Abstract
Abstract 1244
Voriconazole (V) treatment has been shown to improve the 12 week (W) survival rate of hematological patients (pts) with invasive aspergillosis (IA), including recipients of allogeneic hematopoietic stem cell transplants (HSCT). We investigated whether this early survival advantage could translate into a significant increase in overall survival.
We retrospectively reviewed all consecutive pts who received a transplant between Sept. 1997 and Dec. 2008 at Saint-Louis Hospital and were diagnosed as having IA. The temporal origin of the study was the date of IA diagnosis for each patient. Factors associated with survival were analyzed using Cox proportional hazard models. Separate models were estimated for survival up to 12 W and for survival between 12 W and 24 months (M) in pts surviving longer than 12 W. The deaths of pts with and without IA were analyzed with a competing risk framework. Cumulative incidence curves were compared using Gray's tests.
Our study examined 89 IA pts. The median follow-up was 70 M (range, 11–130 M). Two pts did not receive any antifungal treatment and were excluded from subsequent analyses. Of the 87 pts, 42 received first-line V and 45 primarily received a lipid formulation of amphotericin B (n=25), amphotericin B deoxycholate (n=10), caspofungin (n=8) or itraconazole (n=2). The primary characteristics of pts with IA and their causes of death, separated by V as first-line treatment, are shown in the table below.
| . | No voriconazole (n=45) . | Voriconazole (n=42) . |
|---|---|---|
| Median age | 40 | 33 |
| Good risk transplant (%) | 21 (48) | 18 (43) |
| Stem cell source (%) | ||
| Bone marrow | 29 (64) | 21 (50) |
| Peripheral blood stem cells | 10 (22) | 15 (36) |
| Cord blood | 6 (13) | 6 (14) |
| Donor type: identical sibling (%) | 23 (51) | 9 (21) |
| Recipient CMV positive (%) | 25 (60) | 25 (61) |
| Myeloablative conditioning (%) | 35 (81) | 25 (60) |
| Progressive GVHD at IA diagnosis (%) | 25 (56) | 18 (43) |
| ANC/mm3, median (range) | 1000 (0 to 12000) | 1805 (100 to 7000) |
| Corticosteroid dose (mg/kg) | ||
| At diagnosis, median (range) | 1 (0 to 7) | 1 (0 to 4) |
| Cumulated dose over the last 7 days, median (range) | 7 (0 to 48) | 8 (0 to 32) |
| Years of IA diagnosis | ||
| Up to 2002 | 32 (71) | 5 (12) |
| From 2003 | 13 (29) | 37 (88) |
| Type of IA diagnosis (EORTC/MSG 2008) (%) | ||
| Proven | 8 (18) | 5 (12) |
| Probable | 32 (71) | 33 (79) |
| Possible | 5 (11) | 4 (10) |
| IA onset within 30 days from transplant (%) | 9 (20) | 6 (14) |
| Bilateral pulmonary lesions (%) | 27 (71) | 16 (46) |
| Number of deaths (%) | 36 (80) | 35 (83) |
| Main cause of death (%) | ||
| IA | 21 (58) | 8 (23) |
| GVHD | 4 (11) | 6 (17) |
| Relapse | 2 (6) | 5 (14) |
| Infection (not IA) | 4 (11) | 7 (17) |
| Acute respiratory distress syndrome | 4 (11) | 3 (9) |
| Other | 1 (3) | 6 (17) |
| . | No voriconazole (n=45) . | Voriconazole (n=42) . |
|---|---|---|
| Median age | 40 | 33 |
| Good risk transplant (%) | 21 (48) | 18 (43) |
| Stem cell source (%) | ||
| Bone marrow | 29 (64) | 21 (50) |
| Peripheral blood stem cells | 10 (22) | 15 (36) |
| Cord blood | 6 (13) | 6 (14) |
| Donor type: identical sibling (%) | 23 (51) | 9 (21) |
| Recipient CMV positive (%) | 25 (60) | 25 (61) |
| Myeloablative conditioning (%) | 35 (81) | 25 (60) |
| Progressive GVHD at IA diagnosis (%) | 25 (56) | 18 (43) |
| ANC/mm3, median (range) | 1000 (0 to 12000) | 1805 (100 to 7000) |
| Corticosteroid dose (mg/kg) | ||
| At diagnosis, median (range) | 1 (0 to 7) | 1 (0 to 4) |
| Cumulated dose over the last 7 days, median (range) | 7 (0 to 48) | 8 (0 to 32) |
| Years of IA diagnosis | ||
| Up to 2002 | 32 (71) | 5 (12) |
| From 2003 | 13 (29) | 37 (88) |
| Type of IA diagnosis (EORTC/MSG 2008) (%) | ||
| Proven | 8 (18) | 5 (12) |
| Probable | 32 (71) | 33 (79) |
| Possible | 5 (11) | 4 (10) |
| IA onset within 30 days from transplant (%) | 9 (20) | 6 (14) |
| Bilateral pulmonary lesions (%) | 27 (71) | 16 (46) |
| Number of deaths (%) | 36 (80) | 35 (83) |
| Main cause of death (%) | ||
| IA | 21 (58) | 8 (23) |
| GVHD | 4 (11) | 6 (17) |
| Relapse | 2 (6) | 5 (14) |
| Infection (not IA) | 4 (11) | 7 (17) |
| Acute respiratory distress syndrome | 4 (11) | 3 (9) |
| Other | 1 (3) | 6 (17) |
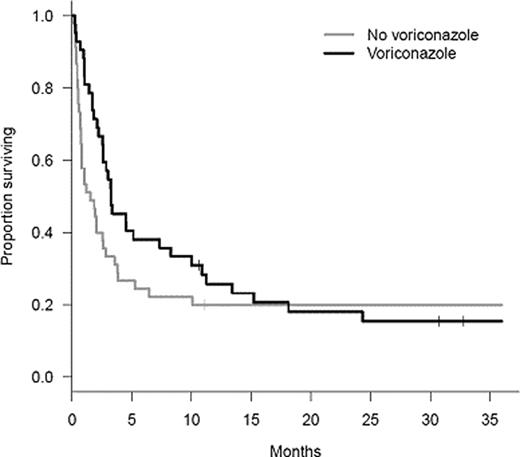
The median survival was 2.6 M, and the overall survival at 24 M was 19% (95% CI 12–30 M) (see figure).
Overall, the survival rates of the two groups were significantly different (P= 0.010). However, the differences in survival were quite dramatic prior to 10 M, whereas both survival curves became very close after one year. At 18 M, the numbers of surviving pts were almost identical in the two groups [19% (95% CI: 11–34%) in pts who did not receive V as first-line treatment vs. 21% (95% CI 11–38%) in pts who did]. Pts who did not receive V as a first-line treatment displayed a higher probability of dying from IA than those who did (P=0.004), whereas opposite results were found for mortality in pts without IA (P=0.006). The 24-M cumulative incidence of death from IA was 47% (95% CI 31–61%) in the no V group and 19% (95% CI 9–33%) in the group treated with V. The 24-M cumulative incidence of death in pts without IA was 4% (95% CI 7–14%) in the no V group and 27% (95% CI 14–42%) in pts treated with V. The probability of death from another cause, with IA, was similar in both groups (29% vs. 36% at 24 M; P=0.46). After adjusting for donor type, conditioning regimen, progressive GVHD at diagnosis of IA and cumulated steroid dose (mg/kg) in the W preceding IA diagnosis, administration of V as first-line treatment was found to decrease the risk of death during the first 12 W by approximately 70% [HR=0.31 (95% CI 0.16–0.60); P=0.0005]. Conversely, analysis of mortality between 12 W and 24 M failed to identify any significant predictor of risk of death; however, only 24 pts died during this period.
The finding that first-line treatment with V, which is associated with a tremendous improvement in IA outcome, does not translate into an increase in overall survival (even in the context of early diagnosis) is striking. Diagnosis of IA following HSCT, whatever the outcome, appears to be a strong marker for poor long-term prognosis.
Bergeron:Pfizer: Speakers Bureau, none; Merck: Speakers Bureau, none; Schering: Speakers Bureau, none. Sulahian:Pfizer: Research Funding, non; Merck: Research Funding, none. Ribaud:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau, none; Schering: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau, none; Gilead: Speakers Bureau, none.
Author notes
Asterisk with author names denotes non-ASH members.