Abstract
Polyphosphate, a linear polymer of inorganic phosphate, is secreted by activated platelets and accumulates in many infectious microorganisms. We recently showed that polyphosphate modulates the blood coagulation cascade at 3 steps: it triggers the contact pathway, it accelerates factor V activation, and it enhances fibrin polymerization. We now report that polyphosphate exerts differential effects on blood clotting, depending on polymer length. Very long polymers (≥ 500mers, such as those present in microorganisms) were required for optimal activation of the contact pathway, while shorter polymers (∼ 100mers, similar to the polymer lengths released by platelets) were sufficient to accelerate factor V activation and abrogate the anticoagulant function of the tissue factor pathway inhibitor. Optimal enhancement of fibrin clot turbidity by polyphosphate required ≥ 250mers. Pyrophosphate, which is also secreted by activated platelets, potently blocked polyphosphate-mediated enhancement of fibrin clot structure, suggesting that pyrophosphate is a novel regulator of fibrin function. In conclusion, polyphosphate of the size secreted by platelets is very efficient at accelerating blood clotting reactions but is less efficient at initiating them or at modulating clot structure. Microbial polyphosphate, which is highly procoagulant, may function in host responses to pathogens.
Introduction
Polyphosphate (polyP)—a linear polymer of inorganic phosphate—accumulates in a variety of microorganisms1 and is secreted by activated human platelets.2,3 We recently showed that polyP is a potent modulator of the human blood-clotting system.3-6 The polymer lengths of polyP are known to vary substantially among different organisms and cell types, with relatively short polymers being secreted by human platelets (∼ 60-100 phosphate units long)2,3 and very long polymers accumulating in microorganisms (many hundreds to more than 1000 phosphate units long).1 In this study, we demonstrate that shorter versus longer polymers of polyP have differential effects on the blood clotting system, with important physiologic/pathophysiologic implications.
PolyP has been widely described in unicellular organisms such as bacteria, fungi, algae, and protozoa, where it plays diverse physiologic roles, including regulating growth, stress responses, and virulence.1,7 Comparatively less is known about the metabolism or physiologic roles of polyP in mammalian cells,8 although polyP is reported to induce apoptosis in plasma cells,9 promote calcification in osteoblasts,10 block metastasis of melanoma cells in a mouse model,11 and possibly serve as a regulatory factor in proliferative signaling pathways.12
PolyP is present at high concentrations in dense granules of human platelets and is secreted upon platelet activation.2,3 PolyP has a half-life in plasma of approximately 90 minutes, because of degradation by phosphatases.4,13 We recently showed that polyP is a potent hemostatic regulator, acting at 3 points in the blood clotting cascade: it initiates the contact pathway of blood clotting,3,4 it accelerates the activation of factor V (FV) by thrombin and factor Xa (FXa),4 and it enhances the thickness of fibrin fibers.5 Our previous studies were conducted with heterodisperse polyP preparations, so the precise size dependence of the actions of polyP on blood clotting was unknown. In the present study, we isolated polyP preparations of carefully defined polymer lengths and used them to investigate the effects of polyP on the blood clotting system. We now report that initiation of the contact phase of blood clotting, accelerating FV activation, and enhancing fibrin clot structure exhibited markedly different polyP size requirements. We further report that inorganic pyrophosphate (PPi), which is also secreted by activated human platelets, abrogated the polyP-mediated enhancement of fibrin-clot structure. These findings have implications for the role of microbial versus endogenous (ie, platelet-derived) polyP in modulating the blood clotting system in health and disease.
Methods
Materials
Sodium phosphate, sodium PPi, sodium triphosphate, kaolin, adenosine diphosphate (ADP), adenosine triphosphate (ATP), soluble polyP preparations of varying polymer size ranges (marketed as “sodium phosphate glass”), and high MW polyP (marketed as “phosphate glass, water insoluble”) were from Sigma-Aldrich. In this paper, we use the naming convention of the supplier (Sigma-Aldrich) for the purchased polydisperse polyP preparations: type 25, type 45, type 65, and type 75+, with nominal mean polymer lengths of 25, 45, 65, and > 75 phosphates, respectively. (In previous studies,4-6 we referred to polyP type 75+ as “polyP75.”) In this paper, we refer to size-fractionated polyP preparations of very narrow size distributions (described in “Size-fractionation of polyP”) by their measured polymer length, followed by “mer” (eg, 105mers).
Citrated, pooled normal human plasma was from George King Biomedical, and FXII-deficient plasma was from Hematologic Technologies. Purified human fibrinogen, FXa, and α thrombin were from Enzyme Research Laboratories. Phospholipid vesicles consisting of 20% phosphatidylserine and 80% phosphatidylcholine (Avanti Polar Lipids) were made by sonication. Recombinant tissue-factor pathway inhibitor (TFPI) was a kind gift from Dr George Broze (Washington University, St Louis, MO).
Isolation of polyP from bacteria
PolyP was extracted from an exopolyphosphatase-deficient strain of Salmonella enterica serovar Typhimurium provided by Dr James Slauch (University of Illinois). Extraction and purification of polyP from bacteria was adapted from Ault-Riche et al14 as follows: Bacteria were grown at 37°C in lysogeny broth (LB) in shaking cultures to the mid-log phase (A600 = 0.9), collected by centrifugation, resuspended in [N-morpholino]propanesulfonic acid (MOPS) medium,15 containing 0.1mM NaH2PO4 and 4 mg/mL glucose, and incubated with shaking at 37°C for an additional 1-2 hours to induce polyP accumulation. Bacteria were collected by centrifugation, resuspended in 10mM Tris (pH 8.0), 2.7 kU/mL Ready-Lyse lysozyme (Epicentre), 1.4mM EDTA (ethylenediaminetetraacetic acid), and sonicated for 5 minutes. The lysate was supplemented with 3.5mM MgCl2 and 30 U/mL Benzonase nuclease (Novagen) and incubated for 30 minutes at 37°C, after which solid guanidinium isothiocyanate (4M final) was dissolved in the lysate and the mixture heated for 5 minutes at 100°C. The mixture was cooled and the pH was adjusted to 5.5 with sodium acetate, followed by the addition of cold ethanol (30% final). Glass milk16 was added (2 μL of 50% glass milk slurry per mL of original culture), and polyP was allowed to bind for 10 minutes at 25°C with mixing. Glass milk was collected by centrifugation, then washed thrice with 5mM Tris HCl (pH 7.4), 50mM NaCl, and 50% ethanol. PolyP was eluted by resuspending the glass milk in 50mM Tris (pH 8.74) at 95°C for 5 minutes. Any contaminating nucleic acids were digested by incubating with 100 U/mL benzonase and 0.2mM ammonium molybdate for 30 minutes at 37°C, after which polyP was precipitated by adding NaCl (50mM final), followed by 2 volumes of ice-cold acetone. After incubating at −80°C for 30 minutes, the precipitate was collected by centrifugation at 10 000g, air-dried for 10 minutes, and dissolved in purified water. Yield was 61 μg polyP per L of bacterial culture.
