL at day 12 (tumor volume 63 ± 39 mm3; n = 3; supplemental Figure 6B). The MicroPET analysis was performed in mice harboring A20 B lymphoma–engrafted mice at day 12 after engraftment, when tumor volume ranged within 63 (± 39) mm3, using 10 μg of [18F]–Id-peptides corresponding to 100 to 20 molar excess over the A20-IgG serum concentration. MicroPET analysis showed a clear tumor uptake of [18F]–pA20-36 in the BALB/c mice bearing subcutaneously A20 tumors (Figure 3C left; supplemental Video 1). All time points were monitored from 10 to 60 minutes; mice bearing subcutaneously 5T33MM tumors tested negative for [18F]–peptides (Figure 3C right), indicating a specific accumulation of [18F]–pA20-36 in the A20 tumor. Conversely, the [18F]–pCNT did not show tumor uptake in the BALB/c mice bearing subcutaneously A20 tumors (Figure 3C middle panels; supplemental Video 2). This analysis also showed a nonspecific accumulation of all radiolabeled peptides in the kidney, urinary bladder, and gastrointestinal tract, which is indicative for a clearance of radiolabeled peptides through the urinary bladder and gastrointestinal tracts.
pA20-36 specifically binds primary and metastatic A20 tumor cells. (A) The pA20-36–FITC peptide specifically detects A20 tumor cells ex vivo. Cells (1 × 106) derived from the site of subcutaneously injection (top) or spleen (middle) from A20 B lymphoma–engrafted BALB/c mice at day 12 were incubated with FITC-conjugated pA20-36 or pCNT peptides (10 μg/mL), and phycoerythrin-labeled anti–mouse B220 antibody and analyzed by flow cytometry. Control splenocytes from tumor-free mice were also analyzed (bottom). Numbers in the quadrants indicate the percentage of cells. (B) The pA20-36–FITC peptide detects niches of metastatic A20 tumor cells in the spleen of A20 B lymphoma–engrafted BALB/c mice. Spleens from A20 B lymphoma–bearing mice were stained with pA20-36–FITC, anti–mouse B220 antibody, and the nuclear dye TOPO-3 and analyzed by confocal microscopy. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. (C) MicroPET images showing the specific retention of [18F]-pA20-36 in the A20 tumor. BALB/c mice bearing a palpable A20 tumor mass on the flank (5-6 mm in maximal diameter) were injected intravenously with 10 μg (8 MBq peptide) of [18F]-pA20-36 (left) or [18F]-pCNT (middle). Mice bearing a palpable 5T33MM tumor mass on the flank were used as control for the specific retention of pA20-36 (right). Images shown are coronal (top) or axial (bottom) sections of static scans of a single mouse for each group collected at 10, 30, and 60 minutes after peptide injection (see also supplemental Videos). Tumors are indicated by white arrows in all cases.
pA20-36 specifically binds primary and metastatic A20 tumor cells. (A) The pA20-36–FITC peptide specifically detects A20 tumor cells ex vivo. Cells (1 × 106) derived from the site of subcutaneously injection (top) or spleen (middle) from A20 B lymphoma–engrafted BALB/c mice at day 12 were incubated with FITC-conjugated pA20-36 or pCNT peptides (10 μg/mL), and phycoerythrin-labeled anti–mouse B220 antibody and analyzed by flow cytometry. Control splenocytes from tumor-free mice were also analyzed (bottom). Numbers in the quadrants indicate the percentage of cells. (B) The pA20-36–FITC peptide detects niches of metastatic A20 tumor cells in the spleen of A20 B lymphoma–engrafted BALB/c mice. Spleens from A20 B lymphoma–bearing mice were stained with pA20-36–FITC, anti–mouse B220 antibody, and the nuclear dye TOPO-3 and analyzed by confocal microscopy. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. (C) MicroPET images showing the specific retention of [18F]-pA20-36 in the A20 tumor. BALB/c mice bearing a palpable A20 tumor mass on the flank (5-6 mm in maximal diameter) were injected intravenously with 10 μg (8 MBq peptide) of [18F]-pA20-36 (left) or [18F]-pCNT (middle). Mice bearing a palpable 5T33MM tumor mass on the flank were used as control for the specific retention of pA20-36 (right). Images shown are coronal (top) or axial (bottom) sections of static scans of a single mouse for each group collected at 10, 30, and 60 minutes after peptide injection (see also supplemental Videos). Tumors are indicated by white arrows in all cases.
pA20-36 inhibits the growth of A20 B lymphoma in engrafted mice
To evaluate the in vivo toxicity of Id-peptides, groups of tumor-free BALB/c mice were daily inoculated intraperitoneally with either pA20-36– or pCNT-peptide (200 mg ≃ kg−1 ≃ d−1) for 2 weeks. Both vehicle- and peptide-treated mice showed normal levels of the inflammatory cytokine interleukin-1β (IL-1β) and the hepatotoxicity marker glutathione and lactate dehydrogenase (Table 2).
