Abstract
Radioimmunotherapy (RIT) with α-emitting radionuclides is an attractive approach for the treatment of minimal residual disease because the short path lengths and high energies of α-particles produce optimal cytotoxicity at small target sites while minimizing damage to surrounding normal tissues. Pretargeted RIT (PRIT) using antibody-streptavidin (Ab-SA) constructs and radiolabeled biotin allows rapid, specific localization of radioactivity at tumor sites, making it an optimal method to target α-emitters with short half-lives, such as bismuth-213 (213Bi). Athymic mice bearing Ramos lymphoma xenografts received anti-CD20 1F5(scFv)4SA fusion protein (FP), followed by a dendrimeric clearing agent and [213Bi]DOTA-biotin. After 90 minutes, tumor uptake for 1F5(scFv)4SA was 16.5% ± 7.0% injected dose per gram compared with 2.3% ± .9% injected dose per gram for the control FP. Mice treated with anti-CD20 PRIT and 600 μCi [213Bi]DOTA-biotin exhibited marked tumor growth delays compared with controls (mean tumor volume .01 ± .02 vs. 203.38 ± 83.03 mm3 after 19 days, respectively). The median survival for the 1F5(scFv)4SA group was 90 days compared with 23 days for the control FP (P < .0001). Treatment was well tolerated, with no treatment-related mortalities. This study demonstrates the favorable biodistribution profile and excellent therapeutic efficacy attainable with 213Bi-labeled anti-CD20 PRIT.
Introduction
Non-Hodgkin lymphoma (NHL) is the sixth most common type of cancer, with over 74 000 new cases diagnosed annually in the United States.1 Following conventional treatment with chemotherapy or radiation therapy, patients with advanced stage indolent NHL inevitably relapse, with death occurring a median of 5 years after recurrence.2 The introduction of rituximab, a monoclonal antibody against CD20, has led to improved survival in patients with NHL.3-5 Despite the encouraging clinical results with anti-CD20 antibodies, however, the majority of patients with indolent NHL who respond to immunochemotherapy eventually relapse with recurrent lymphoma.6,7 Recently, radioimmunotherapy (RIT) has emerged as a promising treatment option for lymphoma. RIT with iodine-131(131I) tositumomab or yttrium-90 (90Y) ibritumomab tiuxetan as a single agent has yielded excellent overall response rates of 50% - 80%, with complete response rates of 20% - 40% in patients with relapsed or refractory indolent NHL.8-13 Even more notable response rates have been observed when RIT is used as front-line treatment in patients with indolent NHL.14 In a recent large phase 3 trial, the addition of 90Y-ibritumomab tiuxetan in first remission after chemotherapy significantly improved response rates and remission durations in patients with advanced-stage follicular lymphoma,15 presumably by killing residual tumor cells that survived the induction chemotherapy.16 Based on this data, 90Y-ibritumomab tiuxetan has been approved by the FDA for first line consolidation therapy in follicular lymphoma. However, the β-emitting radionuclides used in current RIT schemes may not be ideal for irradiating microscopic tumors and isolated tumor cells present in the setting of minimal residual disease (MRD). It is estimated that the fraction of energy deposited in a tumor measuring 200 μm in diameter is only 1.5% and 17% for 90Y-labeled and 131I-labeled antibodies (Abs), respectively.17,18 The remainder of the β energy is deposited in surrounding normal tissues, resulting in dose-limiting toxicities. Furthermore, the relatively low decay energies of β-particles result in suboptimal killing of tumor cells, ultimately contributing to relapse in the majority of treated patients. In contrast, α-emitting radionuclides impart high-linear-energy-transfer radiation along densely ionized, linear tracks over relatively short distances (40 to 90 μm or few cell diameters), which are highly effective in cell-killing. Alpha-particles are associated with as much as 400-fold greater linear energy transfer as β-particles, and cell death may result from transversal of just 1-5 α-particle emissions through the nucleus.19,20 In addition, α-particles induce irreparable double-stranded DNA breaks, which are not amenable to most DNA repair mechanisms present in tumor cells.21 These physical characteristics of α-particles may afford optimal cytotoxicity for small foci of chemoresistant tumor cells while minimizing damage to the surrounding normal tissues in MRD settings.
Our group has successfully demonstrated that pretargeted RIT (PRIT) using antibody-streptavidin (Ab-SA) conjugates and related fusion proteins (FP), followed by radiolabeled biotin provides rapid specific localization of radioactivity at tumor sites.22-27 PRIT is particularly attractive for use of short half-lived α-emitting radionuclides, because it allows the delivery of radioactivity to tumor sites before the activity decays. This is important, because 2 of the most promising α-emitting radionuclides in clinical studies, bismuth-213 (213Bi, t1/2 = 45.6 minutes) and astatine-211 (211At, t1/2 = 7.21 hours), have short half-lives. In this study, we evaluated the biodistributions of 213Bi-labeled DOTA-biotin when using a specific (anti-CD20) FP and a non-specific FP in a PRIT protocol. Those results were compared with biodistribution data from 213Bi in a conventional RIT protocol, where the Ab was labeled directly. The biodistributions were conducted in mice bearing B-cell NHL (Ramos) xenografts. The efficacy of an orally administered chelator of 213Bi (2,3-dimercapto-1-propanesulfonic acid; DMPS) was evaluated as an approach to reduce nonspecific radiation uptake in the kidney. Using the most favorable PRIT schemes defined in the biodistribution experiments, we conducted a preclinical study to evaluate the therapeutic efficacy and toxicity of 213Bi in mice bearing small or large tumor burdens.
Methods
Cell lines
The human Ramos Burkitt lymphoma cell line was obtained from American Type Culture Collection. Cell lines were maintained in log phase growth in RPMI1640 medium supplemented with 10% heat-inactivated fetal calf serum in a 5% CO2 incubator. Cell viability exceeded 95% by trypan blue exclusion.
Antibody, fusion proteins, and pretargeting reagents
The 1F5 (murine anti-CD20) and HB8181 (murine isotype matched nonbinding control) Abs were produced from the respective hybridomas using a hollow fiber bioreactor system in the monoclonal Ab production facility at the Fred Hutchinson Cancer Research Center. The production and purification of 1F5(scFv)4SA fusion protein (FP) has been previously described.26 Briefly, 1F5 scFvSA fusion genes were produced by molecularly fusing the single-chain variable regions (scFv) of the murine anti-CD20 1F5 Ab to the full-length genomic SA of Streptomyces avidinii. The SA gene was then joined to the scFv region using a GSGSA peptide linker. The FP was expressed from an IPTG-inducible lac promoter. The resultant FP was then expressed in the periplasmic space of Escherichia coli and spontaneously formed stable soluble tetramers 1F5(scFv)4SA with a molecular mass of 174 kDa. The 1F5-SA FPs were formulated at a concentration of 2.3 mg/mL in phosphate buffer saline (PBS) containing 5% sorbitol and stored at −80°C. CC49(scFv)4SA FP (negative control) was produced by the same method described above. CC49(scFv)4SA recognizes the TAG-72 antigen expressed on most human adenocarcinomas but not on lymphomas.