Isolation of PolyP from platelets
PolyP was purified from human pheresis platelets (∼ 3-4 × 1011 platelets/pheresis unit) obtained from the Regional Blood Center of Central Illinois. PolyP isolation was performed as previously described,3 with the following minor modifications: The lysis buffer contained 0.5% sodium dodecyl sulfate (SDS); proteinase K digestion was conducted at 50°C; the pH of the solution after Dowex resolubilization was neutralized with 40mM Tris base; and the final polyP preparation was dialyzed overnight, using 1000 molecular weight (MW) cutoff dialysis tubing, against 20mM Tris (pH 7.4) plus Chelex 100 resin (Bio-Rad) to ensure the removal of any bound metal ions.
Size-fractionation of polyP
PolyP was size-fractionated by preparative polyacrylamide gel electrophoresis (PAGE), based on the method of Clark and Wood.17 For shorter polymers (< 150mers), a mixture of 5 mg polyP type 25 plus 5 mg polyP type 75+ was resolved on 18 × 16 cm 15% polyacrylamide or 40 × 16 cm 20% polyacrylamide gels in Tris-borate/EDTA (TBE; 90mM Tris, 90mM borate, 2.7mM EDTA; pH 8.3) until xylene cyanol FF migrated 7 cm and bromophenol blue, 14 cm. For longer polymers (> 150mers), “insoluble” high-MW polyP was washed twice with purified water on a fritted glass funnel to deplete very short polymers, then resuspended in 250mM LiCl and stirred for 2 hours at room temperature, after which residual insolubles were removed by centrifugation (5000g for 10 minutes). Dissolved polyP was precipitated from the supernatant by slowly adding 2.5 volumes of acetone, collected by centrifugation (10 000g for 20 minutes), dried overnight, and dissolved in purified water, resulting in a highly disperse population of polyP polymer lengths. Approximately 20 mg of this preparation was resolved on 18 × 16-cm 4% or 6% polyacrylamide gels (in TBE) until xylene cyanol FF migrated 7 cm and bromophenol blue, 14 cm. Narrowly size-fractionated polyP preparations were eluted from individual 1-cm-wide slices of the 4%, 6%, 15%, or 20% gels, while heterogeneous preparations containing very long polymers greater than either 400 or 1000mers (termed polyP400+ and polyP1000+, respectively) were obtained by pooling several 1-cm-wide gel slices from 4% gels.
PolyP was eluted from gel slices by crushing and agitating overnight in 2.5 mL purified water. Fragments of polyacrylamide were removed by centrifugation using Handee centrifuge columns (Pierce), and the eluted polyP was dialyzed against purified water overnight using Snakeskin dialysis tubing (MW cutoff, 3500) for polymers > 100mers, or Slide-A-Lyzer mini dialysis units (MW cutoff, 1000; both from Pierce) for polymers < 100mers.
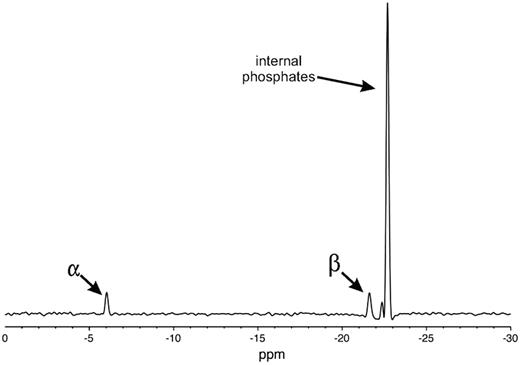
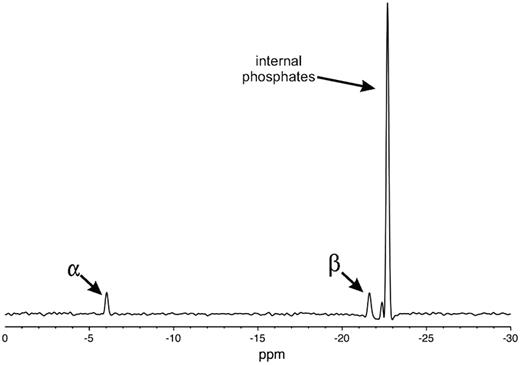
31P-nuclear magnetic resonance (NMR) spectra of polyP preparations were acquired at 20°C with a Varian Unity INOVA 600 spectrometer using a 5-mm Varian AutoTuneX 1H/X PFG Z probe, 13.5-μs (90°) pulse excitation, 16-kHz spectral width, and 5-second recycle time. Chemical shifts were referenced to 0 ppm using an external phosphoric acid standard. Spectra were signal averaged until the phosphate α peak (∼ −6 ppm) achieved a signal-to-noise ratio of at least 5:1 (typically 20 000 to 45 000 scans, depending on sample size and concentration) and processed with baseline correction and 25-Hz line broadening prior to Fourier transformation. Each spectrum showed a peak corresponding to external phosphates (α peak at ∼ −6 ppm) and a second peak corresponding to internal phosphates (from −22 to −24 ppm); for some samples, a third peak was also observed, corresponding to phosphates neighboring the external residues (β peak at from −21 to −22 ppm; see Figure 1 for a typical spectrum). Quantitation was performed by integrating the area under the curves for each peak. Because each polyP polymer has 2 α residues, the number of internal phosphorus atoms, N, was determined using the equation, N/2 = t/α, where α is the integration of the α peak and t the integration of the internal phosphate peak (plus the β peak, when present). PolyP polymer lengths equaled N+2.