In vivo toxicity of Id-peptides
| Group . | IL-1β, pg/mL . | GSH, mM . | LDH, IU/L . |
|---|---|---|---|
| Untreated | 59 ± 0.01 | 0.006 ± 0.001 | 80.0 ± 15.1 |
| pCNT-treated | 60 ± 0.02 | 0.006 ± 0.001 | 68.6 ± 23.8 |
| pA20-36–treated | 59 ± 0.01 | 0.006 ± 0.001 | 73.2 ± 19.2 |
| Group . | IL-1β, pg/mL . | GSH, mM . | LDH, IU/L . |
|---|---|---|---|
| Untreated | 59 ± 0.01 | 0.006 ± 0.001 | 80.0 ± 15.1 |
| pCNT-treated | 60 ± 0.02 | 0.006 ± 0.001 | 68.6 ± 23.8 |
| pA20-36–treated | 59 ± 0.01 | 0.006 ± 0.001 | 73.2 ± 19.2 |
Three groups of tumor-free BALB/c mice (5 mice/group) were left untreated, or intraperitoneally inoculated daily with either pA20-36– or pCNT-peptide (20 mg ≃ kg−1 ≃ d−1) for 2 weeks, after which blood samples were collected and analyzed for IL-1β, glutathione (GSH), and lactate dehydrogenase (LDH). Values represent the mean ± SEM of 3 different experiments.
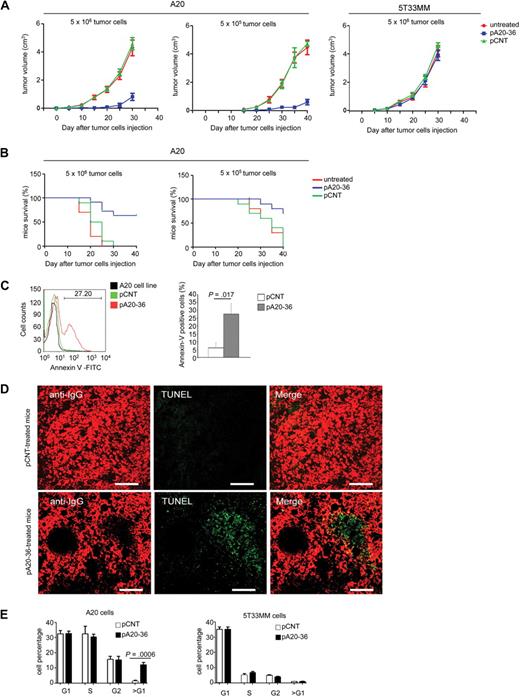
Next, we analyzed the outcome of pA20-36 inoculation on tumor growth in mice engrafted with A20 B lymphoma. After subcutaneous injection of a large tumor load (5 × 106 tumor cells) a detectable tumor mass appeared at day 10 from engraftment. Daily administration of pA20-36 (20 mg ≃ kg−1 ≃ d−1) starting 24 hours after engraftment delayed the appearance of tumor masses up to 25 days (Figure 4A left), whereas it did not affect the subcutaneous growth of 5T33MM control tumor (Figure 4A right); differently, the administration of pCNT did not affect the A20 tumor growth (Figure 4A left). Injection of a reduced tumor load (5 × 105 tumor cells) resulted in increased tumor growth inhibition on treatment with the pA20-36 peptide (Figure 4A middle). A similar outcome was observed in the case of the pA20-36 treatment starting at 2 weeks after engraftment (supplemental Figure 7). Mice survival was significantly affected by the pA20-36 treatment. In fact, mice engrafted with a large (5 × 106 tumor cells) or small (5 × 105 tumor cells) tumor load and treated with pA20-36 showed 64% or 70% survival rates, respectively, at day 40, compared with the death of control groups treated with the pCNT peptide or left untreated (Figure 4B).
Figure 4. pA20-36 inhibits tumor growth in vivo. (A) Daily administration of pA20-36 results in inhibition of tumor growth in mice engrafted with A20 B lymphoma. Tumor volumes were measured in 6- to 8-week-old female BALB/c mice (n = 5 per group) that had been subcutaneously injected with tumor cells (5 × 106, left; 5 × 105, middle) and treated with daily intravenous administration of pA20-36 or scrambled pCNT (20 mg ≃ kg−1 ≃ d−1 in phosphate-buffered saline), or left untreated, beginning the day after tumor cell injection. As control, tumor volumes were also measured in 6- to 8-week-old C57BL/KaLwRij mice (n = 5 per group) that have been subcutaneously injected with 5T33MM tumor cells (5 × 106, right). One representative of 2 independent experiments with similar results is shown. Values are the mean volumes ± SEMs per group of animals (n = 5/group). Statistical analysis was performed by 2-way analysis of variance. In case of large tumor cell load (5 × 106), P < .001 for pA20-36 group versus untreated; P < .001 for pA20-36 group versus pCNT group. In the case of small tumor cell load (5 × 105), P < .001 for pA20-36 group versus untreated; P < .001 for pA20-36 group versus pCNT group. (B) The pA20-36 treatment enhances survival of mice engrafted with A20 B lymphoma. Kaplan-Meier survival curves of A20 B lymphoma–engrafted mice on peptide treatments. BALB/c mice (n = 5 per group) were subcutaneously inoculated with tumor cells and treated as described in panel A and killed when the tumor volume reached a size of 500 mm3, according to the ethical guidelines. Statistical analysis was performed by log-rank Mantel-Cox test; statistically significant difference was observed between pA20-36–treated and pCNT-treated groups (P < .001 for mice grafted with 5 × 105 tumor cells, or P < .003 for mice engrafted with 5 × 106 tumor cells). One representative of 2 independent experiments with similar results is shown. (C) Flow cytometry of apoptotic cells derived from pA20-treated and pCNT-treated mice. Apoptosis was evaluated at day 24 of peptide treatment by cell staining with annexin V–FITC and propidium iodide (PI). Apoptosis was measured as annexin V–positive/PI-negative cell population. Viable A20 cells from culture were used as control. A representative experiment of 4 independent experiments is shown. Quantitative analysis of annexin V–positive tumor cells from pA20-36– and pCNT-treated mice is also shown. Values are the mean ± SD (n = 4/group); statistical analysis was performed according to the Student t test. (D) Cell apoptosis in tumor masses of A20 B lymphoma. Tissue sections of A20 tumor masses were labeled by terminal deoxyuridine nick-end labeling (TUNEL) and anti-IgG to identify apoptotic DNA and B cells, respectively. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. Scale bar = 80 μm. (E) Cell-cycle profiles of A20 and 5T33MM tumor cells after peptide challenge in vivo. To track tumor cell in vivo, A20 (left) or 5T33MM (right) cells were stained with CFSE and injected intraperitoneally in BALB/c mice. After 3 days, mice were injected intraperitoneally with pCNT or pA20-36 peptide (100 μg/100 μL phosphate-buffered saline). Six hours later mice were killed, and single-cell suspensions were prepared from draining lymph nodes. Cell-cycle profile was analyzed by flow cytometry on CFSE-gated tumor population; data are the mean ± SD (n = 4/group); statistical analysis was performed according to the Student t test.