Radiolabeling
As previously described, 1F5 and HB8181 Abs were conjugated with isocyanatobenzyl-CHX-A″.28 Briefly, each Ab was demetallated by dialyzing against 50mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer pH 8.5 that had been treated on a Chelex-100 column to remove metals. The Ab was then reacted overnight with 12 equivalents of isothiocyanatobenzyl-CHX-A″ (Macrocyclics). The mixture was dialyzed against 150mM saline that had been demetallated by treating with Chelex-100. The final preparation was stored in acid washed vials. The 213Bi was obtained from actinium-225 (225Ac, t1/2 ∼ 10 days) on a MP-50 ion exchange resin from the Department of Energy. The 213Bi was eluted into an acid washed vial from the 225Ac column with 0.5 mL of fresh, metal free 0.1M HI (0.1M NaI dissolved in 0.1M HCl). The pH of the eluant was increased by adding 100-250 μL of 500mM metal free Ammonium acetate pH 5.0. For PRIT experiments, 2-10 μL of 3 mg/mL DOTA-biotin was added to the 213Bi eluant and reacted for 5 minutes at 80°C. The mixture was then cooled to room temperature and 10 μL of 100mM DTPA was added. After 2 minutes, 100 μL of 1M NaOH was added to neutralize the pH, and the mixture was diluted to injection volume with PBS. An aliquot was removed and checked for binding by an avidin bead assay. For directly labeled Ab, 200-500 μL of 1F5-CHX-A″ (3.7 mg/mL) or HB8181-CHX-A″ (5.9 mg/mL) was added to the 213Bi eluant and allowed to react for 5 minutes at room temperature. This mixture was passed over a PD-10 column that had been equilibrated in PBS. The protein con-taining fractions were combined and diluted to the final injection volume in PBS. HB8181 was added at 200 μg/dose to block non-specific binding. Purity was determined by instant thin-layer chromatography (80% MeOH with 20% 100mM DTPA in water) and/or immunoprecipitation in MeOH/Water (80/20).
Mouse RIT and PRIT studies
Female FoxN1Nu athymic nude mice, aged 6-8 weeks, were obtained from Harlan Sprague-Dawley and housed under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. For all pretargeting experiments, animals were placed on a biotin-deficient diet (Purina Mills) 4-5 days before treatment and maintained on the diet up to 7 days following radiobiotin administration.
Biodistribution studies
Ramos cells (10 × 106) were injected subcutaneously in the right flank 7 days before FP injection to obtain lymphoma xenografts. Groups of 5 mice with similar, palpable tumors were chosen and were injected intravenously with 2.8 nmol of either 1F5(scFv)4SA or the negative control CC49(scFv)4SA FP. After 20 hours, 50 μg of a biotinylated N-acetyl-galactosamine clearing agent (NAGB CA)22 was administered intravenously followed 4 hours later by 1.2 nmol (1 μg) DOTA-biotin labeled with 20 μCi (0.74 MBq) of 213Bi. Blood samples, tumors, and normal organs were obtained and counted for 213Bi activity as described previously.22
Renal uptake studies
Groups of 5 mice received DMPS (Sigma-Aldrich) in the drinking water (1.2 mg/mL) 24 hours before [213Bi]DOTA-biotin. The control animals received regular drinking water. The biodistribution experiments were performed as described above.
Dosimetry
All biologic retention data in percentage of injected dose per gram (% ID/g) for each organ or tissue were “un-decay corrected” to effective retention by applying the physical decay constant for 213Bi. The time-sequential data for each organ or tissue were then plotted graphically. The data were fitted to biexponential functions of the form y = a*exp(−b*x) + c*exp(−d*x) by linear least-squares regression analysis, with all data points weighted equally. Each best-fit biexponential function was then integrated analytically from time zero to infinity to calculate the area-under-curve, equivalent to the total decays taking place in the organ or tissue per unit administered activity (μCi-hours per μCi). The area-under-curve values were then multiplied by the mean energy emitted per decay for 213Bi (19.44 g-cGy/μCi-hour). This equilibrium dose constant includes all alpha and electron emissions by 213Bi and its decay products thallium-209 and lead-209. All particle emissions were assumed to be absorbed locally in the tissue of origin. The product of this multiplication is the radiation absorbed dose per unit administered activity, in units of cGy per μCi 213Bi administered to the mouse.
Therapy studies
The therapeutic efficacy of 213Bi using the pretargeted approach was evaluated in groups of 5-10 mice. CC49(scFv)4SA FP was used as a negative control. Ramos cells (10 × 106) were injected subcutaneously in the right flank 7 days before FP injection to obtain lymphoma xenografts measuring 6-10 mm in diameter. Mice were injected intraperitoneally with anti-asialo GM1 antiserum (30 μL; WAKO) 8 days and 1 day before FP injection to minimize natural killer cell activity in athymic mice and prevent spontaneous tumor regression. Mice with similar, palpable tumors were chosen and randomized for the studies. Mice received DMPS in the drinking water (1.2 mg/mL) 24 hours before [213Bi]DOTA-biotin and continued for another 24 hours after 213Bi injection. Mice were given 2.8 nmol of either 1F5(scFv)4SA or the negative control CC49(scFv)4SA FPs followed by 5.8 nmol (50 μg) CA 20 hours later. A single dose of 1.2 nmol (1 μg) DOTA-biotin labeled with 200, 600, or 800 μCi (7.4, 22.2, and 29.6 MBq, respectively) [213Bi]DOTA-biotin was administered 4 hours later. In a second set of experiments, mice were injected with 5 × 106 of Ramos cells in the right flank. Mice with similar, visible tumors (≤ 4 mm) were chosen for the studies and randomized into various treatment groups of 5 to 10 mice. After 3 days, mice received DMPS in the drinking water (1.2 mg/mL) and 2.8 nmol of either 1F5(scFv)4SA or the negative control CC49(scFv)4SA FP. Twenty hours later, 5.8 nmol (50 μg) CA was administered to each animal, followed 4 hours later by 1.2 nmol (1 μg) DOTA-biotin labeled with either 200 μCi or 600 μCi 213Bi (7.4 and 22.2 MBq, respectively). Injections were given intravenously in all therapy studies. Mice were assessed every other day for tumor volume measurements, weight change, and general appearance. Mice were euthanized if xeno-grafts exceeded 10% of total body weight, caused obvious discomfort, or impaired ambulation.