PolyP preparations whose mean polymer lengths had been determined by NMR (and found to be 10, 34, 51, 60, 98, 110, 257, and 1350 phosphates long) were employed as size standards for estimating polymer lengths of the rest of the polyP preparations by analytical PAGE,17 using 5% or 15% polyacrylamide gels with 7M urea in TBE (Ready Gels; Bio-Rad), and detecting polyP by toluidine blue staining or DAPI (4′,6 diamidino-2-phenylindole)–negative staining.18
PolyP concentrations
PolyP concentrations were measured by quantifying inorganic phosphate19 after complete hydrolysis in 1M HCl at 100°C for 30 minutes. Briefly, a 50-μL hydrolyzed phosphate sample was mixed with 100 μL malachite green reagent (0.075% malachite green, 0.045% Tween-20, 4.2% ammonium molybdate, 5M HCl) in polypropylene multiwell plates (Corning) and incubated for 20 minutes at room temperature, after which A660 was measured in a Spectramax microplate reader (Molecular Devices) and phosphate concentrations determined by reference to a standard curve. Note that all polyP concentrations reported in this study are given in terms of phosphate monomer concentration (monomer formula: NaPO3).
Clotting assays
Clotting times of citrated human plasma were quantified at 37°C using a STart4 coagulometer (Diagnostica Stago). Tests of the contact pathway of blood clotting were conducted using final concentrations of 33% plasma, 25μM phospholipid, 0-40μM polyP, 41.7mM imidazole (pH 7.0), and 8.33mM CaCl2. Prewarmed polyP in imidazole buffer was incubated in coagulometer cuvettes with prewarmed plasma and phospholipid for 3 minutes, after which clotting was initiated by the addition of CaCl2. For comparison, a standard curve was prepared using various concentrations of kaolin (0.04-3333 μg/mL), and no polyP, in the same clotting assay (supplemental Figure 1C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The specific activities of polyP preparations were then calculated, by reference to this standard curve, to yield kaolin equivalents20 (ie, the kaolin concentration that yields the same clotting time as polyP, when both are plotted in terms of μg/mL).
Clotting reactions initiated by FXa contained 33% plasma, 33μM phospholipid, 0-40μM polyP, 16.7mM Tris (pH 7.4), 33.3mM NaCl, 0.03% bovine serum albumin, 333 pM FXa, and 8.33mM CaCl2. Plasma was prewarmed separately from polyP and FXa components to minimize inhibition of FXa by plasma inhibitors and to prevent contact activation of plasma by polyP. Components were then mixed simultaneously in prewarmed coagulometer cuvettes to initiate clotting. As the ability of polyP to shorten FXa clotting times has been attributed to the acceleration of FV activation, we converted these shortened FXa clotting times into polyP specific activities, by comparison to a standard curve, in which the FXa-initiated clotting reaction was conducted in the absence of polyP but with varying concentrations (0-6.7nM) of FVa added to the plasma. The specific activities of polyP preparations were calculated, by reference to this standard curve, to yield FVa equivalents (ie, comparing the FVa concentration that would yield the same clotting time as polyP, when both are plotted in terms of molar concentrations).
In some experiments, the ability of polyP to antagonize the anticoagulant activity of TFPI was quantified by adding TFPI to plasma (400 ng/mL, final) and initiating clotting with 0.1nM FXa. For comparison, a standard curve was prepared in the absence of polyP, but with varying TFPI concentrations (0-400 ng/mL) added to the plasma. This standard curve was then used to calculate the percent inhibition of TFPI activity.
Fibrin clot turbidity
Fibrin clots were formed in medium-binding, polystyrene 96-well plates as previously described.5 Briefly, mixtures of fibrinogen, polyP, and calcium ions were preincubated for 15 minutes at room temperature, after which thrombin was added to trigger clot formation. Final (160 μL) reactions contained 2.6 mg/mL fibrinogen, 10nM thrombin, 0-400μM polyP, 50mM Tris-HCl (pH 7.4), 150mM NaCl, 0.02% NaN3, and 2.5mM CaCl2. Turbidities (A405) of fibrin clots were quantified using a Spectramax microplate reader 60 minutes after thrombin addition.
Results
We previously reported that heterodisperse polyP preparations are potent modulators of blood clotting.3-5 In particular, we showed that heterodisperse, commercially available polyP type 75+, whose polymer lengths encompass the polyP sizes secreted by human platelets, was more potent in triggering the contact pathway than was polyP type 25. However, both polyP type 25 and 75+ contain some polymers that are considerably longer (and shorter) than their nominal mean polymer lengths, so the minimum polyP polymer lengths required to modulate blood clotting were not known. This is important because polyP secreted by platelets (60 to 100mers)2 are considerably shorter than the polyP found in infectious microorganisms (frequently in excess of 1000 phosphate units long).1 We therefore tested the hypothesis that very long polyP polymers (500 to 1500mers) exert differential effects on blood clotting, compared with the much shorter polyP polymers that are secreted by platelets. To test this hypothesis, we isolated polyP preparations with narrow size distributions.
Size-fractionation of polyP and determination of polymer length
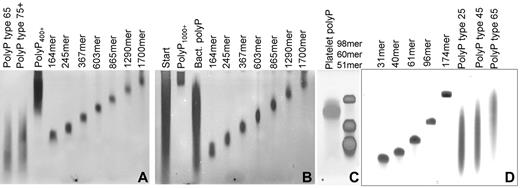
PolyP was size-fractionated by preparative PAGE, and the polymer sizes of selected fractions were determined by NMR (Figure 1). We also used NMR analyses to determine the mean polymer lengths for polyP type 65 (67 phosphates) and type 75+ (158 phosphates). Figure 2 shows the size distributions of several commercial, heterodisperse polyP preparations, compared with narrowly size-fractionated polyP preparations used in this study. We also prepared heterodisperse polyP with polymer sizes ≥ 400mers (polyP400+; Figure 2A) and ≥ 1000mers (polyP1000+; Figure 2B). PolyP purified from S typhimurium was very heterodisperse, with polymers ranging in size from approximately 100mers to approximately 1300mers (Figure 2B), while platelet-derived polyP was fairly homogeneous in size (Figure 2C) and comparable to the size range reported previously.2,3
One-dimensional 31P NMR spectrum of 34mer polyP. Peaks corresponding to the 2 external phosphates (α), phosphates adjacent to the external phosphates (β), and internal phosphates, as indicated.
