Abstract
Abstract 1301
Allogeneic transplantation in MM is pursued in the hope of establishing an immune mediated graft vs. MM effect (allogeneic effect) in order to prevent relapse. After RIC allogeneic transplantation, donor immune function (donor chimerism) is established over time in the recipient. Long term B lymphocyte recovery may be delayed by graft vs. host disease (GVHD). GVHD may also be associated with the beneficial graft vs. MM effect. The recovery pattern of the original light chain associated with MM clone (involved free light chain) and the alternate light chain (uninvolved in MM and donor derived after allotransplant) serve as markers for MM progression vs. establishment of donor derived B cell function respectively after allotransplant. The serum FLC assay is a marker for both the progression of the recipient's original MM clone (rising involved FLC) as well as establishment of donor derived immune recovery (restoration of uninvolved FLC to normal).We hypothesized that the FLC assay is influenced by the competing effects of progression of the MM clone vs. the beneficial impact of alloimmunity/GVHD on relapse.
Forty seven RIC allogeneic peripheral blood hematopoietic stem cell transplant recipients (45 matched sibling donors) for MM at the Medical College of Wisconsin (2005-2009) were studied. Conditioning regimen was,(200 cGY TBI +/− fludarabine). Median follow up was 2 years. Baseline and follow-up serum free light chain assay results at 3 monthly intervals were correlated with outcomes. The light chain associated with MM clone was designated as “involved” (IFLC) and the complementary light chain designated as the “uninvolved” (UFLC).
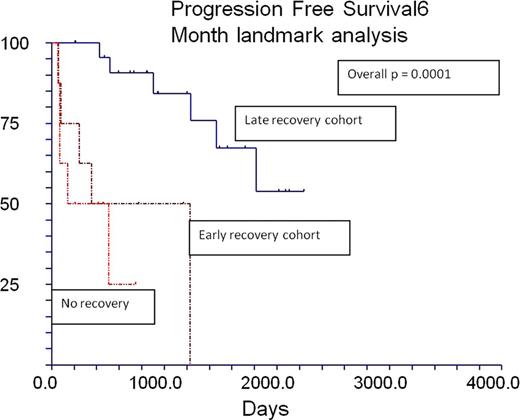
Overall (OS) and progression free (PFS) survival of cohort at 4.5 years was 54% (CI 36 – 72%) and 38% (CI 15– 61%) respectively. Treatment related mortality was 14.5%. All patients, except those with rapidly progressive MM (group 1 below), had suppression of both IFLC and UFLC immediately after RIC. Based on pattern of recovery of UFLC we identified 3 distinct groups. Group 1(n=12) – those who did not have any UFLC recovery, Group 2(n=10) those UFLC returned to normal levels within 6 months of RIC (early recovery) and a group 3 (n=25) where the UFLC recovery was delayed beyond 6 months (late recovery). Outcomes for all the three groups are shown in the table 1. The incidence of clinical GVHD was similar across the groups. Median PFS was significantly superior in the late recovery group (not reached at 5 years, vs. 4.6 mo and 11.8 mo in the other groups) (p=0.0001). In order to eliminate “survival by response” bias, we performed a landmark analysis, restricting the analysis to those alive at 6 mo after RIC and confirmed these findings (Fig 1.).
Outcomes and UFLC recovery after RIC allografts for MM
| Group . | UFLC Response Group . | Any GVHD . | Median PFS . | PFS . | Relapsed Death . | Relapsed Alive . |
|---|---|---|---|---|---|---|
| N | 47 | 23 | 10 | 7 | ||
| 1 | No Recovery (12) | 9 (75%) | 4.6 mo | 0 | 6 | 0 |
| 2 | Early Recovery (10) | 8 (80%) | 11.8 mo | 4 | 3 | 2 |
| 3 | Late Recovery (25) | 18 (72%) | NR @ 5 yrs | 19 | 1 | 5 |
| P=NS | P=0.0001 |
| Group . | UFLC Response Group . | Any GVHD . | Median PFS . | PFS . | Relapsed Death . | Relapsed Alive . |
|---|---|---|---|---|---|---|
| N | 47 | 23 | 10 | 7 | ||
| 1 | No Recovery (12) | 9 (75%) | 4.6 mo | 0 | 6 | 0 |
| 2 | Early Recovery (10) | 8 (80%) | 11.8 mo | 4 | 3 | 2 |
| 3 | Late Recovery (25) | 18 (72%) | NR @ 5 yrs | 19 | 1 | 5 |
| P=NS | P=0.0001 |
Our data suggest that after RIC, with immunosuppressive conditioning, late recovery of UFLC is a marker of the efficacy of the allogeneic effect. The antineoplastic effect of donor alloimmunity likely explains the delayed recovery of UFLC (mediated by clinical/subclinical GVH activity) and paradoxically, better anti-MM effect in the late UFLC recovery group. Rapid progression of MM clone and non-establishment of a donor anti-MM effect explains the rapid relapse in those with no UFLC recovery. In the early recovery group, a higher risk of relapse is likely mediated by the absence of an alloimmune effect sufficient enough to delay B cell recovery and also insufficient for beneficial graft vs. MM effect. Since GVHD rates were similar across groups, the beneficial “allo immune effect” is likely independent of clinical GVHD. The FLC assay may be a sensitive marker of donor B cell recovery after allotransplant and needs further validation as a measure of donor derived antineoplastic effect in MM and other malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.