Figure 4. pA20-36 inhibits tumor growth in vivo. (A) Daily administration of pA20-36 results in inhibition of tumor growth in mice engrafted with A20 B lymphoma. Tumor volumes were measured in 6- to 8-week-old female BALB/c mice (n = 5 per group) that had been subcutaneously injected with tumor cells (5 × 106, left; 5 × 105, middle) and treated with daily intravenous administration of pA20-36 or scrambled pCNT (20 mg ≃ kg−1 ≃ d−1 in phosphate-buffered saline), or left untreated, beginning the day after tumor cell injection. As control, tumor volumes were also measured in 6- to 8-week-old C57BL/KaLwRij mice (n = 5 per group) that have been subcutaneously injected with 5T33MM tumor cells (5 × 106, right). One representative of 2 independent experiments with similar results is shown. Values are the mean volumes ± SEMs per group of animals (n = 5/group). Statistical analysis was performed by 2-way analysis of variance. In case of large tumor cell load (5 × 106), P < .001 for pA20-36 group versus untreated; P < .001 for pA20-36 group versus pCNT group. In the case of small tumor cell load (5 × 105), P < .001 for pA20-36 group versus untreated; P < .001 for pA20-36 group versus pCNT group. (B) The pA20-36 treatment enhances survival of mice engrafted with A20 B lymphoma. Kaplan-Meier survival curves of A20 B lymphoma–engrafted mice on peptide treatments. BALB/c mice (n = 5 per group) were subcutaneously inoculated with tumor cells and treated as described in panel A and killed when the tumor volume reached a size of 500 mm3, according to the ethical guidelines. Statistical analysis was performed by log-rank Mantel-Cox test; statistically significant difference was observed between pA20-36–treated and pCNT-treated groups (P < .001 for mice grafted with 5 × 105 tumor cells, or P < .003 for mice engrafted with 5 × 106 tumor cells). One representative of 2 independent experiments with similar results is shown. (C) Flow cytometry of apoptotic cells derived from pA20-treated and pCNT-treated mice. Apoptosis was evaluated at day 24 of peptide treatment by cell staining with annexin V–FITC and propidium iodide (PI). Apoptosis was measured as annexin V–positive/PI-negative cell population. Viable A20 cells from culture were used as control. A representative experiment of 4 independent experiments is shown. Quantitative analysis of annexin V–positive tumor cells from pA20-36– and pCNT-treated mice is also shown. Values are the mean ± SD (n = 4/group); statistical analysis was performed according to the Student t test. (D) Cell apoptosis in tumor masses of A20 B lymphoma. Tissue sections of A20 tumor masses were labeled by terminal deoxyuridine nick-end labeling (TUNEL) and anti-IgG to identify apoptotic DNA and B cells, respectively. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. Scale bar = 80 μm. (E) Cell-cycle profiles of A20 and 5T33MM tumor cells after peptide challenge in vivo. To track tumor cell in vivo, A20 (left) or 5T33MM (right) cells were stained with CFSE and injected intraperitoneally in BALB/c mice. After 3 days, mice were injected intraperitoneally with pCNT or pA20-36 peptide (100 μg/100 μL phosphate-buffered saline). Six hours later mice were killed, and single-cell suspensions were prepared from draining lymph nodes. Cell-cycle profile was analyzed by flow cytometry on CFSE-gated tumor population; data are the mean ± SD (n = 4/group); statistical analysis was performed according to the Student t test.
Treatment of B-cell lymphoma with anti-idiotype antibodies can give arise to idiotype variants.23 Thus, we evaluated whether the residual A20 B lymphocytes in engrafted mice on pA20-36 treatment could result in the emergence of A20 idiotype variants. By flow cytometry, B220+/pA20-36+ B cells were detected at a similar frequency after 24 days of tumor treatment with pA20-36 or pCNT peptides (supplemental Figure 8A), which indicated that the antigenicity of B220+ tumor cells was not affected by pA20-36 peptide treatment. Further, gene sequence analysis of the IgH variable regions (complementary determinant regions) of A20 tumor cells from both pCNT- and pA20-36–treated mice showed a unique nucleotide sequence of both the framework and the complementary determinant regions (supplemental Figure 8B). The nucleotide sequence overlapped with the one expressed by the original A20 cell clone,24 indicating that the minimal residual disease observed in pA20-36–treated mice did not result from mutant, idiotypic escape of tumor IgG at day 24 after treatment.