Toxicity studies
Weight loss was used as a measure of general well being. Mice were analyzed for “huddling” behavior, diarrhea, lethargy, and other signs of debility. Toxicity assessments measuring leukocyte count, hemoglobin, and platelet counts, plus aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN) levels, were performed on days 14, 28, and 120 from the 213Bi-labeled reagent injection, using mice treated as described above with methods previously defined. Averages and standard errors for hematology and chemistry data were calculated and reported for each group of mice studied. Age-matched normal athymic mice were used for the comparison of hematology and chemistry data.
Results
Radiolabeling of the antibodies and DOTA-biotin
213Bi-labeled DOTA-biotin was prepared in low specific activity for biodistribution studies and high specific activity for therapy studies. Preparation of 213Bi-labeled DOTA-biotin in low specific activity (20-40 μCi/μg) gave radiochemical yields of 86%-98% (by avidin bead assay). At high specific activity (200-800 μCi/μg), radiochemical yields of [213Bi]DOTA-biotin were 82%-98%. The radiochemical purity of the isolated 213Bi-labeled Ab-CHX-A″ was 94%-97% as determined by instant thin-layer chromatography and immunoprecipitation.
Biodistributions
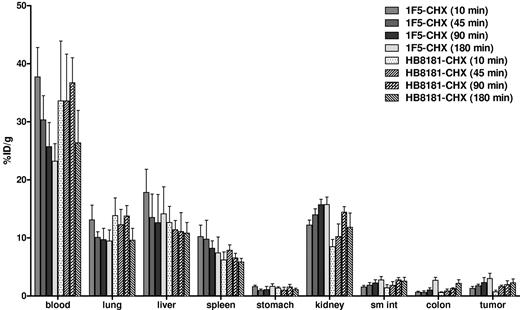
The biodistribution of [213Bi]DOTA-biotin after pretargeting with 1F5(scFv)4SA FP was evaluated in athymic mice bearing B-cell NHL xenografts. Groups of 5 mice were administered 2.8 nmol of either 1F5(scFv)4SA or CC49(scFv)4SA (negative control) FP intravenously. Twenty hours later, mice received 5.8 nmol (50 μg) of CA, followed 4 hours later by 1.2 nmol of [213Bi]DOTA-biotin. At 10, 45, 90, or 180 minutes after injection of [213Bi]DOTA-biotin, mice were killed, and tissues were harvested and counted in a gamma counter to determine the percentage of injected dose per gram (% ID/g) for each tissue (Figure 1). Mice pretargeted with 1F5(scFv)4SA FP exhibited rapid localization of 213Bi in tumors within 10 minutes after [213Bi]DOTA-biotin administration (8.4% ± 1.2% ID/g). Peak radioactivity in tumors was reached after 90 minutes (16.5% ± 7.0% ID/g) and maintained at a high level (13.7% ± 6.6% ID/g) up to 180 minutes (approximately 3 half-lives of 213Bi) after injection. Control mice, which received nonspecific CC49(scFv)4SA FP, had negligible tumor uptake of 213Bi (1.7% ± 0.6% and 1.4% ± 0.3% ID/g after 90 and 180 minutes, respectively), demonstrating the specificity of pretargeting with the anti-CD20 Ab-SA (P = .0001). The biodistribution of [213Bi]DOTA-biotin in normal organs was similar for 1F5(scFv)4SA and CC49(scFv)4SA. For mice pretargeted with 1F5(scFv)4SA FP, the blood exhibited rapid clearance of radioactivity within 45 minutes after injection (0.6% ± 0.1% ID/g). The normal organ with the most nonspecific uptake of 213Bi was the kidney, with 10.5% ± 1.1% after 10 minutes and 6.2% ± 1.0% ID/g after 90 minutes.
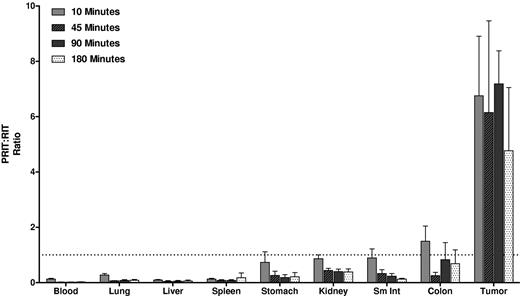
Biodistributions of [213Bi]DOTA-biotin in tumor, blood, and normal organs after PRIT with 1F5-SA or CC49-SA FPs. Athymic mice bearing Ramos xenografts were injected with 2.8nM each unlabeled FP followed 20 hours later by 5.8nM CA, and 4 hours after that by 1.2nM [213Bi]DOTA-biotin. Groups of 5 mice were euthanized 10, 45, 90, and 180 minutes after the injection of [213Bi]DOTA-biotin. The radioactivity in the tissues were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.
Biodistributions of [213Bi]DOTA-biotin in tumor, blood, and normal organs after PRIT with 1F5-SA or CC49-SA FPs. Athymic mice bearing Ramos xenografts were injected with 2.8nM each unlabeled FP followed 20 hours later by 5.8nM CA, and 4 hours after that by 1.2nM [213Bi]DOTA-biotin. Groups of 5 mice were euthanized 10, 45, 90, and 180 minutes after the injection of [213Bi]DOTA-biotin. The radioactivity in the tissues were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.