One-dimensional 31P NMR spectrum of 34mer polyP. Peaks corresponding to the 2 external phosphates (α), phosphates adjacent to the external phosphates (β), and internal phosphates, as indicated.
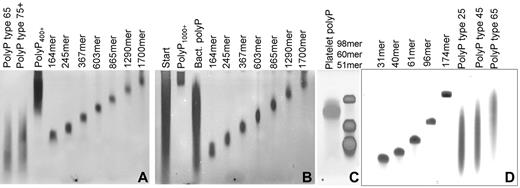
Size distributions of polyP preparations used in this study. PolyP was resolved by electrophoresis on 5% polyacrylamide gels with 7M urea (A-B) or on 15% polyacrylamide gels with 7M urea (C) and detected by DAPI-negative staining, or resolved on 15% polyacrylamide gels with 7M urea and detected by toluidine blue staining (D). Lanes containing size-fractionated polyP preparations are labeled by estimated polymer length, while the heterodisperse polyP preparations are labeled polyP type 25, type 45, type 65, type 75+, polyP400+, polyP1000+, “Platelet” (human platelet-derived polyP), “Bact” (S typhimurium polyP), and “Start” (the solubilized “insoluble” polyP preparation that was used as the starting material for obtaining very long-chain polyP). Note that polyP types 25, 45, 65, and 75+ refer to the nominal mean polymer lengths (given by the supplier), while the rest of the polyP sizes indicated in this figure were determined either by NMR analyses or were estimated from the sample's migration on analytical PAGE using polyP standards whose mean polymer lengths were determined by NMR (analyses not shown).
Size distributions of polyP preparations used in this study. PolyP was resolved by electrophoresis on 5% polyacrylamide gels with 7M urea (A-B) or on 15% polyacrylamide gels with 7M urea (C) and detected by DAPI-negative staining, or resolved on 15% polyacrylamide gels with 7M urea and detected by toluidine blue staining (D). Lanes containing size-fractionated polyP preparations are labeled by estimated polymer length, while the heterodisperse polyP preparations are labeled polyP type 25, type 45, type 65, type 75+, polyP400+, polyP1000+, “Platelet” (human platelet-derived polyP), “Bact” (S typhimurium polyP), and “Start” (the solubilized “insoluble” polyP preparation that was used as the starting material for obtaining very long-chain polyP). Note that polyP types 25, 45, 65, and 75+ refer to the nominal mean polymer lengths (given by the supplier), while the rest of the polyP sizes indicated in this figure were determined either by NMR analyses or were estimated from the sample's migration on analytical PAGE using polyP standards whose mean polymer lengths were determined by NMR (analyses not shown).
Very long polyP polymers are most efficient at initiating clotting via the contact pathway
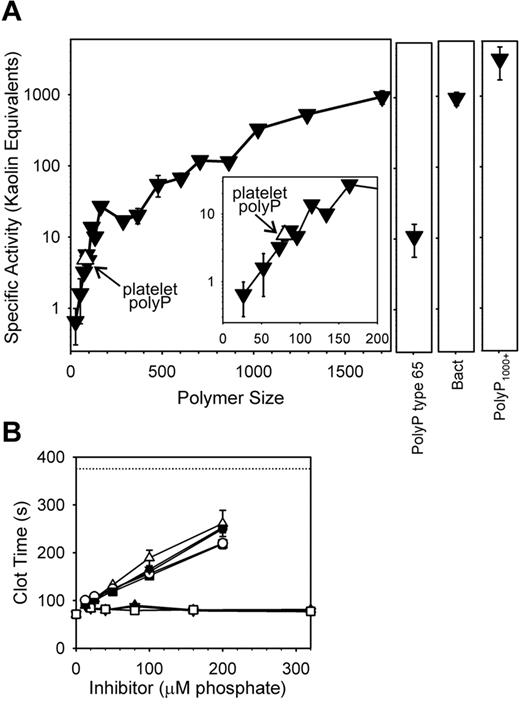
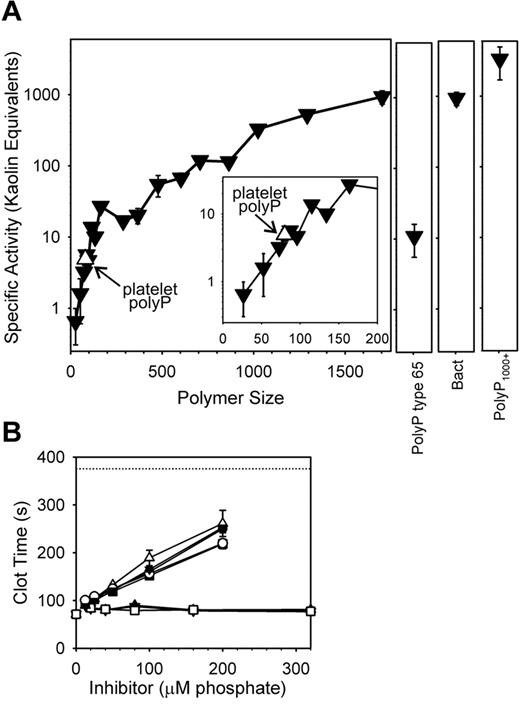
Using polyP preparations of narrowly defined polymer size, we found that polyP from monophosphate to 30mers did not shorten the clotting time of recalcified plasma, when tested at 20μM polyP (supplemental Figure 1A-B). 53mers detectably accelerated plasma clotting, with clotting times decreasing as polyP polymer length increased. To understand the relative potencies of these different polyP preparations, we converted their clotting times into specific activities, based on “kaolin equivalents.”20 This was accomplished by determining the concentration, on a mass basis, of kaolin that would yield the same clotting time observed with a given concentration of polyP (see supplemental Figure 1C for the kaolin standard curve). The results (Figure 3A) show that polyP-specific activities increased as polymer length increased. Thus, polyP purified from human platelets (size range, ∼ 60-100mers) had a specific activity in this clotting assay that was, on a mass basis, 4.9 ± 0.9-fold higher than kaolin. Note that polyP purified from human platelets had essentially the same specific activity as synthetic polyP of the same size (Figure 3A). Bacterial polyP had a very high specific activity, while the strongest procoagulant activity in this assay was exhibited by polyP1000+, which had a specific activity > 3000-fold higher than that of kaolin.