To address the mechanism of tumor growth inhibition by pA20-36, we analyzed the levels of apoptosis in pA20-36–treated tumor tissues. Flow cytometry of single tumor cell suspensions showed a statistically significant, higher number of annexin V–positive cells in pA20-36–treated mice compared with pCNT-treated mice at day 23 (Figure 4C). Accordingly, the histology of fresh tumor masses confirmed the presence of large areas of apoptotic cells in pA20-36–treated tumors, which were undetected in tumor treated with pCNT (Figure 4D). To analyze the effect of pA20-36 on apoptosis in vivo, syngenic BALB/c mice were injected intraperitoneally with CFSE-labeled A20 tumor cells. At day 3 after inoculation, mice were injected intraperitoneally with pA20-36 or control peptide (100 μg/100 μL phosphate-buffered saline), and 6 hours later single-cell suspensions were isolated from lymph nodes. We observed a significant increase of sub-G1 DNA content in mice inoculated with pA20-36 compared with control mice (Figure 4E left). Control CFSE-labeled 5T33MM cells were not significantly affected by the pA20-36 peptide treatment (Figure 4E right), indicating that pA20-36 specifically induced apoptosis of A20 B cells in vivo.
Analysis of tumor microenvironment
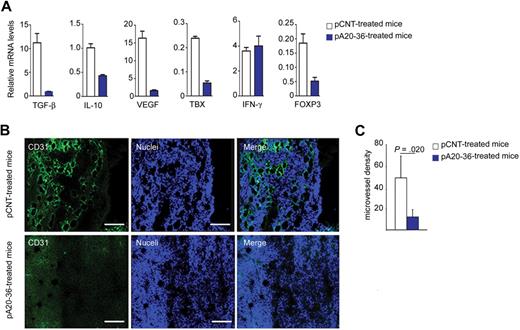
Next, we addressed the effect of the Id-peptide treatment on antitumor immunity within the tumor microenvironment of the A20 B lymphoma–engrafted mice. First, we evaluated the expression levels of cytokines in tumor tissues by real-time polymerase chain reaction of mRNAs. The pA20-36 treatment resulted in a reduced expression of immunosuppressive cytokines genes (Tgfb1 and Il10), and proangiogenic genes (Vegf, Tbxas1) in the B-cell lymphoma–engrafted mice, whereas no significant effect was observed in the case of Ifng (Figure 5A). The immunosuppressive cytokines IL-10 and transforming growth factor-β (TGF-β) promote the development and activity of Foxp3+ CD4+ regulatory T (Treg) cells.25 Consistently with the reduced expression of IL-10 and TGF-β, Foxp3 expression was reduced in pA20-36–treated mice (Figure 5A), indicating that the pA20-36 treatment could affect the number of tumor-infiltrating Treg cells.
Analysis of tumor microenvironment in A20 B lymphoma–engrafted mice. (A) Cytokine expression levels in tumor tissues from mice injected intravenously with pA20-36 or pCNT peptides (200 mg ≃ kg−1 ≃ d−1) at day 24 after treatment. Mean values ± SDs of 3 independent experiments are shown. Statistical analysis was performed according to the Student t test (n = 4 tumors per group). (B) Inhibition of tumor angiogenesis by the pA20-36 peptide. Sections of fresh tumor tissues were incubated with antibody to CD31 to stain endothelial cells, together with TOPO-3 staining followed by confocal microscopy. Panels are representative of samples from 4 mice per group with similar staining profiles. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. (C) Quantitative analysis of vessel density. The mean number of vessels per field (56.25 μm2) of tumor sections described in panel B is shown; mean values ± SDs (n = 4/group); statistical analysis was performed according to the Student t test.
Analysis of tumor microenvironment in A20 B lymphoma–engrafted mice. (A) Cytokine expression levels in tumor tissues from mice injected intravenously with pA20-36 or pCNT peptides (200 mg ≃ kg−1 ≃ d−1) at day 24 after treatment. Mean values ± SDs of 3 independent experiments are shown. Statistical analysis was performed according to the Student t test (n = 4 tumors per group). (B) Inhibition of tumor angiogenesis by the pA20-36 peptide. Sections of fresh tumor tissues were incubated with antibody to CD31 to stain endothelial cells, together with TOPO-3 staining followed by confocal microscopy. Panels are representative of samples from 4 mice per group with similar staining profiles. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. (C) Quantitative analysis of vessel density. The mean number of vessels per field (56.25 μm2) of tumor sections described in panel B is shown; mean values ± SDs (n = 4/group); statistical analysis was performed according to the Student t test.
The reduced expression of proangiogenic cytokines in tumor tissues from pA20-36–treated mice was investigated by assessing the outcome of pA20-36 treatment on tumor angiogenesis. Immune fluorescence analysis with anti-CD31 MAb as marker of endothelial cells showed a 25% reduction of the microvessel density in tumor tissues of pA20-36–treated mice, compared with pCNT mice (Figure 5B-C). Altogether, these results indicated that the decreased expression of proangiogenic cytokines in pA20-36–treated tumors resulted in reduced tumor angiogenesis.
Next, we analyzed the tumor-infiltrating immune cell populations. The pA20-36 treatment did not significantly affect the absolute number of both CD11c+ dendritic cells and CD11b+ macrophage cells in tumor tissues, whereas we observed a significant decrease in CD11b+Gr-1+ cells, which includes the myeloid-derived suppressor cells that are involved in immune suppression26 (Figure 6A). The pA20-36 treatment also decreased the number of CD4+, CD8+ T, and Treg cells (Figure 6A).