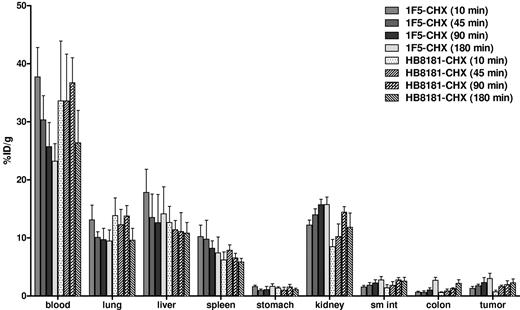
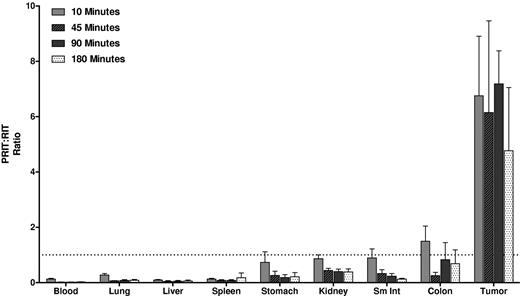
Biodistributions using conventional RIT with directly labeled [213Bi]1F5-CHX-A″ were also evaluated (Figure 2). Athymic mice with Ramos xenografts received 213Bi-labeled 1F5-CHX-A″. HB8181-CHX-A″ labeled with 213Bi was used as a nonbinding control. Maximum tumor uptake for 1F5 Ab was 3.0% ± 0.9% ID/g after 180 minutes versus 2.3% ± 0.7% ID/g for the control Ab (P = .171). The blood concentration of [213Bi]1F5-Ab was 37.7% ± 5.0% ID/g after 10 minutes and remained elevated up to 180 minutes after injection (23.2% ± 3.0% ID/g), indicating that the majority of radioactivity was retained in blood during this period. Because of high activity in blood, the content of 213Bi was also relatively high in vascular organs, such as lung, liver, spleen, and kidney. Overall, there were no significant differences in tumor uptake and normal organ distributions between the 2 Abs within 180 minutes of radiolabeled Ab injection, consistent with our previous data suggesting that it requires 20 to 24 hours for an intact Ab to localize optimally to target tumor sites. Figure 3 depicts the PRIT to RIT ratio for tissue concentrations in comparative biodistributions of PRIT and conventional 1-step RIT (P < .001 for all tissues, except P = .28 for colon). Tumor-to-blood ratios were 58- to 426-fold higher with PRIT than conventional RIT, and tumor-to-normal organ ratios of nearly 100:1 were observed with PRIT compared with 3:1 or less with conventional RIT (Table 1).
Biodistribution of radioactivity in tumor, blood, and normal organs of athymic mice bearing Ramos xenografts injected with either 1F5 or HB8181 Abs directly labeled with 213Bi. Groups of 5 mice were injected with 1.4nM either conventional trace-labeled 1F5 or HB8181 Abs. Mice were euthanized 10, 45, 90, and 180 minutes after injection of each 213Bi-labeled Abs. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.
Biodistribution of radioactivity in tumor, blood, and normal organs of athymic mice bearing Ramos xenografts injected with either 1F5 or HB8181 Abs directly labeled with 213Bi. Groups of 5 mice were injected with 1.4nM either conventional trace-labeled 1F5 or HB8181 Abs. Mice were euthanized 10, 45, 90, and 180 minutes after injection of each 213Bi-labeled Abs. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.
PRIT:RIT ratios obtained from comparative biodistributions of PRIT and RIT in the tumors, blood, and normal organs of athymic mice bearing Ramos xenografts. The % ID/g of tissue were obtained in 2 separate experiments using 1F5 Ab in a conventional RIT and PRIT schemes. The ratio of PRIT to RIT for % ID/g of each tissue was calculated for direct comparison.
PRIT:RIT ratios obtained from comparative biodistributions of PRIT and RIT in the tumors, blood, and normal organs of athymic mice bearing Ramos xenografts. The % ID/g of tissue were obtained in 2 separate experiments using 1F5 Ab in a conventional RIT and PRIT schemes. The ratio of PRIT to RIT for % ID/g of each tissue was calculated for direct comparison.
Renal uptake and dosimetry
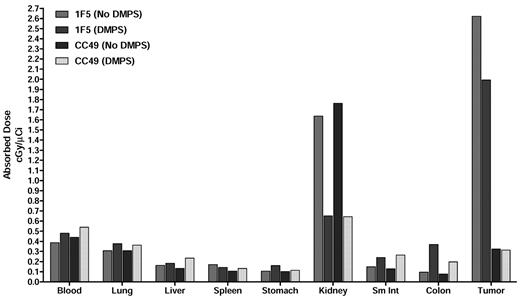
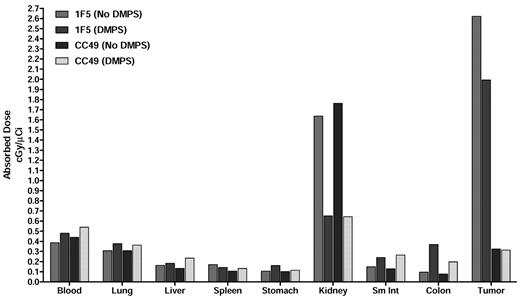
The efficacy of chelation of 213Bi with DMPS administered in the drinking water was evaluated to reduce nonspecific uptake in the kidney. Earlier studies have shown that DMPS, a metal chelator, is effective in clearing the radioactive alpha daughters of 225Ac, including 213Bi.29 In this study, mice were treated with DMPS orally 24 hours before [213Bi]DOTA-biotin injection, and the biodistribution was evaluated. DMPS-treated mice displayed significantly lower levels of radioactivity in the kidneys compared with the untreated controls at all time points (Figure 4). The renal uptake of 213Bi was 2.3 ± 0.3 for mice treated with DMPS, compared with 6.1 ± 0.8 for mice in the standard PRIT group after 45 minutes. The tumor uptake in DMPS-treated mice appeared to be slightly lower than in untreated mice (P = .025), but the tumor-to-kidney ratios were more favorable for the DMPS-treated group (P = .008). The radiation-absorbed dose per unit of administered activity was calculated based on the biodistribution data using the Medical Internal Radiation Dose method (Figure 5). In DMPS-treated mice, the total absorbed dose was estimated to be 0.7 cGy/μCi in the kidneys, compared with 1.6 cGy/μCi for the mice in the standard PRIT group without DMPS. This corresponds to a > 60% reduction in the renal radiation dose for DPMS-treated mice. The total absorbed dose in tumor was slightly lower in the DMPS group (2.0 cGy/μCi), compared with the untreated group (2.6 cGy/μCi); however, the tumor-to-kidney ratio was approximately 2 times higher in the DMPS group. The mean absorbed doses in other tissues were comparable with and without DMPS.
Graphs depicting the effect of DMPS on biodistributions in tumors, kidneys, blood, lungs, livers, and spleens of athymic mice bearing Ramos xenografts injected with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) The activity of 213Bi following 1F5(scFv)4SA in the tissues of mice not treated with DMPS (B) The effect of the chelating agent DMPS on the normal organ and tumor uptake of 213Bi when administered in the drinking water. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay and expressed as the % ID/g of tissue. The % ID/g is shown as function of time after injection.
Graphs depicting the effect of DMPS on biodistributions in tumors, kidneys, blood, lungs, livers, and spleens of athymic mice bearing Ramos xenografts injected with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) The activity of 213Bi following 1F5(scFv)4SA in the tissues of mice not treated with DMPS (B) The effect of the chelating agent DMPS on the normal organ and tumor uptake of 213Bi when administered in the drinking water. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay and expressed as the % ID/g of tissue. The % ID/g is shown as function of time after injection.
Graph showing the absorbed doses for blood, tissues and tumor without DMPS and with DMPS being provided in the drinking water. The total radiation absorbed dose per unit administered activity is shown in units of cGy per μCi 213Bi administered to the mouse.