Initiation of clotting via the contact pathway is most efficient with very long polyP polymers. (A) Clotting was initiated by preincubating 5μM polyP with citrated plasma for 3 minutes at 37°C, after which calcium chloride was added and the time to clot formation recorded. PolyP specific activities were calculated by comparing polyP-initiated clotting times to a standard curve in which clotting was initiated by varying kaolin concentrations (see supplemental Figure 1C), yielding “kaolin equivalents” on a mass basis. Activities of sized-fractionated polyP preparations (▾) are compared with platelet-derived polyP (▿; also indicated with arrows), bacterial-derived polyP (“Bact”), polyP type 65, and PolyP1000+. The inset focuses on polymers shorter than 200mers. The point for platelet polyP was plotted at its mean polymer length (80mers). (B) Inhibition of contact-pathway–initiated clotting by an excess of small polyP polymers. Plasmas were preincubated for 3 minutes 37°C with a combination of 10μM polyP1000+ and the indicated concentrations of shorter phosphate/polyP preparations (identified as “inhibitor” on the x-axis), after which calcium chloride was added to allow clotting. The short phosphates were: monophosphate (▿), PPi (▴), and triphosphate (□) (none of which had any effect); and 11mers polyP (▾), 16mers polyP (○), 35mers polyP (■), 53mers polyP (▵), and 83mers (●) (which prolonged the clotting times in a concentration-dependent manner). The dotted line represents the clotting time in the absence of polyP. Data in all panels are mean ± SE (n = 3).
Initiation of clotting via the contact pathway is most efficient with very long polyP polymers. (A) Clotting was initiated by preincubating 5μM polyP with citrated plasma for 3 minutes at 37°C, after which calcium chloride was added and the time to clot formation recorded. PolyP specific activities were calculated by comparing polyP-initiated clotting times to a standard curve in which clotting was initiated by varying kaolin concentrations (see supplemental Figure 1C), yielding “kaolin equivalents” on a mass basis. Activities of sized-fractionated polyP preparations (▾) are compared with platelet-derived polyP (▿; also indicated with arrows), bacterial-derived polyP (“Bact”), polyP type 65, and PolyP1000+. The inset focuses on polymers shorter than 200mers. The point for platelet polyP was plotted at its mean polymer length (80mers). (B) Inhibition of contact-pathway–initiated clotting by an excess of small polyP polymers. Plasmas were preincubated for 3 minutes 37°C with a combination of 10μM polyP1000+ and the indicated concentrations of shorter phosphate/polyP preparations (identified as “inhibitor” on the x-axis), after which calcium chloride was added to allow clotting. The short phosphates were: monophosphate (▿), PPi (▴), and triphosphate (□) (none of which had any effect); and 11mers polyP (▾), 16mers polyP (○), 35mers polyP (■), 53mers polyP (▵), and 83mers (●) (which prolonged the clotting times in a concentration-dependent manner). The dotted line represents the clotting time in the absence of polyP. Data in all panels are mean ± SE (n = 3).
The procoagulant activity of polyP in this clotting assay was dependent on the contact pathway, because FXII-deficient plasma did not clot (all clotting times were > 1000 seconds with 20μM polyP; data not shown).
Adding an excess of short-chain polyP of a variety of sizes inhibited plasma clotting initiated by long-chain polyP (Figure 3B). On the other hand, a similarly large excess of very short phos-phates (monophosphate, PPi, and triphosphate) had no effect on polyP-initiated clotting via the contact pathway (Figure 3B).
Shorter polyP polymers accelerate FXa-initiated clotting
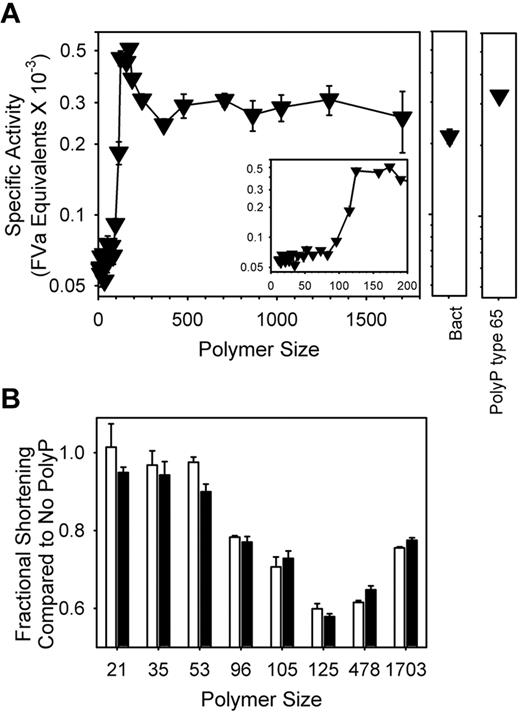
We previously reported that polyP type 75+ shortened plasma-clotting times initiated by either tissue factor or FXa, an effect that we demonstrated was due to accelerating FV activation.4 In the present study, we evaluated this aspect of polyP's procoagulant effect using polyP polymers of defined lengths in clotting assays initiated by FXa. Short polymers did not appreciably shorten FXa-initiated plasma-clotting times, but longer polyP polymers (> 60mers) detectably accelerated plasma clotting (supplemental Figure 2A-B). We converted these clotting times into specific activity (FVa equivalents, on a molar basis) by reference to a standard curve, in which plasma was spiked with varying FVa concentrations (standard curve not shown). The results (Figure 4) show that maximal specific activities in this clotting assay were achieved with polyP polymers that were approximately 125-200 phosphates long, while progressively longer polymers (especially ≥ 400mers, including bacterial polyP) had somewhat reduced specific activities, compared with 125mers. A large excess of monophosphate, PPi, or triphosphate did not antagonize the procoagulant activity of longer chain polyP (not shown). PolyP shortened FXa-initiated clotting times equally well in normal and FXII-deficient plasma (Figure 4B), indicating that this effect of polyP is independent of the contact pathway of blood clotting.