Tumor-infiltrating immune cells in pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice. (A) The pA20-36 treatment affects the number of tumor-infiltrating CD4+, CD8+ T, and Treg cells. Cell suspensions were prepared from tumor tissues at day 14 after subcutaneously tumor injection and analyzed by flow cytometry on staining with specific antibodies. Values are the mean ± SD (n = 4/group); statistical analysis was performed by Student t test. (B) Tumor B220+ cells show an increased expression of the activation markers on pA20-36 treatment. Expression of activation markers in CD11c+ (top), CD11b+ (middle), and B220+ (bottom) antigen-presenting cells derived from tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice as assessed by flow cytometry. Histograms are representative of sample from 5 mice with similar staining profiles. (C) Tumor-infiltrating CD8+ T cells from pA20-36–treated mice show an activated phenotype. Expression of activation markers in CD4+ (top) and CD8+ (bottom) T cells from tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice as assessed by flow cytometry. Histograms are representative of sample from 5 mice with similar staining profiles. (D) Granzyme-positive CD8+ T cells infiltrating tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice. Sections of fresh tumor tissues were incubated with antibody to CD8, anti–granzyme B, and with TOPO-3. Pictures were captured with a Leica TCS SP2 confocal microscope with a HCX PL APO 63.0×/1.40 oil UV objective (NA 1.40) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. Scale bar = 14.2 μm. CTLA4 indicates cytotoxic T-lymphocyte–associated antigen 4.
Tumor-infiltrating immune cells in pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice. (A) The pA20-36 treatment affects the number of tumor-infiltrating CD4+, CD8+ T, and Treg cells. Cell suspensions were prepared from tumor tissues at day 14 after subcutaneously tumor injection and analyzed by flow cytometry on staining with specific antibodies. Values are the mean ± SD (n = 4/group); statistical analysis was performed by Student t test. (B) Tumor B220+ cells show an increased expression of the activation markers on pA20-36 treatment. Expression of activation markers in CD11c+ (top), CD11b+ (middle), and B220+ (bottom) antigen-presenting cells derived from tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice as assessed by flow cytometry. Histograms are representative of sample from 5 mice with similar staining profiles. (C) Tumor-infiltrating CD8+ T cells from pA20-36–treated mice show an activated phenotype. Expression of activation markers in CD4+ (top) and CD8+ (bottom) T cells from tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice as assessed by flow cytometry. Histograms are representative of sample from 5 mice with similar staining profiles. (D) Granzyme-positive CD8+ T cells infiltrating tumor masses of pA20-36– or pCNT-treated A20 B lymphoma–engrafted mice. Sections of fresh tumor tissues were incubated with antibody to CD8, anti–granzyme B, and with TOPO-3. Pictures were captured with a Leica TCS SP2 confocal microscope with a HCX PL APO 63.0×/1.40 oil UV objective (NA 1.40) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. Scale bar = 14.2 μm. CTLA4 indicates cytotoxic T-lymphocyte–associated antigen 4.
Furthermore, we analyzed the activation status of tumor-infiltrating immune cells. No significant difference was observed in the expression of activation markers (CD80, CD86, major histocompatibility complex II, programmed death ligand 1 (PD-L1), and CD40) in CD11c+ dendritic and CD11b+ macrophage tumor-infiltrating cells between pCNT- and pA20-36–treated mice (Figure 6B). Remarkably, B220+pA20-36+ cells from pA20-36–treated tumors cells showed an increased expression of the activation markers CD80, CD86, major histocompatibility complex II, PD-L1, and CD40, thus pointing to an increased antigen-presenting capability of tumor B cells in vivo on pA20-36 treatment (Figure 6B). The activation status of A20 tumor cells occurred selectively in the context of the tumor microenvironment, because it was not detected in cultured A20 cells stimulated with pA20-36 (supplemental Figure 9).
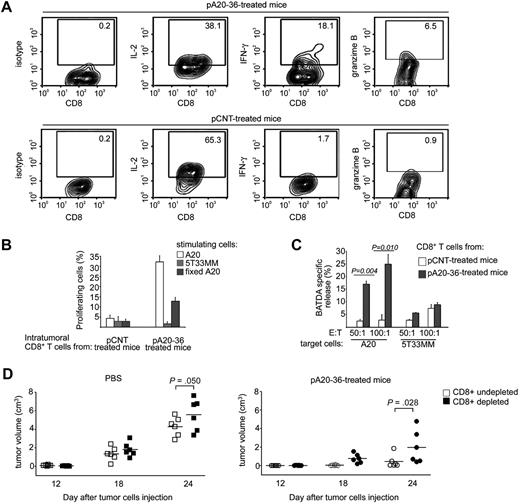
Tumor-infiltrating CD8+ T cells expressed lower levels of the negative costimulatory molecules cytotoxic T lymphocyte–associated antigen 4 and PD-1 in pA20-36–treated mice compared with pCNT-treated mice; CD62L, a marker of naive and central memory T cells,27 was also down-regulated in pA20-36–treated mice (Figure 6C top). Differently, tumor-infiltrating CD4+ T cells showed similar expression levels of cytotoxic T lymphocyte–associated antigen 4, PD-1, and CD62L in pA20-36– and pCNT-treated mice (Figure 6C bottom). These findings indicated that tumor tissues from pA20-36–treated mice were infiltrated by activated CD8+ T cells. Consistently, CD8+ granzyme B+ T cells were selectively detected in tumor tissues of pA20-36–treated mice (Figure 6D and Figure 7A).