Graph showing the absorbed doses for blood, tissues and tumor without DMPS and with DMPS being provided in the drinking water. The total radiation absorbed dose per unit administered activity is shown in units of cGy per μCi 213Bi administered to the mouse.
Therapy and toxicity
Two RIT studies were performed to evaluate the therapeutic efficacy of 213Bi using 2 different xenograft models with low and high tumor burdens. In the first therapy study, female athymic mice were injected subcutaneously with Ramos cells (10 × 106), resulting in development of large palpable tumors (∼ 6-10 mm in diameter) 7 days after tumor cell inoculation. Mice were randomized into 5 different groups, all of which received oral DMPS 24 hours before treatment with 213Bi. Animals were then injected with either 1F5(scFv)4SA FP or the negative control CC49(scFv)4SA FP followed 20 hours by NAGB clearing agent. Treatment groups of 5 to 10 mice each received a single injection of 200, 600, or 800 μCi [213Bi]DOTA-biotin 4 hours after the administration of clearing agent. The CC49 negative control group was only tested at the highest dose (800 μCi).
The mean tumor sizes were not statistically significantly different between the groups at the initiation of the experiment (P > .2). As expected, the untreated control mice showed exponential growth of the lymphoma xenograft, requiring euthanasia in all mice by day 20 (Figure 6). Mice treated with 1F5(scFv)4SA FP followed by [213Bi]DOTA-biotin exhibited dose-dependent tumor responses. Tumor volumes 19 days after 213Bi treatment were 35.0 ± 20.9 mm3, 26.2 ± 24.8 mm3, and 11.6 ± 12.6 mm3 for 200, 600, and 800 μCi of 213Bi treatment groups, respectively, which were significantly smaller than the untreated group (237.9 ± 58.5 mm3). In comparison, mice treated with nonbinding control CC49(scFv)4SA FP followed by [213Bi]DOTA-biotin showed tumor growth patterns similar to the untreated mice, confirming the specificity of PRIT efficacy. All mice treated with the negative control CC49(scFv)4SA FP and 800 μCi [213Bi]DOTA-biotin were euthanized by day 20 due to uncontrolled tumor growth. Mice treated with 1F5(scFv)4SA FP followed by 200 or 400 μCi [213Bi]DOTA-biotin experienced longer survivals than the control groups (P < .05), though tumors rapidly recurred, leading to euthanasia by day 45 in all mice. Three of the 10 mice treated with 1F5(scFv)4SA FP and 800 μCi [213Bi]DOTA-biotin achieved complete regression of tumors by day 20, and one mouse experienced prolonged progression-free survival without tumor recurrence for the duration of the experiment (> 120 days). There was no evidence of significant toxicity or weight loss in mice treated with 200 or 600 [213Bi]DOTA-biotin; however, one mouse treated with 800 μCi [213Bi]DOTA-biotin showed signs of early toxicity with a loss of approximately 10% of its body weight (Figure 6C).
Analysis of tumor size, cumulative survival, and toxicity of mice bearing large-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of large tumors (6-10 mm in diameter) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing Ramos lymphoma xenografts (∼ 6-10 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) of [213Bi]DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this figure correspond to treatment groups designated in panels A and B.
Analysis of tumor size, cumulative survival, and toxicity of mice bearing large-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of large tumors (6-10 mm in diameter) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing Ramos lymphoma xenografts (∼ 6-10 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) of [213Bi]DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this figure correspond to treatment groups designated in panels A and B.
The second therapy study was designed to more closely mimic a MRD setting by using a xenograft model with smaller tumor burdens. Athymic mice were injected with 5 × 106 Ramos cells in the flank. Three days after cell inoculation, tumors were present and animals with similar-sized, small tumors (≤ 4 mm in diameter) were randomized into 5 groups. Mice were treated with oral DMPS and FP followed 20 hours later by NAGB CA as described above. A single dose of 200 or 600 μCi [213Bi]DOTA-biotin was injected 4 hours after the CA in groups of 5 to 10 mice. Mice received no higher than 600 μCi of 213Bi due to possible early toxicities with weight loss observed at 800 μCi dose in the earlier experiment described above.
Control mice receiving either no treatment or treatment with the non-binding CC49(scFv)4SA FP followed by [213Bi]DOTA-biotin exhibited exponential growth of tumors as before, necessitating euthanasia by day 25 in all mice (Figure 7). Mice receiving CC49(scFv)4SA FP and 600 μCi of Bi-213-labeled DOTA biotin exhibited a modest delay in tumor growth (95.5 ± 39.4 mm3 after 16 days) compared with the untreated mice (275.8 ± 67.6 mm3; P = .0018). Mice treated with 1F5(scFv)4SA FP followed by 200 or 600 μCi [213Bi]DOTA-biotin exhibited significant delays in tumor growth (1.1 ± 2.5 mm3 and 9.2 ± 9.5 mm3, respectively, at day 16) compared with the control groups (P < .01). Complete tumor regressions were achieved in 8 of 10 animals receiving 1F5(scFv)4SA FP and 600 μCi [213Bi]DOTA-biotin and in 6 of 10 mice treated with 200 μCi [213Bi]DOTA-biotin. The median survival for the group receiving 1F5(scFv)4SA FP and 600 μCi [213Bi]DOTA-biotin was 90 days compared with median survivals of 19 days (P < .0001) and 23 days (P < .0001) for untreated mice and mice treated with CC49(scFv)4SA FP and 600 μCi [213Bi]DOTA-biotin, respectively. The treatment was well tolerated, with no treatment-related mortalities in any group. Leukocyte counts, hemoglobin, and platelet counts in mice receiving 1F5(scFv)4SA FP and 600 μCi [213Bi]DOTA-biotin were similar to those of the age matched controls on days 14, 28, and 120 of treatment (Table 2). Transaminase levels appeared slightly higher in the treated mice (P < .01), but there were no significant differences in creatinine or BUN (data not shown) detected between the treated and the age-matched control mice at days 14, 28, and 120 after the 213Bi injection. All laboratory values remained stable in the treated mice until the end of observation in this study (> 120 days), suggesting the absence of significant hematologic, hepatic, or renal toxicities during the period of observation.
Analysis of tumor size, cumulative survival, and toxicity of mice bearing small-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of small tumors (≤ 4 mm) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing small Ramos lymphoma xenografts (≤ 4 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. Survival curves in this panel correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this panel correspond to treatment groups designated in panels A and B.
Analysis of tumor size, cumulative survival, and toxicity of mice bearing small-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of small tumors (≤ 4 mm) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing small Ramos lymphoma xenografts (≤ 4 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. Survival curves in this panel correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this panel correspond to treatment groups designated in panels A and B.