FXa-initiated clotting is accelerated by shorter polyP polymers than are required for initiating clotting via the contact pathway. PolyP (5μM) was added simultaneously to normal plasma together with 333 pM FXa and calcium chloride, and time to clot formation was measured. (A) PolyP specific activities were calculated by comparing polyP clot times to a standard curve in the absence of polyP, but in which varying FVa concentrations were added to plasma (not shown). The FVa concentration (in nM FVa) that yielded the same clotting time as a given polyP preparation was then divided by the polyP concentration (5000 nM). Sized-fractionated polyP preparations (▾) are compared with bacterial-derived polyP (“Bact”) and polyP type 65. The inset focuses on polymers shorter than 200mers. (B) Shortening of the FXa clotting time by polyP is independent of FXII. PolyP preparations of the indicated polymer sizes (all at 20μM phosphate) were added to FXa-initiated clotting reactions conducted with either pooled normal plasma (white bars) or FXII-deficient plasma (black bars). Clotting times with polyP were normalized to the clotting time of the respective plasma without polyP. Data in all panels are mean ± SE (n = 3).
FXa-initiated clotting is accelerated by shorter polyP polymers than are required for initiating clotting via the contact pathway. PolyP (5μM) was added simultaneously to normal plasma together with 333 pM FXa and calcium chloride, and time to clot formation was measured. (A) PolyP specific activities were calculated by comparing polyP clot times to a standard curve in the absence of polyP, but in which varying FVa concentrations were added to plasma (not shown). The FVa concentration (in nM FVa) that yielded the same clotting time as a given polyP preparation was then divided by the polyP concentration (5000 nM). Sized-fractionated polyP preparations (▾) are compared with bacterial-derived polyP (“Bact”) and polyP type 65. The inset focuses on polymers shorter than 200mers. (B) Shortening of the FXa clotting time by polyP is independent of FXII. PolyP preparations of the indicated polymer sizes (all at 20μM phosphate) were added to FXa-initiated clotting reactions conducted with either pooled normal plasma (white bars) or FXII-deficient plasma (black bars). Clotting times with polyP were normalized to the clotting time of the respective plasma without polyP. Data in all panels are mean ± SE (n = 3).
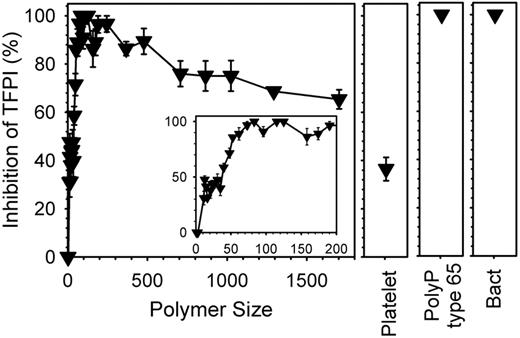
Shorter polyP polymers abrogate TFPI anticoagulant activity
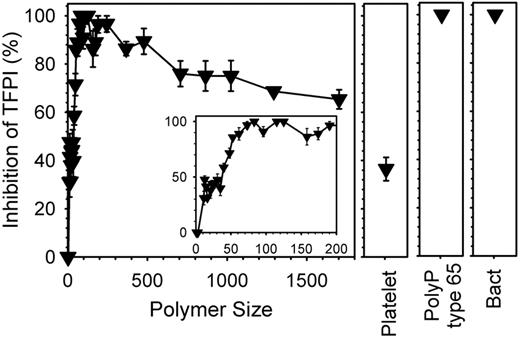
We previously reported that polyP abrogated the anticoagulant function of TFPI, which was largely attributed to the ability of polyP to accelerate FV activation4 ; FVa is known to protect FXa from TFPI inhibition,21 which we previously confirmed.4 When we tested the ability of polyP preparations of defined polymer lengths to abrogate TFPI anticoagulant activity, we found that polymers as short as 11mers detectably accelerated clotting in the presence of TFPI, while longer polymers were more potent in antagonizing TFPI anticoagulant function (supplemental Figure 3A-B). Maximal abrogation of TFPI anticoagulant activity was observed with polyP preparations in the size range from approximately 70mers to approximately 250mers (Figure 5). Platelet-derived polyP exhibited an intermediate potency in antagonizing TFPI function, while bacterial polyP and polyP type 65 were very potent in this assay. Adding a large excess of monophosphate, PPi, or triphosphate did not reverse the inhibition of TFPI anticoagulant activity by longer chain polyP (data not shown).
Accelerating FXa-initiated clotting requires even shorter polyP polymers in the presence of added TFPI. Clotting reactions were conducted as in Figure 4A, except that plasma contained 400 ng/mL TFPI, clotting was initiated with 0.1nM FXa and polyP, when included, was at 20μM. Percent TFPI inhibition was calculated by comparing polyP clot times to a standard curve in the absence of polyP, but in which 0-400 ng/mL TFPI had been added to plasma (not shown). Sized-fractionated polyP preparations (▾) are compared with platelet-derived polyP (“Platelet,” at 150μM), bacterial-derived polyP (“Bact”), and polyP type 65. The inset focuses on polymers shorter than 200mers. Data are mean ± SE (n = 3).
Accelerating FXa-initiated clotting requires even shorter polyP polymers in the presence of added TFPI. Clotting reactions were conducted as in Figure 4A, except that plasma contained 400 ng/mL TFPI, clotting was initiated with 0.1nM FXa and polyP, when included, was at 20μM. Percent TFPI inhibition was calculated by comparing polyP clot times to a standard curve in the absence of polyP, but in which 0-400 ng/mL TFPI had been added to plasma (not shown). Sized-fractionated polyP preparations (▾) are compared with platelet-derived polyP (“Platelet,” at 150μM), bacterial-derived polyP (“Bact”), and polyP type 65. The inset focuses on polymers shorter than 200mers. Data are mean ± SE (n = 3).