The CD8+ T activity significantly contributes to pA20-36–induced tumor growth inhibition. (A) Intracellular cytokines expression of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells from pCNT- or pA20-36–treated tumor masses were incubated with A20 tumor cells for 3 hours and then analyzed for intracellular expression of IL-2, interferon-γ (IFN-γ), and granzyme B by flow cytometry. A representative experiment of 2 independent experiments with similar results is shown. Numbers in the quadrants indicate the percentage of cells. (B) Antigen-specific proliferation of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells from tumor mass of pCNT- or pA20-36–treated mice were stained with CFSE, incubated with A20 or 5T33MM tumor cells (5 × 103), and 1 week later measured by flow cytometry. Percentage of proliferating CD8+ T cells was calculated from CFSE profiles with the use of FlowJo software, as described in “Methods.” Values are the mean ± SD (n = 3). A representative experiment of 2 independent experiments with similar results is shown. (C) Cytotoxic activity of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells (effector cells, E) from pCNT- or pA20-36–treated tumor masses were incubated with BATDA-labeled A20 or 5T33MM cells (5 × 103) (target cells, T), at ratio of 50:1 and 100:1. BATDA-specific release was measured by time-resolved fluorometry. Maximal release of BATDA was measured by incubating target cells in lysis buffer containing 1% Triton X-100; spontaneous release was measured by incubating cells in medium alone. Cytolytic activity was calculated as follows: (experimental release − spontaneous release)/(Triton X-100 release × spontaneous release) × 100. Values are the mean ± SD (n = 3); statistical analysis was performed by Student t test. A representative experiment of 2 independent experiments with similar results is shown. (D) CD8+ T cells contributed to anti-tumor activity of pA20-36 peptide. BALB/c mice (n = 6/group) were antibody-mediated depleted of CD8+ T cells or left undepleted and were subcutaneously injected with A20 tumor cells (5 × 106); mice were then treated intravenously with pA20-36 or scrambled pCNT (200 mg ≃ kg−1 ≃ d−1), beginning the day after tumor injection. Tumor volumes were evaluated at the indicated time after tumor injection. A representative experiment of 2 independent experiments is shown. Symbols represent the tumor volume of each individual mouse; horizontal lines indicate the mean. Statistical analysis was performed by 2-way analysis of variance.
The CD8+ T activity significantly contributes to pA20-36–induced tumor growth inhibition. (A) Intracellular cytokines expression of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells from pCNT- or pA20-36–treated tumor masses were incubated with A20 tumor cells for 3 hours and then analyzed for intracellular expression of IL-2, interferon-γ (IFN-γ), and granzyme B by flow cytometry. A representative experiment of 2 independent experiments with similar results is shown. Numbers in the quadrants indicate the percentage of cells. (B) Antigen-specific proliferation of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells from tumor mass of pCNT- or pA20-36–treated mice were stained with CFSE, incubated with A20 or 5T33MM tumor cells (5 × 103), and 1 week later measured by flow cytometry. Percentage of proliferating CD8+ T cells was calculated from CFSE profiles with the use of FlowJo software, as described in “Methods.” Values are the mean ± SD (n = 3). A representative experiment of 2 independent experiments with similar results is shown. (C) Cytotoxic activity of tumor-infiltrating CD8+ T cells. Purified CD8+ T cells (effector cells, E) from pCNT- or pA20-36–treated tumor masses were incubated with BATDA-labeled A20 or 5T33MM cells (5 × 103) (target cells, T), at ratio of 50:1 and 100:1. BATDA-specific release was measured by time-resolved fluorometry. Maximal release of BATDA was measured by incubating target cells in lysis buffer containing 1% Triton X-100; spontaneous release was measured by incubating cells in medium alone. Cytolytic activity was calculated as follows: (experimental release − spontaneous release)/(Triton X-100 release × spontaneous release) × 100. Values are the mean ± SD (n = 3); statistical analysis was performed by Student t test. A representative experiment of 2 independent experiments with similar results is shown. (D) CD8+ T cells contributed to anti-tumor activity of pA20-36 peptide. BALB/c mice (n = 6/group) were antibody-mediated depleted of CD8+ T cells or left undepleted and were subcutaneously injected with A20 tumor cells (5 × 106); mice were then treated intravenously with pA20-36 or scrambled pCNT (200 mg ≃ kg−1 ≃ d−1), beginning the day after tumor injection. Tumor volumes were evaluated at the indicated time after tumor injection. A representative experiment of 2 independent experiments is shown. Symbols represent the tumor volume of each individual mouse; horizontal lines indicate the mean. Statistical analysis was performed by 2-way analysis of variance.
CD8+ T lymphocytes contributed to the antitumor activity of the Id-peptide
Because a consistent number of CD8+ T lymphocytes infiltrated the A20 B lymphoma in pA20-36–treated mice, we addressed the role of T cells in the observed tumor inhibition. Increased intracellular production of interferon-γ and granzyme B together with a decreased production of IL-2 was observed in CD8+ T lymphocytes isolated from pA20-36–treated mice, compared with pCNT-treated mice (Figure 7A), which was indicative of cell differentiation toward activated T cells. Furthermore, intratumor CD8+ T cells were stained with CFSE as a marker of cell proliferation, incubated with A20 cells, and analyzed by flow cytometry. After 1 week of CFSE staining, proliferating CD8+ T lymphocytes were greater than 30% in pA20-36–treated tumors compared with 4.0% in pCNT-treated tumors (Figure 7B). CD8+ T-cell proliferation was specifically induced by A20 B cells because incubation with 5T33MM was unable to stimulate CD8+ T lymphocytes (Figure 7B). These results show that the high proliferation rate was restricted to tumor-infiltrating CD8+ T cells derived from pA20-36–treated mice in the presence of A20 tumor cells. Because the A20-stimulating cells used in the proliferation assay had never encountered the pA20-36 peptide, these results underscored that CD8+ T cells from the pA20-36–treated mice recognized tumor epitopes of A20 cells. Consistently, formaldehyde-fixed A20 B cells stimulated a CD8+ T-cell proliferation albeit in a smaller cell percentage, as a probable outcome of a reduced antigenicity of tumor cell membrane proteins caused by the formaldehyde treatment (Figure 7B).