Discussion
In this study, we assessed the biodistribution, therapeutic efficacy, and toxicity profile of the α-emitting radionuclide 213Bi targeted to the CD20 antigen in a mouse lymphoma xenograft model. Our study showed that conventional RIT with directly labeled [213Bi]1F5-Ab was not effective in delivering 213Bi to the tumor due to the protracted circulating half-life of radiolabeled Ab, making 213Bi less suitable for conventional RIT because of the short half-life of the radionuclide. These results were concordant with our previous experiments using other radionuclides, which showed maximal targeting of radiolabeled intact Ab to tumor xenografts requires 20 to 24 hours. In marked contrast, the pretargeted approach delivered high levels of radiation rapidly to tumor sites while minimizing nonspecific radiation exposure to normal organs. Furthermore, our study demonstrated that anti-CD20 PRIT using 213Bi could effectively kill lymphoma cells with regression of tumors and significant prolongation of survivals in all treated mice. As expected, the α-emitting radionuclide 213Bi was more effective for small tumors with approximately 40% of mice achieving long term remissions with 600 μCi of 213Bi compared with no long term remissions in mice with larger tumor volumes treated at this dose. Systemic therapy with ≤ 600 μCi 213Bi was well tolerated with minimal toxicities during the period of observation, as indicated by serial weight and blood test monitoring on days 14, 28, and 120 after treatment.
Recently, increasing data have accrued supporting the importance of eliminating small residual numbers of lymphoma cells following induction chemotherapy in order to achieve long-term disease remissions.30-32 Even after achieving a complete clinical and radiologic remission, microscopic foci of tumor cells may escape the cytotoxic effects of chemotherapy and result in MRD leading to recurrent disease and the eventual emergence of drug-resistant tumors. High-dose myeloablative chemotherapy regimens given with stem cell support significantly reduces the risk of relapse by potentially eliminating MRD, but this approach is associated with significant treatment-related mortality and morbidity.33 Immunotherapy with the anti-CD20 Ab, rituximab, has been evaluated as consolidation or maintenance therapy after first-line chemotherapy with some promising results34-36 ; however, resistance to rituximab is common and the safety of long-term rituximab therapy remains unknown. The exquisite sensitivity of lymphoma cells to radiation makes RIT an especially promising option for consolidation after chemotherapy to eradicate small numbers of chemotherapy-resistant tumor cells, as indicated by results of a recent multicenter randomized trial.15,16
Although encouraging results have been obtained with conventional RIT using β-emitting radionuclides such as 131I and 90Y, it is conceivable that alternative radionuclides will prove superior for clinical situations in which multiple micrometastases or isolated tumor cells are present. Alpha-emitting radionuclides with high-liner energy transfer and short path lengths have many theoretical advantages in the setting of MRD37-41 and have produced exciting results in intraperitoneal and xenograft tumor models and patients with AML, ovarian cancer, and gliomas.42-47 Supiot et al48 conducted a study comparing the efficacy of the B-B4 (anti-syndecan-1) Ab labeled with either 213Bi or 131I for therapy of multiple myeloma. RIT with [213Bi]B-B4 demonstrated cell killing that was dose-dependent, whereas 131I-labeled B-B4 exhibited minimal cytotoxicity. Behr et al evaluated CO17-1A Fab′ fragments labeled with either 213Bi or 90Y in a metastatic GW39 human colon cancer xenograft model in athymic mice.49 At maximally tolerated doses, cure rates were 95% for 213Bi and 20% for 90Y. The results are consistent with the results reported by Bloomer et al, demonstrating better therapeutic results with an α-emitting (211At) radionuclide than with β-emitting radionuclides for therapy of malignant ascites.50 Taken together, these results demonstrate that α-emitting radionuclides may be superior to β-emitting radionuclides for treatment of microscopic disseminated diseases.
Due to availability and decay properties, only a few α-emitting radionuclides are considered suitable for in vivo applications. The α-emitting radionuclides, 211At (t1/2 = 7.2 hours), 212Bi (t1/2 = 60.6 minutes) and 213Bi (t1/2 = 45.6 minutes) have been considered most favorable37,40 because they do not produce daughter radionuclides that also decay by α-emission. However, the very short half-lives and limited availability of those radionuclides may pose challenges in clinical settings. Despite these hurdles, several investigators have successfully conducted clinical trials with α-emitters. Jurcic and Scheinberg have treated 18 acute myeloid leukemia (AML) patients in a phase 1 trial with a 213Bi-labeled humanized anti-CD33 Ab (HuM195) demonstrating reduction in bone marrow leukemic burden in 14 of 18 evaluable patients. Myelosuppression and transaminitis were routinely observed, but reversible.44 In a subsequent study, 20 AML patients were safely treated with [213Bi]HuM195 following cytarabine with significant reductions in leukemic blast levels after the delivery of the radiolabeled Ab in the majority of patients.21 Despite these encouraging pilot trials, several investigators have concluded that the optimal application of short-lived α-emitters will require novel methods of isotope delivery such as PRIT.21,40 Initial investigations exploring the utility of pretargeting for 213Bi used an anti-CD25 scFv-SA FP and 213Bi-labeled DOTA-biotin.51,52 This study demonstrated the curative potential of this approach for mice with acute T-cell leukemia and the superiority of 213Bi compared with 90Y in a disseminated leukemia model. Other investigators have reported tumor growth inhibition and prolongation of survival in mice bearing A431 epidermoid carcinomas that were treated with anti-Ley B3 Ab-SA conjugates followed by 213Bi-labeled DOTA-biotin.53 Our study demonstrates that systemic therapy with 213Bi using the PRIT approach can lead to prolonged complete remissions of lymphoma xenografts with minimal toxicities in a preclinical model.