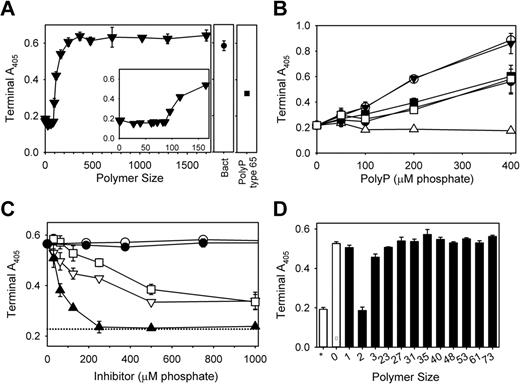
Longer polyP polymers enhance fibrin clot turbidity
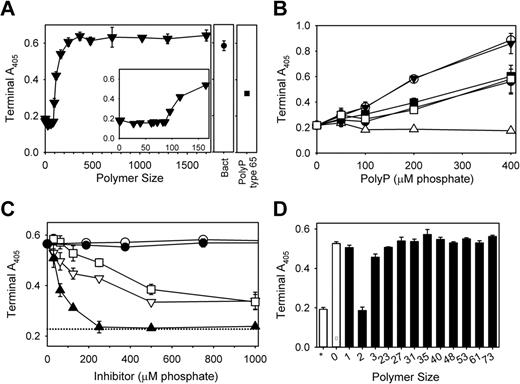
We recently described an additional procoagulant effect of polyP downstream of thrombin generation. When polyP was added to clotting reactions consisting of purified fibrinogen, thrombin, and calcium ions, the resulting fibrin fibrils had markedly increased diameters.5 Our previous study was performed with a heterodisperse polyP preparation (type 75+), so here we tested the ability of polyP preparations of defined polymer lengths to enhance fibrin clot structure, as assessed by increased clot turbidity (Figure 6A). Shorter polymers (< 100mers) did not influence fibrin clot turbidity, while progressively longer polymers increased clot turbidity, with ≥ 250mers supporting maximal turbidity increases. Bacterial polyP promoted similarly large increases in fibrin turbidity (Figure 6A). Notably, the magnitude of the increase in fibrin clot turbidity elicited by long-chain polyP (700mers and polyP400+) was roughly double that elicited by polyP type 65 or 75+ (Figure 6A-B).
Optimal enhancement of fibrin clot turbidity requires relatively long polyP polymers. Fibrin clots were prepared by adding thrombin to fibrinogen that had been preincubated with Ca2+ and polyP, and clot turbidity (A405) was quantified 60 minutes after thrombin addition. (A) Clot turbidity as a function of polyP polymer size (all at 150μM phosphate). The value for x = 0 is in the absence of added polyP, while “Bact” indicates S typhimurium–derived polyP. The inset focuses on polyP preparations shorter than 180mers. (B) Clot turbidity as a function of polyP concentration, using the following polyP polymer lengths: 65mers (▵), 158mers (■), 700mers (○), polyP type 65 (●), polyP type 75+ (□), and polyP400+ (▾). (C) PPi abrogates the ability of polyP to enhance clot turbidity. Fibrinogen was preincubated with calcium ions, 150μM polyP400+, and the indicated concentrations of phosphate-containing substances, after which thrombin was added. The substances tested were: monophosphate (▿), PPi (▴), triphosphate (□), ADP (○), and ATP (●). The dotted line represents clot turbidity in the absence of polyP. (D) PPi, but not other polyP, polymers abrogates the ability of polyP400+ to enhance clot turbidity. Phosphate polymers of the indicated lengths (all at 150μM phosphate) were included in fibrin-clotting reactions as performed in panel C. The open bar with the asterisk represents clot turbidity in the absence of polyP, while the open bar with “0” represents clot turbidity with polyP400+, but no added small phosphate polymer.
Optimal enhancement of fibrin clot turbidity requires relatively long polyP polymers. Fibrin clots were prepared by adding thrombin to fibrinogen that had been preincubated with Ca2+ and polyP, and clot turbidity (A405) was quantified 60 minutes after thrombin addition. (A) Clot turbidity as a function of polyP polymer size (all at 150μM phosphate). The value for x = 0 is in the absence of added polyP, while “Bact” indicates S typhimurium–derived polyP. The inset focuses on polyP preparations shorter than 180mers. (B) Clot turbidity as a function of polyP concentration, using the following polyP polymer lengths: 65mers (▵), 158mers (■), 700mers (○), polyP type 65 (●), polyP type 75+ (□), and polyP400+ (▾). (C) PPi abrogates the ability of polyP to enhance clot turbidity. Fibrinogen was preincubated with calcium ions, 150μM polyP400+, and the indicated concentrations of phosphate-containing substances, after which thrombin was added. The substances tested were: monophosphate (▿), PPi (▴), triphosphate (□), ADP (○), and ATP (●). The dotted line represents clot turbidity in the absence of polyP. (D) PPi, but not other polyP, polymers abrogates the ability of polyP400+ to enhance clot turbidity. Phosphate polymers of the indicated lengths (all at 150μM phosphate) were included in fibrin-clotting reactions as performed in panel C. The open bar with the asterisk represents clot turbidity in the absence of polyP, while the open bar with “0” represents clot turbidity with polyP400+, but no added small phosphate polymer.
We investigated the ability of small phosphate-containing molecules to abrogate the enhancement of fibrin clot turbidity by long-chain polyP. Neither ADP nor ATP diminished the turbidity of fibrin clots formed in the presence of polyP400+, but monophosphate, PPi, and triphosphate—to varying extents—inhibited the ability of polyP400+ to enhance fibrin clot turbidity (Figure 6C). In particular, PPi potently blocked the polyP-mediated enhancement of fibrin clot turbidity, with half-maximal inhibition at 58μM and complete abrogation at 250μM PPi. Monophosphate and triphosphate were far less potent than PPi; even at 1mM, they decreased the polyP-mediated enhancement of fibrin clot turbidity by only 65% (Figure 6C). PPi had no measurable effect on fibrin clot turbidity in the absence of polyP (not shown). PolyP polymers from 23mers to 73mers (tested at 150μM) did not detectably diminish the enhancement of fibrin clot turbidity induced by 100μM polyP400+ (Figure 6D).
Discussion
We previously reported that heterodisperse polyP preparations modulate the human blood-clotting system by initiating the contact pathway of blood clotting,3,4 accelerating the activation of FV by thrombin and FXa,4 and enhancing the thickness of fibrin fibers.5 Here, we demonstrate that polyP polymers of different lengths exert differential effects on blood coagulation. In a separate study, we recently showed that polyP purified from human platelets triggered plasma clotting via the contact pathway, promoted activation of FXII and prekallikrein in plasma, promoted bradykinin formation in plasma, caused bradykinin-mediated edema when injected subcutaneously in mice, and triggered thrombosis when injected intravenously in mice.3 That study showed that FXII-deficient mice were protected from the lethal effects of injected polyP, and also that phosphatase treatment of activated platelets (to hydrolyze released polyP) abrogated their ability to trigger clotting via the contact pathway.3 In the present study, we found that polyP polymers of the sizes secreted by platelets (60-100mers) initiated clotting via the contact pathway with a potency, on a mass basis, that was approximately 5-fold greater than that of kaolin, a potent trigger of the contact pathway of blood clotting. Thus, the results in the present study are fully consistent with our previous demonstration that polyP secreted from platelets exerts procoagulant and proinflammatory activity in vivo.3 On the other hand, longer polyP polymers, including those isolated from bacteria, are even more potent at activating the contact pathway of blood clotting—and, in fact, these long polyP polymers are, on a mass basis, thousands of times more potent than kaolin in triggering blood clotting.