Next, we investigated the cytolytic activity of tumor-infiltrating CD8+ T cells. The CD8+ T cells isolated from pA20-36–treated mice showed a dose-dependent killing activity against cultured A20 cells, whereas they did not significantly react against the 5T33MM control cell line (Figure 7C). Conversely, CD8+ T cells from pCNT-treated mice showed a weak cytolytic activity against both A20 and 5T33MM cells, which indicated the level of nonspecific bystander effect (Figure 7C). Collectively, these results show that tumor-infiltrating CD8+ T cells from mice treated with pA20-36 elicited an effective immune response toward A20 tumor antigens in vitro.
We next evaluated the in vivo contribution of CD8+ T cells to antitumor activity by antibody-mediated depletion of CD8+ T cells. To this end, mice depleted of CD8+ T lymphocytes were subcutaneously injected with A20 B lymphoma and analyzed for tumor growth compared with undepleted animals. Then, mice were randomly assigned to receive either pA20-36 treatment, or vehicle, beginning at day 1 after tumor engraftment. In the absence of pA20-36 treatment, depletion of CD8+ T cells affected the unchecked development of A20 tumors, as shown by the significant increase in tumor growth in CD8+-depleted mice, compared with undepleted mice at day 24 (Figure 7D left). In pA20-36–treated groups of mice, we observed a further significant increase of tumor growth in CD8+ T cell–depleted mice, compared with undepleted mice (Figure 7D right), pointing to a relevant contribution of CD8+ T lymphocytes to the antitumor activity of the pA20-36 peptide.
Discussion
Tumorigenic B-cell lymphomas are sensitive to anticancer treatments, including conventional chemotherapy, radiation therapy, and corticosteroids.28 Nevertheless, the disease is associated with incomplete response to clinical treatments that result in a minimal residual disease in which a few neoplastic cells undetected in vivo replenish the cancer cell reservoir. This grim scenario calls for novel strategies to target the anatomic homing of tumorigenic B cells. Here, we have reported the targeting specificity and the therapeutic properties of an idiotype-specific peptide toward a murine B lymphoma engrafted in syngenic, immune competent mice. Our results show a highly specific targeting of the selected A20 idiotype–specific peptide, which allowed the in vivo detection of an early stage of metastatic disease by using sensitive imaging techniques, such as fluorescence and MicroPET analysis (Figure 3).
The in vitro functional analysis of the Id-peptide used in this study underscores some relevant activities: (1) the pA20-36 Id-peptide was specifically internalized by target cells with a kinetic shared with the surface A20-Ig on BCR cross-linking, suggesting a mechanism of receptor-mediated endocytosis (supplemental Figure 3); and (2) the pA20-36 Id-peptide triggered a BCR-mediated signaling that induced apoptosis of the target cells (Figure 2). Accordingly, B-cell apoptosis is observed in various types of both immature and mature neoplastic B cells after cross-linking of the sIg-BCR with surrogate antigens.29 Moreover, effective immunotherapy of B-cell lymphoma based on passive transfer of Id-specific monoclonal antibodies correlated with anti-idiotype–induced signal transduction in lymphoma cells.30 The apoptotic pathway activated on BCR cross-linking has not been univocally clarified31 ; however, evidence for mitochondria as central executors of BCR-induced apoptosis and for caspase-2, -3, and -9 as effectors of the apoptotic program downstream of mitochondria was reported.32-34 Our results indicate that the BCR oligomerization by multimeric presentation of Id-peptide recruited the apoptosis-initiating protease caspase-9, which in turn activated the executioner proteases caspase-3 and -7, thus resulting in apoptotic cell death of the targeted tumor cells (Figure 2D). Of interest, we also observed a significant apoptotic effect in vivo in the case of the monomeric Id-peptide (Figure 4), suggesting that pA20-36 could acquire both monomeric and multimeric conformations. Although this possibility needs to be further investigated, previous studies have shown that receptor peptide ligands can mediate the dimerization of the cognate receptors; in support of this possibility, the crystal structure of a 20-residue cyclic mimetic peptide of erythropoietin showed a simple, compact homodimer of β-hairpin structure that induced cross-linking of the erythropoietin receptor, resulting in signal transduction and cell proliferation.35
Although cell apoptosis induced by chemotherapy or radiotherapy has been associated with a silent or tolerogenic immune response, recent studies have underscored the immunogenic properties of apoptotic cell death induced by chemotherapeutic compounds.36-38 In this study, we report that an A20 cell–specific Id-peptide induced a consistent antitumor immunity in immune-competent mice harboring A20 B lymphoma cells. Indeed, we observed an increased number of armed CD8+ T cells that colonized the tumor masses in pA20-36–treated mice (Figures 6D and 7A). Consistently, CD8+ T cells showed an A20 cell–specific proliferative and cytolytic activity (Figure 7B-C). Consistently, the CD8+ T-cell depletion in mice strongly counteracted the tumor inhibition by pA20-36, thus pointing to the relevant role for CD8+ T cells in the mechanism of tumoricidal activity mediated by pA20-36 in vivo.