Toxicity and pharmacokinetic studies with 225Ac-Hu195 in mice and cynomologous monkeys and with pretargeted α-emitters including 213Bi and 212Pb53,54 suggest that renal tubular damage may be the principal toxicity of this approach.29,55 Therefore, we evaluated the efficacy of DMPS to reduce nonspecific uptake of 213Bi in the kidney by providing it in the drinking water. Jaggi et al reported that DMPS could reduce approximately 60% of 213Bi accumulation in the kidney after intravenous injection of an 225Ac “nano-generator.”29 The current study demonstrates similar efficacy of DMPS with approximately 60% reduction of the total absorbed radiation dose in the kidneys. A recent study by Bäck et al reported that a mean absorbed dose to the kidney of approximately 10 Gy seemed to be acceptable, corresponding to approximately 50% reduction in GFR, in RIT using α-emitting radionuclides.56 In our study where DMPS was administered in the drinking water, the absorbed dose to the kidneys per unit administered activity was 0.7 cGy/μCi (4.2 Gy at 600 μCi 213Bi), which delivered substantially less radiation than the reported tolerable α-particle dose of 10 Gy. The lack of significant nephrotoxicity documented here by evaluating serum BUN and creatinine is encouraging. Last, the generally shorter half-lives, shorter range, and higher linear energy transfer of α-emitters could complicate the measurement of dosimetry.21 Conventional Medical Internal Radiation Dose methods that estimate mean absorbed dose over a specific organ volume may not always yield biologically meaningful information because the high energy of α particles delivered over a short range may result in targeted cells receiving high doses whereas neighboring cells may receive no radiation exposure. Therefore, we are currently evaluating a novel method using α-particle imaging to better determine the small-scale dosimetry of α-emitting radionuclides.57
In summary, the current study demonstrates that systemic therapy with α-emitting radionuclides using a pretargeted approach is feasible and effective for treatment of non-Hodgkin lymphoma. The results appear promising with significant tumor regressions and prolonged survivals especially in mice bearing relatively small albeit macroscopic tumors. We anticipate that this treatment method would be even more effective in eliminating microscopic foci of residual lymphoma cells and micrometastases. We are currently evaluating studies using α-PRIT directly compared with β-PRIT following initial chemotherapy in a true MRD setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A.K.G. is a scholar in clinical research of the Leukemia & Lymphoma Society.
This work was supported by National Institutes of Health grants PO1 CA44991 and RO1 CA109663, the Lymphoma Research Foundation (O.W.P.) and gifts from David and Patricia Giuliani, Mary and Geary Britton-Simmons, James and Sherry Raisbeck, the Wyner-Stokes Foundation, and the Hext Family Foundation. S.I.P. is the recipient of a Lymphoma Research Foundation Fellowship Award.
National Institutes of Health
Authorship
Contribution: S.I.P. designed and performed research, analyzed data, and wrote the paper; J.S. performed research and analyzed data; J.M.P. and D.S.W. interpreted research and analyzed and interpreted data; D.K.H. contributed vital reagents and performed research; N.O., A.L.K., S.F., and A.A. performed research and collected data; T.B. conceived and designed research; Y.L. contributed vital reagents; D.R.F., A.K.G. and D.J.G. interpreted data; and O.W.P. conceived and designed research, analyzed and interpreted data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oliver W. Press, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M/S D3-395, Seattle, WA 98109; e-mail: press@u.washington.edu.

![Figure 1. Biodistributions of [213Bi]DOTA-biotin in tumor, blood, and normal organs after PRIT with 1F5-SA or CC49-SA FPs. Athymic mice bearing Ramos xenografts were injected with 2.8nM each unlabeled FP followed 20 hours later by 5.8nM CA, and 4 hours after that by 1.2nM [213Bi]DOTA-biotin. Groups of 5 mice were euthanized 10, 45, 90, and 180 minutes after the injection of [213Bi]DOTA-biotin. The radioactivity in the tissues were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470001.jpeg?Expires=1769316709&Signature=hwK382UiCCqtWOWo4Ju4p0GE0tw3ApPL2I8jtZAR3ZPAkju4Wfcs2dQ5yEsZjrw2B~EDn~S-pYtcLry1wPPiFv3pdYr~by-I1cSrbj0qn8DsIGmYw-ZYhFtVVzfACzU9~JX5Ej87Q2QS8CLug0kAtuiWlDAbli0jq8k7TR3o7GKl74Wx9fugraxYWkV1p-1rjfvjtLeu1D~f5PI1Mza6jo47BUajInMUle83Leny9HOsmVMDvzeRYhTGNfwHz80yaFho-aCKPliCYaFxlAIlV2lkJZVt0APGScYEnr0Bc4seUVGzGNJy~1BSDvDmJPTAvUx71szMsiZzn5sd7HTlEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Graphs depicting the effect of DMPS on biodistributions in tumors, kidneys, blood, lungs, livers, and spleens of athymic mice bearing Ramos xenografts injected with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) The activity of 213Bi following 1F5(scFv)4SA in the tissues of mice not treated with DMPS (B) The effect of the chelating agent DMPS on the normal organ and tumor uptake of 213Bi when administered in the drinking water. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay and expressed as the % ID/g of tissue. The % ID/g is shown as function of time after injection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470004.jpeg?Expires=1769316709&Signature=lnPavA1ljOfCtalFaujERAs5UuA5d0ybBhxzHvqDdOUxS5a0TJpALmednn8qO02U8pwOLM8fn4tg2IIHmokb7H9UXFQJuNup12Qc1HjV172Bz~hUkPIFFxbaJCUFZFXN3xqh3YUW-7ElfGDbTxfQNvUXNIy4ipwDXohqLYYRlP8~ehkaV8vMwgTutReneVJG9YZsscPA9Ncr5wGgJyGzeMMgzr~pRwm2fYlAqDEkludww~PiTI5nQdxw-6AywOql1b0JwVoE2oNLb6Znoc2CZpCXuSrJzTiJZqaZpccIgOSWU2QXfroJwZPR1rT8lXOdJr8qI6GVIqabupJ~NXRgBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Analysis of tumor size, cumulative survival, and toxicity of mice bearing large-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of large tumors (6-10 mm in diameter) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing Ramos lymphoma xenografts (∼ 6-10 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) of [213Bi]DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this figure correspond to treatment groups designated in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470006.jpeg?Expires=1769316709&Signature=2dv0134zvek2KOJj5k2aAoqep2X2GYX8ZEr1jfufOm~C-MbAzsAs1G--u9Xb8PWvEjFsFsUTJD62A~Zw7N08rvrNpizhwSlWFhdmmefpEKRdc~T3pAUdjdv8mAAXq0ghMVULK5l9frcCJp9pb-HdZ0VbPrlVQeleglgQ~quCK9jQfKSl5wxvWzNIyD8jEjRRv~NdosCYSYXGxZ8tlg7cz0kjKCMnyheQNHPoste2fzegNtLizoblcdwU1jYfmLQWzQXjkaLkF-V3ZOtInjojT0mU1Crav5ekYhB2LusGT5HYTx2q8jFaN4vKEOhvqcRA44lVsLVodhcdubpsAt3V3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Analysis of tumor size, cumulative survival, and toxicity of mice bearing small-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of small tumors (≤ 4 mm) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing small Ramos lymphoma xenografts (≤ 4 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. Survival curves in this panel correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this panel correspond to treatment groups designated in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470007.jpeg?Expires=1769316709&Signature=bQia0CwY9NLdv~hgWnQifAyK0v~~HT7m0uBWzmcXbZdv0u3-LatwYkazfC8AfkKs0gR~n7Dnrb2oBSEz42nytMkHW98l5XLiL7q9fzeAmPtWn9QWXFHBHSTMAo0SGEsnKe1AfNNWSTduy7~pOp8WA9QDRH9xYFVkmhwVMz-yBr~jCj~JttEpciJ163nCG0EOlPhAAFGPUMiDFiBK8mmfOqoyj8QhULM5UHSqIDsOE4wG6FoVDE5E9ewC6xMmt6y4hgxZwWLFHczamMM3IK30HFM9hrfja4NVLmViM6-9B4~yS6ttjyrPGwI6iFfQoQljLlHml221xoU3xRWvT1LUwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Biodistributions of [213Bi]DOTA-biotin in tumor, blood, and normal organs after PRIT with 1F5-SA or CC49-SA FPs. Athymic mice bearing Ramos xenografts were injected with 2.8nM each unlabeled FP followed 20 hours later by 5.8nM CA, and 4 hours after that by 1.2nM [213Bi]DOTA-biotin. Groups of 5 mice were euthanized 10, 45, 90, and 180 minutes after the injection of [213Bi]DOTA-biotin. The radioactivity in the tissues were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470001.jpeg?Expires=1770150318&Signature=Rhr5aBPHfoAq0Oy3OQ9rMJs6zw6ooNyyETLksDfjaAJIWRBkvymJiQYlm6nisHRB~26CUy1ofv8Uz1kolpKs5rAjYvQAqXgdbgCslL2xqSFC6wso4nL49LV2mlhFer1v77kw9vOVMdxa2-JVHla7lwfGQpgp1--WmCPZjT~mSDYmhN-ahb0nUeWz2KqvLQF0vH5T6ZoY1dqFdj1Be4~KCjt7Y49zHzAPOvgjdqcBd71Xcd~p8UbHu8EaaUyB0VCkSVWlXzYtxGTAsWutLOR-AMicsiLJlNbglRrMetTaj-~D8D69SyeHzjL6WWUqOzM-WubsgzGwCAbOEl0bE2nq~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Graphs depicting the effect of DMPS on biodistributions in tumors, kidneys, blood, lungs, livers, and spleens of athymic mice bearing Ramos xenografts injected with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) The activity of 213Bi following 1F5(scFv)4SA in the tissues of mice not treated with DMPS (B) The effect of the chelating agent DMPS on the normal organ and tumor uptake of 213Bi when administered in the drinking water. The radioactivity in tumor, blood, and normal organs were quantified by gamma counting, corrected for decay and expressed as the % ID/g of tissue. The % ID/g is shown as function of time after injection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470004.jpeg?Expires=1770150318&Signature=oWeWqtuiO2DO84Bf3K3J92ONecMtEM~EKBxgbTd4RBYoVqBSv~6KUh8WOMGi~HqjbcuNPBOOXng5enb6tvIxo-T00beKEmnp2Zv8Xs3js62fER4mWzzQv~Y8~s9dwCFROeyXQQWpMZmdnvrhtAL9dDZe2Wx7fXeajET0bOtPvLgufioSaUbqSOEzmWD~cWf-dVdTetPUPH2eZ5fGWEb2YjzKfC-hKPfPUAzkQy4mtgh-7Tpq2PLT2Uqwi7PyHWtxD4IWRY08qq6FDnYJA~PxZE71uR18CffMALJ0hRGJ2sjxAsJe8aaudcjn~XDNv94yP65NwQlhyjMDJ8wMQnlSoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Analysis of tumor size, cumulative survival, and toxicity of mice bearing large-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of large tumors (6-10 mm in diameter) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing Ramos lymphoma xenografts (∼ 6-10 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200, 600, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively) of [213Bi]DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this figure correspond to treatment groups designated in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470006.jpeg?Expires=1770150318&Signature=yTqh8Z~flG78r4OLzWJY-PF~9LUpOtw9LSsjkemF4AztlDIvgVaZRAAPloG0RzceXTAyepmVtLAqzNLEdCPhi~51CkFEBi9zsblIaGjmqYh7zpy7ok4SLI3lCPHgor47ZkBLPf1-qWJiTpsTD3d-72Lu9rhZLw1eAcVuPi3ac0lsqvXRA9AONCd-n9bxdODHnDPlwUWfehH9I45s9~jo35QjDbKInFjQX2A2LqiPw-YeH0MtE7MFBxHTwK2~Yxwb8g5xsXFNOYvOqXFFx4fboOmUGAgOxMD3FqK2FrZ6z2S4OV5dnvskj8deLIlrPO6N98~J6uFUOeeo4k0zH0OH6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Analysis of tumor size, cumulative survival, and toxicity of mice bearing small-sized Ramos lymphoma xenografts treated with 1F5 or CC49 FPs followed by a CA and [213Bi]DOTA-biotin. (A) Responses of small tumors (≤ 4 mm) to PRIT. The sizes of Ramos tumor xenografts were serially measured in athymic mice treated with either 1F5 or CC49 FP followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. An additional control group bearing xenograft tumors received no treatment. Animals were killed when tumors reached > 10% of the body weight. To minimize fluctuations in the graph and facilitate interpretation of the data, the mean tumor volume for each group was plotted until > 50% of the mice in the group were killed. (B) Kaplan-Meier survival curves of mice bearing small Ramos lymphoma xenografts (≤ 4 mm in diameter) treated with either 1F5 or CC49 FPs followed by NAGB CA and 200 or 600 μCi (7.4 or 14.8 MBq, respectively) [213Bi]DOTA-biotin. Survival curves in this panel correspond to treatment groups designated in panel A. (C) Weight as assessment of general health in mice bearing Ramos lymphoma xenografts treated as described in panels A and B. Weight curves in this panel correspond to treatment groups designated in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-05-282327/7/m_zh89991060470007.jpeg?Expires=1770150318&Signature=eaKZqJJw5Sk6ZacNtTW--~ejzw1qqwAsL6hLZbAenY5Y2ebEQOGSHjCyczWFhSpCw-6ZQQt3ZMp-vQ1QZxulFfaz~j6pD2CYbnz32-j9PIxrqcF3clL4X7iqVSij9nzAA5JesY-KPLZCyfJPaZOiI4xJIzp7d5XYJZLVxYcmVOdXxN6qVN6RJRHGvbQwnypIX6Z08cPFH0uEYuGebUBlMW4iZEfjMHKTI9j77McLFtRakUfzYzNP-x5IJxQ8PhX-uT0X5pyANqRIvEeRC5jaPDVPyJA2GgJv0qgYM8XaSqjR8zUYmejQy6tSZjlfelWGdIgJT4GqbZYqBXp4reCe~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)