We now report that short polyP polymers antagonized the ability of very long polyP polymers to trigger blood clotting via the contact pathway. Long polyP polymers likely act as templates to assemble the multiple proteins necessary to fully activate the contact pathway, and, in fact, we recently showed that polyP binds to FXII, high MW kininogen, FXI, and prekallikrein (with the tightest binding to FXII and high MW kininogen).3 We speculate that shorter polyP polymers also bind to these individual clotting proteins but are not long enough to bind multiple proteins simultaneously, thereby acting as competitors that keep these proteins from assembling effectively together on polymers long enough to promote the mutual activation of FXII and prekallikrein. Notably, polyP isolated from human platelets spans a narrow size range, and in particular, platelet polyP lacks polymers shorter than 60mers, which could inhibit the activation of the contact pathway.2,3 On the other hand, commercial polyP preparations are typically very heterodisperse, with polyP type 65 and type 75+ containing significant quantities of very short polyP polymers. This may explain a recent report that polyP type 65 (from the same commercial source as in this study) was very effective at promoting FXII autoactivation in plasma, but much less effective at promoting FXI or prekallikrein activation.22 Enzymes of the contact pathway participate in a variety of (patho)physiologic processes other than triggering blood clotting, including blood-pressure regulation as well as modulating fibrinolysis, angiogenesis, and apoptosis; in fact, this system has been described as the plasma kallikrein-kinin system, because prekallikrein can be activated on cell surfaces independently of FXII.23 It is possible that shorter polyP polymers may promote the activation of some of the enzymes of the contact pathway, even when this does not lead to actual clot formation, an idea that we are investigating further.
In this study, we showed that bacterial polyP was very potent at triggering the contact pathway of blood clotting. Damaged microorganisms might release intracellular polyP, and organisms such as Neisseria meningitidis and N gonorrhoeae express large amounts of long-chain polyP on the exterior of their cells.24,25 We previously showed that exposure of long-chain bacterial polyP to plasma also leads to elaboration of inflammatory mediators, such as bradykinin.3 We therefore propose that the response of the clotting system to very long-chain polyP may be a part of the host response to pathogens.
Long-chain polyP may also come from endogenous sources in mammals; many tissues contain polyP with a size range of 50mers to 800mers, while brain contains primarily long-chain polyP (∼ 800mers).8 Release of long-chain polyP molecules from intracellular compartments in association with ischemia-triggered cell injury might play a role in contact activation and subsequent thrombus formation, as well as contact-pathway–mediated inflammatory responses. In mouse models of cerebral artery ischemia-reperfusion, deficiency of contact pathway clotting factors was neuroprotective.26
PolyP enhances the turbidity of fibrin clots by causing the formation of thicker fibrin fibrils that appear to incorporate polyP into the clot.5 In the present study, we found that polyP polymers shorter than 100mers had no measurable effect on fibrin clot turbidity, suggesting that polyP of the size secreted by platelets will not modulate fibrin clot structure. On the other hand, polyP polymers longer than 250mers maximally enhanced fibrin clot turbidity, as did bacterial polyP. Interestingly, we found that PPi potently inhibited the enhancement of fibrin clot turbidity by long-chain polyP. Platelet-dense granules contain abundant PPi (∼ 1.5 μmol PPi per 1011 platelets), which is secreted in response to platelet agonists.27 PPi released from platelets could readily attain a concentration of 2-7μM in whole blood, with orders of magnitude higher concentrations possible within platelet-rich thrombi. A clear role for platelet-released PPi has not been previously defined, but we propose that PPi is a novel modulator of fibrin clot structure. Further studies of the interactions between PPi and fibrin(ogen), and the ability of PPi to block the effects of polyP on fibrin structure, are clearly warranted.
PolyP may have potential as a procoagulant agent to control bleeding. We previously reported that heterodisperse polyP type 75+ could reverse the anticoagulant effect of a number of anticoagulant drugs, as well as shorten the clotting times of hemophilic plasmas and plasmas from patients on coumadin therapy.6 On the other hand, injection of heterodisperse polyP preparations intravenously in mice can elicit generalized activation of the contact pathway, resulting in the release of vasoactive substances and inflammatory mediators leading to hypotension and edema.3 The present study showed that short polyP polymers had limited ability to activate the contact pathway, but retained ability to accelerate FV activation and abrogate the anticoagulant activity of TFPI. It would therefore be interesting to investigate if suitably size-fractionated polyP preparations could be used pharmacologically to control bleeding while limiting the unwanted side effect of systemic activation of the contact pathway.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr George Broze (Washington University, St Louis, MO) for the kind gift of the recombinant TFPI, Dr James Slauch (University of Illinois) for supplying the exopolyphosphatase-deficient Salmonella, and the University of Illinois School of Chemical Sciences NMR Facility for assistance with data collection.
This work was supported by National Institutes of Health grants R01 HL47014 (to J.H.M.) and R01 GM75937 (to C.M.R.), and by grant 06-2328 from the Roy J. Carver Charitable Trust (to J.H.M.).
National Institutes of Health
Authorship
Contribution: S.A.S., S.H.C., R.D.H., J.H., and J.B. performed experiments and contributed figures; and S.A.S., C.R., and J.H.M. designed the research, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: J.H.M. and S.A.S. are coinventors on pending patent applications covering potential medical uses of polyP. The remaining authors declare no competing financial interests.
Correspondence: James H. Morrissey, Department of Biochemistry, College of Medicine, University of Illinois at Urbana-Champaign, 417 Medical Science Bldg, MC-714, 506 South Mathews Ave, Urbana, IL 61801; e-mail: jhmorris@illinois.edu.