B-cell malignancies may present tumor antigen to the immune system by either a direct presentation,39,40 or by cross-presenting tumor antigens from apoptotic cells.41 We observed that in vivo exposure of tumor cells to pA20-36 resulted in up-regulation of the costimulatory molecules CD80, CD86, and CD40 (Figure 6B), which was restricted to the tumor microenvironment, because their expression on A20 cells on in vitro stimulation with pA20-36 was unaffected (supplemental Figure 9). These observations underscore a role for neoplastic B cells in presenting tumor antigens to T cells. Accordingly, a direct tumor-antigen presentation by A20 B-cell lymphomas resulting in a strong antitumor effect has been recently reported.41
Tumor-specific CD8+ T cells from pCNT-treated mice lacked an effective cytolytic activity, indicating that the tumor microenvironment shifted toward a T-cell suppression. Such inactive CD8+ T cells have been isolated from tumors,42,43 where local suppression of CD8+ T-cell activity was ascribed to the immunosuppressive cytokines TGF-β and IL-10 or Foxp3+ CD4+ Treg cells.44,45 Consistently, the A20 tumor model underscores some interesting properties: (1) tumor growth directly correlated with increased percentages of Treg cells infiltrating tumor, spleen, and tumor-draining lymph nodes46 ; (2) Treg cells promoted an early immune escape mechanism in A20 tumor by suppressing the endogenous antitumor activity of both CD4+ and CD8+ T cells.46 Although the mechanisms that mediate the reduced TGF-β, IL-10, and Treg on Id-peptide treatment need further investigation, the immune analysis of A20 tumors indicates that the shift of the immune response in tumor microenvironment toward an immune-activated status could account for the antitumor CD8+ T-cell activity observed in pA20-36–treated mice. Furthermore, our results highlight the property of fluorochrome- or [18F]-labeled Id-peptide to target in vivo both the tumor masses and micrometastases, as assessed by histology and MicroPET analysis (Figure 3).
Of clinical interest, the selected idiotype peptide also exerted an effective killing activity in vivo. A major limitation of the Id-peptide–based approach is the requirement of a panning procedure for each individual patient. However, there is evidence that chronic antigenic stimulation may contribute to the neoplastic transformation and drives a selective process that shapes the antigenic repertoire of a subset of B-cell proliferation, including chronic lymphocytic leukemia.47 In this setting, Id-peptides will provide a unique tool to define the antigenic specificities of the immunoglobulins isolated from tumor B cells and may devise an effective peptide-based strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), MIUR-PRIN, MIUR-FIRB, ISS (G.S., I.Q., and C.P.).
Authorship
Contribution: C.P. designed and performed research, analyzed data, and wrote the paper; C.F. and E.I. performed research and assisted with data analysis; F.M.T., F.T., and L.L. assisted with the FACS experiments; A.D.L., A.P., M.P., M.S., and E.V. assisted with RPLs construction and ELISA; M.R.P., M.L., and S.G. performed MicroPET experiments; O.F. performed peptide synthesis; F.D.P. performed SPR experiments; N.C., C.A., A.G., G.P., and A.B. assisted with the mouse experiments; M.G. performed MS analysis; I.Q. assisted with data analysis and reviewed the manuscript; and G.S. conceived the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giuseppe Scala, Department of Experimental and Clinical Medicine, University of Catanzaro “Magna Graecia,” 88100 Catanzaro, Italy; e-mail: scala@unicz.it.



![Figure 3. pA20-36 specifically binds primary and metastatic A20 tumor cells. (A) The pA20-36–FITC peptide specifically detects A20 tumor cells ex vivo. Cells (1 × 106) derived from the site of subcutaneously injection (top) or spleen (middle) from A20 B lymphoma–engrafted BALB/c mice at day 12 were incubated with FITC-conjugated pA20-36 or pCNT peptides (10 μg/mL), and phycoerythrin-labeled anti–mouse B220 antibody and analyzed by flow cytometry. Control splenocytes from tumor-free mice were also analyzed (bottom). Numbers in the quadrants indicate the percentage of cells. (B) The pA20-36–FITC peptide detects niches of metastatic A20 tumor cells in the spleen of A20 B lymphoma–engrafted BALB/c mice. Spleens from A20 B lymphoma–bearing mice were stained with pA20-36–FITC, anti–mouse B220 antibody, and the nuclear dye TOPO-3 and analyzed by confocal microscopy. Pictures were captured with a Leica TCS SP2 confocal microscope with a HC PL FLUOTAR 20×/0.50 oil UV objective (NA 0.50) in glycerol and acquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Adobe Photoshop CS. (C) MicroPET images showing the specific retention of [18F]-pA20-36 in the A20 tumor. BALB/c mice bearing a palpable A20 tumor mass on the flank (5-6 mm in maximal diameter) were injected intravenously with 10 μg (8 MBq peptide) of [18F]-pA20-36 (left) or [18F]-pCNT (middle). Mice bearing a palpable 5T33MM tumor mass on the flank were used as control for the specific retention of pA20-36 (right). Images shown are coronal (top) or axial (bottom) sections of static scans of a single mouse for each group collected at 10, 30, and 60 minutes after peptide injection (see also supplemental Videos). Tumors are indicated by white arrows in all cases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/2/10.1182_blood-2009-11-253617/4/m_zh89991053820003.jpeg?Expires=1767748387&Signature=zkIieUeSizen~Bty6r4oAKsX2r1Qeb-n187K-WiOBGi994hEHrC4VsQkJlpJq9EQh8T6AVvL3Ni0tDDeIrNMXuRb6ZxX5Lmq-CUOW8LjzmVg28LWZVJM-IZ2qKwAHLRKVD2OKrYR12vMGP4XkBWCLgHd10eVHQ5BCyE0lkQ-TQcjJ5MGHZVCtsN03J~PNFcq0wDm1j3T8gcOUkUPso0hMaaHoVk6BHBsPSxpC3hKv9HQKk6cA6FMJzfsn3udgUvvdS8IKgJnaThnhssUCd7Q4E~qPcKYEOztR12RcSFQzmMEzL1MufqQjuAVsh3QE-86kw~zZZfz4TTv1xRbJIyCfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal